Abstract
Metastasis tumor antigen 1 (MTA1), a novel candidate metastasis-associated gene, is known to increase the migration and invasion of various tumor cells in vitro. It also plays an important role in tumorigenesis and tumor aggressiveness of breast cancer. Estrogen receptor alpha (ERα) plays an important role in the etiology of breast cancer and has been widely accepted as a prognostic marker for breast cancer and a response predictor for endocrine therapy. The ERα gene methylation has been linked to the lack of ERα expression in breast cancer. The aim of the study is to assess the correlation between the ERα methylation and MTA1 expression in breast cancer and further to investigate whether the repressed ERα methylation can downregulate the expression of MTA1 in vitro. In general, we found ERα methylation had significant correlation with the MTA1 expression (p < 0.05) in female patients of breast cancer (n = 102) by methylation-specific polymerase chain reaction and immunohistochemistry. To gain a deeper insight into the molecular mechanism underlying the relation between MTA1 and ERα methylation, we treated the invasive breast cancer cell lines with the demethylating agent, found the downregulation of MTA1 protein expression, and mRNA with the unmethylation of ERα (p < 0.05). And the invasive ability of breast cancer cells was significantly positively associated with MTA1 expression. These unique findings have greatly extended our current knowledge about the relation between ERα methylation and MTA1 expression. These data strongly support the hypothesis that methylation is involved in the relation between MTA1 and ERα in breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastasis-associated protein 1 (MTA1), a master dual co-regulatory protein, is found to be an integral part of NuRD (nucleosome remodeling and histone deacetylation) complex, which has indispensable transcriptional regulatory functions via histone deacetylation and chromatin remodeling [1]. MTA1 was reported as a metastasis-associated gene [2]. Overexpression of MTA1 has been shown to be closely correlated with aggressiveness in a variety of human cancers including breast cancer [3–5]. Breast cancer is one of the most common malignancies and the leading cause of cancer deaths in women [6]. Estrogen receptor alpha (ERα) plays an important role in the etiology of breast cancer and has been widely accepted as a prognostic marker for breast cancer and a response predictor for endocrine therapy [7, 8]. Usually, the function of ERα could be silenced by ERα gene promoter methylation [9]. It has been reported that MTA1 is a target of heregulin-beta1 and represses ER-mediated transcription by recruiting histone deacetylase [10] and ShRNA targeted against MTA1 which mediate the MTA1 gene silencing and consequentially recover the protein expression of ERα in ER-negative human breast cancer cell lines MDA-MB-231 [11]. However, whether the ER-alpha promoter methylation interacts with the MTA1 expression in breast cancer has not been elucidated. The aim of the study is to assess the correlation between the ERα methylation and MTA1 expression in breast cancer and furthermore to investigate whether the repressed ERα methylation can downregulate the expression of MTA1 in vitro. We treat the invasive ERα methylated breast cancer cell lines with the demethylating agent and then evaluate the MTA1 expression and cell invasive ability with demethylation of ERα.
Materials and methods
Patients and tissues
Breast carcinoma tissue and paired non-tumor portion samples from female patients of breast cancer (n = 102) were collected from the archived tissue bank at the First Affiliated Hospital of China Medical University between 2005 and 2011. Pathology classification was based on the WHO’s criteria published by Tavassoli et al. [12–14]. None of the patients received neoadjuvant chemotherapy or radiotherapy before operation. Clinicopathological information of each patient was reviewed using the hospital medical records. The study protocol was reviewed and approved by the Ethics Committee of China Medical University, Shenyang, China.
