Abstract
Background
Although metastasis-associated protein 1 (MTA1) has been recently demonstrated as a potent angiogenesis-promoting factor in various malignant tumors, its angiogenic property in gastric cancer (GC) remains unclear. This study has detected the expression of MTA1 protein in surgically resected tissues of pathologic N0 (pN0) GC and further investigated its relation with other clinicopathologic factors and tumor angiogenesis and prognosis.
Methods
MTA1 protein expression was detected immunohistochemically in 111 pN0 GC specimens. Its correlations with clinicopathologic factors and tumor prognosis were evaluated. The intratumoral microvessel density (MVD) was assessed based on CD105 antigen immunoreactivity and analyzed for correlation with MTA1 protein expression.
Results
Overexpression of MTA1 was detected in 36.04 % of patients and exhibited a significant association with tumor size and MVD. Survival analysis demonstrated that both overall (OS) and disease-free (DFS) survivals in patients overexpressing MTA1 were significantly poorer than those without MTA1 overexpression 5 years after the operation (both p < 0.001). Multivariate survival analysis demonstrated that MTA1 overexpression was an independent prognosticator for unfavorable OS and DFS (p < 0.001, respectively).
Conclusions
MTA1 overexpression is frequently observed in pN0 GC patients and is significantly associated with increased angiogenesis and poor prognosis. Detection of MTA1 protein expression may help predict the relapse and prognosis of pN0 GC. Also, MTA1 protein may form a novel target for antiangiogenic therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is a major public health problem and the second leading cause of cancer-related death worldwide [1]. In China, its mortality rate ranks the highest among all tumors and represents about 25 % of GC mortality worldwide [2]. Although our understanding of the biologic and clinical nature of GC and the treatment of this disease have been significantly improved, the overall 5-year survival rate after surgical resection has lacked significant improvement during the past three decades [3, 4]. The presence of lymph node metastasis is considered one of the most important prognostic factors for GC [5]. However, even in histologically node-negative (pN0) GC, some patients still suffered a recurrence after complete surgical resection and then have a poor prognosis. To date, there is no available biomarker routinely used to determine the prognosis of pN0 GC. Hence, better defining the pathogenesis of GC and exploring novel biomarkers that could better stratify individuals at increased risk of relapse with pN0 GC are in urgent clinical demand.
Recent advances in GC molecular biology have provided a variety of biomarkers useful for cancer detection, progression monitoring, prognosis, and determining the therapeutic responses [2]. Among them, the MTA1 protein was recently identified by differential screening of a cDNA library from highly metastatic and nonmetastatic rat mammary adenocarcinoma cell lines. An important component of the nucleosome remodeling and deacetylase (NuRD) complex, MTA1 protein is associated with ATP-dependent chromatin remodeling and histone deacetylase activity [6–11]. It has been reported that MTA1 overexpression is closely correlated with the invasion of several human cancers, such as esophageal, lung, breast, prostate, and colorectal cancers [12–16]. However, the role of MTA1 protein in the relapse and prognosis of curatively resected pN0 GC patients remains unknown.
Previous clinical studies indicated that MTA1 expression is closely related to tumor angiogenesis [13, 14]. Essential components of the tumor microenvironment, intratumoral microvessels facilitate rapid tumor growth and potential tumor metastasis [17]. Tumor angiogenesis is also a significant predictor of hematogenous metastasis and tumor relapse. For example, high microvessel density (MVD) was associated with the dismal prognosis of a wide range of human malignant tumors [18]. However, the angiogenic role of MTA1 protein in primary GC has not yet been investigated.
The present study detected MTA1 protein expression by immunohistochemistry in patients with pN0 GC, explored its relation with clinicopathologic features as well as with tumor angiogenesis and prognosis, and clarified the independent prognostic factors affecting long-term survival.
Materials and methods
Patients and follow-up
A total of 111 sequential patients diagnosed with primary GC and who had undergone radical resection without lymph node metastasis at Qilu Hospital of Shandong University between May 2002 and June 2005 were enrolled in this study. None of the patients had received adjuvant therapy before surgery. The tumor specimens were collected and fixed in 10 % buffered formalin solution immediately after resection. Serial sections (5 μm) were cut from each tissue block and reviewed histologically with hematoxylin-eosin staining. The tumors were classified histologically based on the classification system of the World Health Organization [19] and postoperatively staged according to the tumor-node-metastasis (TNM) classification of the International Union Against Cancer [20]. The serial sections of each lymph node specimen were pathologically examined by meticulous histopathologic analysis to ensure the accuracy of pN0 status of each patient. The clinicopathologic characteristics of these patients are listed in Table 1. The patient’s informed consent and approval from the ethics committee of Qilu Hospital of Shandong University were obtained prior to the use of patients’ clinicopathologic information and tumor tissues for research purposes.