DNA extraction from tumor tissues and bisulfite modification
The genomic DNA was extracted from the de-paraffinized samples by standard procedure with proteinase K digestion and phenol/chloroform extraction [15]. The DNA concentration was qualified by determination of OD260/280. Sodium bisulfite conversion was accomplished with 2 μg of extracted DNA per sample, using previously described methods [16]. The alkali-denatured DNA was incubated in a buffer containing 3 mol/l NaHSO3 and 0.5 mmol/l hydroquinone at 54°C for 16 h. The modified DNA was then purified using the Promega Wizard Genomic DNA Purification kit (Promega, Madison, MI, USA) and eluted with 50 μL of sterile water. DNA was precipitated with 0.5 mol/l ammonium acetate (pH 4.6), 1.5 μL of 20 mg/mL glycogen, and ethanol, and then resuspended in the Tris–ethylenediaminetetraacetic acid (EDTA) buffer.
Methylation-specific PCR for ER1, ER3, ER4, and ER5
ER1, ER3, ER4, and ER5 were chosen for the assay of methylation-specific polymerase chain reaction (PCR) (MSP) from the six primer-pairs described previously [17], which covered the most significantly methylated loci. The DNA extracted from lymphocytes of healthy volunteers was treated with SssI methyltransferase (New England Biolabs, USA) and then subjected to bisulfite modification and was used as the positive control for the methylated alleles; H2O was used as template in the negative control. The PCR protocol included the following parameters: an initial 10 min of incubation at 95°C followed by 14 cycles of 94°C for 30 s, 62°C (ER1) or 59°C (ER3, ER4, and ER5) for 45 s (−0.5°C decreased/each cycle), and 72°C for 45 s, ending with a final extension of 72°C for 10 min. The 15-μL reaction products were separated by electrophoresis on a 3% TBE/agarose gel, stained with GeneFinder™, and then visualized under UV light.
Immunohistochemical staining
Formalin-fixed and paraffin-embedded specimens were cut into 4-μm-thick sections, which were subsequently de-waxed and hydrated. Immunohistochemical staining for MTA1 (sc-17773, 1:200, Santa Cruz, USA) was performed using UltraSensitive™ S-P kits (Maixin-Bio; PR China) according to the manufacturer’s instructions and using the reagent supplied with the kit. For the negative control, phosphate-buffered saline (PBS) was used in place of the primary antibodies. We adopted the German semi-quantitative scoring system considering the staining intensity and area extent, which has been widely accepted and used in previous studies [18, 19]. Every lesion was given a score according to the intensity of the staining (no staining = 0, weak staining = 1, moderate staining = 2, strong staining = 3) and the extent of stained cells (0% = 0, 1–10% = 1, 11–50% = 2, 51–80% = 3, 81–100% = 4; negative means 0% area staining). The final immunoreactive score was determined by multiplying the intensity scores with the extent of positivity scores of stained cells, with the minimum score of 0 and a maximum score of 12 [18, 19]. Slides were independently examined by two pathologists. However, if there was a discrepancy in individual scores, both pathologists reevaluated together by reaching a consensus agreement before combining the individual scores. To obtain statistical results, a final score equal to or less than 1 was considered as negative, while scores of 2 or more were considered as positive.
Breast cancer cell lines and cell culture conditions
Invasive breast cancer cell lines including the metastatic MDA-MB-435 s and MDA-MB-231 were chosen for this study and cultured in RPMI 1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (Hyclone, USA) in a 5% CO2-humidified atmosphere at 37°C.
5-Aza-2′-deoxycytidine treatment
MDA-MB-435 s and MDA-MB-231 were exposed to fresh medium (control) or to culture media with 5 μmol/l of the demethylating agent 5-aza-2′-deoxycytidine (5-Aza-dC) (A3536, Sigma, USA) for 72 h at 37°C in a humidified atmosphere containing 5% CO2, and, after exposure, the cells were washed twice with PBS.
Methylation specific PCR of cell lines
The DNA of cell lines treated with 5-Aza-dC or fresh medium (control) were extracted and investigated by MSP as previous mentioned.
Reverse-transcriptase PCR
The cell lines were treated with 5-Aza-dC or fresh medium (control) as previous mentioned. Total RNA was extracted with TRizol reagent (Invitrogen, USA), and the quality of RNA was analyzed by A260/A280 ratio and gel analysis. The reverse transcription was performed with RNA PCR Kit (AMV Ver.3.0, Takara, Japan) according to the manufacturer’s protocols. Primers specific to human MTA1 were forward: 5′-AGCTACGAGCAGCACAACGGGGT-3′ and reverse: 5′-CACGCTTGGTTTCCGAGGAT-3′. The PCR conditions were 94°C (3 min) and 20 × 30 cycles of 94°C (30 s), 58°C (30 s), and 72°C (30 s). PCR products were electrophoresed on a 2% agarose gel and semiquantitative evaluation of MTA1 mRNA expression levels was performed relative to expression of the house-keeping genes glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primers for GAPDH were: forward, 5′-CCACCCATGGCAAATTCCCATGGCA-3′ and reverse, 5′-TCTAGACGGCAGGTCAGGTCCACC-3′. The expected PCR products were 290 bp for MTA1 and 597 bp for GAPDH.
Western blot analysis
After 5-Aza-dC treatments, the cells were harvested, and the cell pellets were washed with ice-cold phosphate-buffered saline and then lysed in lysis buffer containing 10 mM Tris (pH 7.5), 150 mM NaCl, 10 mM EDTA, 1% sodium dodecyl sulfate (SDS), 1 mM sodium orthovanadate and a mixture of protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 μg/mL pepstatin A, 2 μg/mL aprotinin). The lysates were sonicated for 10 s, centrifuged for 20 min at 20,000×g, and then stored at −70°C. Equal amounts (25 μg) of the cell lysates were resolved by 12% SDS-PAGE and transferred to polyvinylidene fluoride membranes. After blocking, blots were incubated mouse anti-MTA1 monoclonal antibody (sc-17773, Santa Cruz, 1:250) or β-actin (Zhongshan Golden Bridge Biotechnology, 1:1,000) overnight at 4°C and followed by each corresponding second antibody at room temperature for 1 h at 37°C. Then the results were developed by ECL (Pierce Biotechnology, USA). The protein bands were then analyzed using the BioImageing System (UVP, USA). The grayscale value of the MTA1 was normalized to the values of the corresponding β-actin band to determine the expression level of the protein. The experiments were repeated at least three times independently.
Matrigel invasion assay
Cell invasive ability was examined using a 24-well Transwell with 8.0-μm pore polycarbonate memberance inserts (Corning Inc., NY, USA) according to the manufacturer’s protocol. The Matrigel (100 μL/mL) was applied to the upper surface of the membranes. After the treatment of 5-Aza-dC for 72 h, cells were seeded on the upper chamber (5 × 104 cells/well) and incubated for 24 h. The cells without treatment served as the control. Cells that had invaded the surface of the membrane were fixed with methanol and stained with hematoxylin. The cells that invaded and moved onto the lower surface of the filter membrane were counted in ten random high-power fields (×400) by an inverted microscope. The experiment was repeated three times, and the data were shown as mean ± standard deviation (SD).
Statistical analysis
SPSS version 13.0 for Windows was used for all analyses. The Pearson’s Chi-Square test was used to analyze the relationship between MTA1 expression and ERα methylation. One-way analysis of variance was performed to compare data from the densitometry analysis of MTT, Western blotting, and reverse transcription polymerase chain reaction (RT-PCR). Statistical significance in this study was set at p < 0.05. The association between MTA1 expression and invasive ability of cells was assessed using the Spearman’s correlation test. All reported p values are two-sided.
Result
The methylation of ERα in breast cancer
ERα methylation was observed in 38 of 102 (37.3%) breast cancer tissues. ERα methylation had no significant correlation with age or menopausal status (p > 0.05), but it was significantly associated with lymph node metastasis and tumor size and TNM stage in breast cancer (p < 0.05), as shown in Table 1.
Expression of MTA1 in breast cancer and non-neoplastic breast tissues
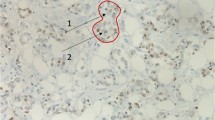
The results of immunhistochemistry revealed the nuclear and cytoplasm staining of the MTA1 protein in the breast cancer whereas the normal breast epithelium showed very weak or even no staining of normal ductal cells (Fig. 1). MTA1 expression in breast cancer (30/102, 29.4%) was higher than that in non-neoplastic breast tissues (6/102, 5.9%) (p < 0.01). The relationship between MTA1 expression and different clinicopathological factors in breast cancer was shown in Table 1. MTA1 expression was significantly associated with tumor size, lymph node metastasis, and TNM stage (p < 0.01) but not with age and menopausal status (p > 0.05).
Immunohistochamical staining of MTA1 in breast cancer and non-neoplastic breast tissues. a Positive MTA1 immunostaining was seen in invasive breast cancer, the patterns of MTA1 expression were mixed nuclear/cytoplasmic staining. b Negative MTA1 expression in the non-neoplastic breast tissues. Original magnification—all ×400
Correlation between MTA1 expression and ERα methylation
Our results indicated that ERα methylation had significant correlation with the MTA1 expression (p < 0.05), as shown in Table 2.
ERα methylation after the treatment of 5-Aza-dC
The methylation status of ERα of breast cancer cells was modified from methylated to unmethylated by the 5-Aza-dC treatment, as shown in Fig. 2.
Treatment of 5-Aza-dC demethylated breast cancer cell lines. MSP was performed on bisulfite-treated DNA from breast cancer cell lines to analysis ER methylation. MSP results from MDA-MB-435 s and MDA-MB-231 cells were shown. DNA bands in lanes labeled with U indicate PCR products amplified with primers recognizing the unmethylated promoter sequence. DNA bands in lanes labeled with M represent amplified products with methylation-specific primers. In vitro methylated DNA (IVD) serves as positive controls for the U and M reactions, respectively
Treatment of 5-Aza-dC downregulated MTA1 expression
Western blotting and RT-PCR results showed that MTA1 protein expression and mRNA were found in MDA-MB-435 s and MDA-MB-231. Furthermore, to explore the relation between MTA1 expression and ERα methylation in breast cancer, we treated the breast cell lines with 5-Aza-dC. The results of RT-PCR and Western blotting showed the downregulation of MTA1 protein expression and mRNA with the unmethylation of ERα with the treatment of 5-Aza-dC in breast cancer cell lines (p < 0.05, Fig. 3).
Treatment of 5-Aza-dC in breast cancer cell lines downregulated MTA1 expression. a RT-PCR showed the downregulation of MTA1 mRNA after the treatment of 5-Aza-dC in breast cancer cell lines. b MTA1 mRNA in breast cancer cell lines was statistically analyzed. c Western blotting showed the downregulation of MTA1 protein expression after the treatment of 5-Aza-dC in breast cancer cell lines. d MTA1 protein expression in breast cancer cell lines was statistically analyzed. Data represented the mean ± SD of three independent experiments.*p < 0.05
Treatment of 5-Aza-dC decreased invasive ability of breast cancer cells was dependent on MTA1
Matrigel invasion assays showed that the invasive ability of the MDA-MB-435 s, and MDA-MB-231 cells were decreased with the treatment of 5-Aza-dC. The MDA-MB-435 s and MDA-MB-231 cells had fewer numbers of cells which invaded onto the lower surfaces of the Transwell filters than cells treated with 5-Aza-dC (p < 0.05) (Fig. 4). The Spearman’s correlation test revealed that the number of invasive cells was significantly positively associated with the MTA1 protein expression (p < 0.01, Correlation coefficient = 0.942) and mRNA in MDA-MB-435 s and MDA-MB-231 cells (p < 0.01, correlation coefficient = 0.891). The invasive ability of breast cancer cell lines was significantly positively associated with MTA1 expression.
Treatment of 5-Aza-dC decreased invasive ability of breast cancer cells. a Matrigel invasion assay showed that the invasive ability of the MDA-MB-435 s and MDA-MB-231 cells was decreased with the treatment of 5-Aza-dC. b Number of cells invading onto the lower surfaces of the filter was counted. Data represented the mean ± SD of three independent experiments. Columns, mean (n = 3); bar, SD; *p < 0.05
Discussion
Breast cancer is a major public problem, according to the World Health Organization; more than 1.2 million people will be diagnosed each year with breast cancer worldwide; breast cancer will be responsible for 3% of deaths among women [20, 21]. The ERα-negative breast cancers are resistant to endocrine therapies and have a worse prognosis than ERα-positive breast cancer. DNA methylation and chromatin remodeling are two epigenetic mechanisms that have been linked with the lack of ERα expression, and in these cases, demethylation of the ERα promoter or treatment with histone deacetylase (HDAC) inhibitors shows promise in restoring ERα expression in ERα-negative breast cancers [22]. ERα is an acetylated protein, the acetylation status of which directly influences its transactivation functions. MTA1 is widely overexpressed in human cancers including breast cancer, and its overexpression has been associated with metastasis. MTA1 is a subunit of the NuRD complex, which is associated with chromatin remodeling and histone deacetylase activity, leading to transcriptional regulation of specific genes. Acetylation is a key posttranslational modification of many proteins and is responsible for the regulation of a number of critical intracellular pathways. And it was reported that MTA1 may also regulate ER-α functions by deacetylating ERα [23]. Mazumdar had identified ER-mediated transcription as a nuclear target of MTA1 and indicated that HDAC complexes associated with the MTA1 corepressor may mediate ERα transcriptional repression by heregulin-beta1 [10]. Kumar demonstrated a pathway that may redirect ER signalling by sequestering ERα in the cytoplasm, blocking the activation of ER-responsive pathways in the nuclear compartment, and stimulating the non-genomic functions of ERα in the cytoplasm [24]. Toh reported that MTA1 converted breast cancer cells to a more aggressive phenotype by repression of the ERα transactivation function through deacetylation of the chromatin in the ER-responsive element of ER-responsive genes [25]. The ERα gene methylation has been linked to the lack of ERα expression in breast cancer [26]. The methylation of CpG islands, cytosineguanine-rich areas located in the 5′ regulatory region of ERα, may block the transcription of ERα, resulting in the loss of ERα expression [27]. What is the relationship of MTA1 expression to DNA methylation? More needs to be further studied.
In our study, we found both MTA1 expression and ERα methylation was correlated positively with lymph node metastasis, tumor size, and TNM stage in breast cancer. Breast cancer patients with positive MTA1 expression had a tendency to ERα methylation. No previous research reported MTA1 expression had relation with ERα methylation. In the present study, our results showed that ERα methylation had significant correlation with the MTA1 expression in breast cancer. So, we hypothesized that ERα methylation might affect MTA1 expression. To gain a deeper insight into the molecular mechanism underlying the relation between MTA1 and ERα methylation, we treated the invasive breast cancer cell lines with the demethylating agent, 5-Aza-dC, further to detect MTA1 protein expression and mRNA level. First, we found that treatment of 5-Aza-dC in the invasive breast cancer cell-induced demethylation of ERα. We also found the downregulation of MTA1 protein expression and mRNA with the unmethylation of ERα. And the invasive ability of breast cancer cells was significantly positively associated with MTA1 expression. So it was acceptable that positive MTA1 expression stimulated breast cancer cell proliferation and is more aggressive.
Treatment of breast cancer cells with the general DNMT inhibitor, 5-Aza-dC, led to demethylation of ERα and reactivation of functional ER protein. Our results also suggested that MTA1 protein expression and mRNA downregulation was accompanied with the demethylation of ERα, but the mechanism of MTA1 downregulation after 5-Aza-dC treatment is not yet clear. No other report elucidates the relation. Maybe DNMT can promote MTA1 expression directly. No report about MTA1 supports this hypothesis. We also thought it is not acceptable. Previous studies had implicated that process of DNA methylation, mediated by Dnmt, may depend on or generate an altered chromatin state via histone deacetylase activity and revealed a direct connection between DNA methylation and histone deacetylation [28]. DNMT was identified as a co-repressor protein carrying deacetylase activity [29]. MTA1 is a part of the NuRD complex and associated with HDAC. Maybe the inhibition of DNMT can alter the function of HDAC. We did not deny this view, but how about the inhibition of HDAC downregulating the MTA1 protein expression and mRNA. On further analysis, the reactivation of ERα can decrease the invisibility of breast cancer cells. Previously, the reactivation of ERα has been shown to increase ability to undergo apoptosis and differentiation, decrease potential to damage DNA, metastasize, and resist chemotherapy [30]. Maybe the demethylation of ERα and reactivation of functional ER protein in breast cancer cells can affect the MTA1 expression. Further investigation about the mechanism is needed.
These unique findings have greatly extended our current knowledge about the relation between ERα and MTA1 expression. These data strongly support the hypothesis that methylation is involved in the relation between MTA1 and ERα in breast cancer.
References
Ghanta KS, Li DQ, Eswaran J, Kumar R. Gene profiling of MTA1 identifies novel gene targets and functions. PLoS One. 2011;6:e17135.
Molli PR, Singh RR, Lee SW, Kumar R. MTA1-mediated transcriptional repression of BRCA1 tumor suppressor gene. Oncogene. 2008;27:1971–80.
Hofer MD, Chang MC, Hirko KA, Rubin MA, Nosé V. Immunohistochemical and clinicopathological correlation of the metastasis-associated gene 1 (MTA1) expression in benign and malignant pancreatic endocrine tumors. Mod Pathol. 2009;22:933–9.
Murakami M, Kaul R, Robertson ES. MTA1 expression is linked to ovarian cancer. Cancer Biol Ther. 2008;7:1468–70.
Tong D, Heinze G, Schremmer M, Schuster E, Czerwenka K, Leodolter S, et al. Expression of the human MTA1 gene in breast cell lines and in breast cancer tissues. Oncol Res. 2007;16:465–70.
Ahmad Z, Khurshid A, Qureshi A, Idress R, Asghar N, Kayani N. Breast carcinoma grading, estimation of tumor size, axillary lymph node status, staging, and Nottingham prognostic index scoring on mastectomy specimens. Indian J Pathol Microbiol. 2009;52:477–81.
Duss S, André S, Nicoulaz AL, Fiche M, Bonnefoi H, Brisken C, et al. An oestrogen-dependent model of breast cancer created by transformation of normal human mammary epithelial cells. Breast Cancer Res. 2007;9:R38.
Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9:R6.
Zhao L, Wang L, Jin F, Ma W, Ren J, Wen X, et al. Silencing of estrogen receptor alpha (ERalpha) gene by promoter hypermethylation is a frequent event in Chinese women with sporadic breast cancer. Breast Cancer Res Treat. 2009;117:253–9.
Mazumdar A, Wang RA, Mishra SK, Adam L, Bagheri-Yarmand R, Mandal M, et al. Transcriptional repression of oestrogen receptor by metastasis-associated protein 1 corepressor. Nat Cell Biol. 2001;3(1):30–7.
Jiang Q, Zhang H, Zhang P. ShRNA-mediated gene silencing of MTA1 influenced on protein expression of ER alpha, MMP-9, CyclinD1 and invasiveness, proliferation in breast cancer cell lines MDA-MB-231 and MCF-7 in vitro. J Exp Clin Cancer Res. 2011;30:60.
Tavassoli FA. Breast pathology: rationale for adopting the ductal intraepithelial neoplasia (DIN) classification. Nat Clin Pract Oncol. 2005;2:116–7.
Tavassoli FA. Correlation between gene expression profiling-based molecular and morphologic classification of breast cancer. Int J Surg Pathol. 2010;18:167S–9S.
Tavassoli FA. Lobular and ductal intraepithelial neoplasia. Pathologe. 2008;29:107–11.
Jing MX, Mao XY, Li C, Wei J, Liu C, Jin F. Estrogen receptor-alpha promoter methylation in sporadic basal-like breast cancer of Chinese women. Tumour Biol. 2011;32(4):713–9.
Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–6.
Lapidus RG, Nass SJ, Butash KA, Parl FF, Weitzman SA, Graff JG, et al. Mapping of ER gene CpG island methylation-specific polymerase chain reaction. Cancer Res. 1998;58:2515–9.
Kok LF, Lee MY, Tyan YS, Wu TS, Cheng YW, Kung MF, et al. Comparing the scoring mechanisms of p16INK4a immunohistochemistry based on independent nucleic stains and independent cytoplasmic stains in distinguishing between endocervical and endometrial adenocarcinomas in a tissue microarray study. Arch Gynecol Obstet. 2010;281:293–300.
Koo CL, Kok LF, Lee MY, Wu TS, Cheng YW, Hsu JD, et al. Scoring mechanisms of p16INK4a immunohistochemistry based on either independent nucleic stain or mixed cytoplasmic with nucleic expression can significantly signal to distinguish between endocervical and endometrial adenocarcinomas in a tissue microarray study. J Transl Med. 2009;7:25.
Dowling EC, Klabunde C, Patnick J, Ballard-Barbash R. International Cancer Screening Network (ICSN). Breast and cervical cancer screening programme implementation in 16 countries. J Med Screen. 2010;17:139–46.
Gaffan J, Dacre J, Jones A. Educating undergraduate medical students about oncology: a literature review. J Clin Oncol. 2006;24:1932–9.
Brinkman JA, El-Ashry D. ER re-expression and re-sensitization to endocrine therapies in ER-negative breast cancers. J Mammary Gland Biol Neoplasia. 2009;14:67–78.
Manavathi B, Singh K, Kumar R. MTA family of coregulators in nuclear receptor biology and pathology. Nucl Recept Signal. 2007;5:e010.
Kumar R, Wang RA, Mazumdar A, Talukder AH, Mandal M, Yang Z, et al. A naturally occurring MTA1 variant sequesters oestrogen receptor-alpha in the cytoplasm. Nature. 2002;418:654–7.
Toh Y, Nicolson GL. The role of the MTA family and their encoded proteins in human cancers: molecular functions and clinical implications. Clin Exp Metastasis. 2009;26(3):215–27.
Flanagan J, Kugler S, Waddell N, Johnstone C, Marsh A, Henderson S, et al. DNA methylome of familial breast cancer identifies distinct profiles defined by mutation status. Breast Cancer Res. 2010;12(Suppl1):O4.
Yoshida T, Eguchi H, Nakachi K, Tanimoto K, Higashi Y, Suemasu K, et al. Distinct mechanisms of loss of estrogen receptor alpha gene expression in human breast cancer: methylation of the gene and alteration of trans-acting factors. Carcinogenesis. 2000;21:2193–201.
Fuks F, Burgers WA, Brehm A, Hughes-Davies L, Kouzarides T. DNA methyltransferase Dnmt1 associates with histone deacetylase activity. Nat Genet. 2000;24:88–91.
Gururaj AE, Singh RR, Rayala SK, Holm C, den Hollander P, Zhang H, et al. MTA1, a transcriptional activator of breast cancer amplified sequence 3. Proc Natl Acad Sci U S A. 2006;103:6670–5.
Sahab ZJ, Man YG, Semaan SM, Newcomer RG, Byers SW, Sang QX. Alteration in protein expression in estrogen receptor alpha-negative human breast cancer tissues indicates a malignant and metastatic phenotype. Clin Exp Metastasis. 2010;27:493–503.
Acknowledgment
This work was supported by Scientific Research Foundation for the Doctoral Program, Ministry of Science and Technology, Liaoning Province (no. 20121126).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mao, Xy., Chen, H., Wang, H. et al. MTA1 expression correlates significantly with ER-alpha methylation in breast cancer. Tumor Biol. 33, 1565–1572 (2012). https://doi.org/10.1007/s13277-012-0410-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0410-7