All patients underwent follow-up at 3- to 6-month intervals. The follow-up studies included physical examination, laboratory analysis, computed tomography, barium esophagography, ultrasonography, and fibrogastroscopy if necessary. The site of tumor relapse and time to recurrence were recorded. Follow-up of all patients was completed up to December 3, 2010, with the follow-up ranging from 14 to 80 months (median 67 months).
Immunohistochemical staining of MTA1 and CD105
The tissue sections were dewaxed with xylene and rehydrated with graded alcohol. Antigen retrieval was done by heating at 92–98 °C for 20 min in citrate buffer (pH 6.0). Sections were then cooled to room temperature and incubated in 3 % hydrogen peroxide for 10 min to inactivate the endogenous peroxidase. After blocking with 10 % goat serum at 37 °C for 20 min, the primary antibodies against MTA1 (sc-9446; Santa Cruz Biotechnology, Santa Cruz, CA USA) and CD105 (sc-20632; Santa Cruz Biotechnology) were applied at dilutions of 1:100 [12] and 1:400 [21] respectively in phosphate buffered solution (PBS) overnight at 4 °C. After incubation with the horseradish peroxidase-conjugated secondary antibody at 37 °C for 30 min, antibody binding was visualized by incubating with substrate 3,3-diaminobenzidine solution. The sections were counterstained with hematoxylin and mounted with glycerol gelatin. Sections stained with PBS instead of the primary antibodies were used as negative controls.
Interpretation of MTA1 protein expression and MVD
Two pathologists (Junhui Zhen and Cuijuan Zhang, Department of Pathology, Qilu Hospital) who were blind to the patients’ clinicopathologic factors and outcomes, independently evaluated the GC specimens for MTA1 protein staining. The nuclear expression of MTA1 was scored semiquantitatively by combining the intensity and the proportion of positively stained tumor cells [12]. The results were reported as follows: 0, no staining; +, slight staining; ++, moderate staining; +++, intense staining. The cancer tissues scored as ++ and +++ were defined as exhibiting overexpression of MTA1 protein.
The MVD was determined by immunostaining for CD105, which was expressed in the cytoplasm and membrane of endothelial cells. Counting of microvessels in tissues was performed according to Weidner’s standards [22]. Briefly, the results were expressed as the mean ± SD number of vessels in one 200× microscopic field. The mean value of the vessel counts in four independent vascular hot spots was considered the final value. At the same time, any stained endothelial cells were identified as independent vessels that must be clearly separated from each other. Furthermore, the blood vessels with a lumen diameter exceeding the size of approximately eight red blood cells were excluded. There was no discrepancy in the immunostaining results between the two clinical pathologists.
Statistical analysis
The SPSS version 13.0 software was used for statistical analyses. Correlations of MTA1 protein expression with clinicopathologic features were evaluated using the χ 2 test. The Mann–Whitney test was used to examine the relation between MTA1 protein expression and MVD. The patient survivals and their differences were calculated by the Kaplan–Meier method and log-rank test. Cox regression was adopted for multivariate analysis of independent prognostic factors. Differences with p < 0.05 were considered significant.
Results
MTA1 protein expression in GC
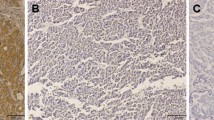
MTA1 protein was expressed at variable levels and clearly stained in the cell nuclei. Among the 111 paraffin-embedded cancer tissues, 40 cases (36.04 %) showed overexpression (moderate or strong) of MTA1 protein, and 71 cases showed negative or low expression. The representative cases with different levels of MTA1 are shown in Fig. 1.
Correlation of MTA1 protein expression with clinicopathologic factors
MTA1 protein overexpression was significantly correlated with tumor size (p < 0.001, Table 1), but not significantly correlated with gender, age, histological differentiation, and invasion depth (p > 0.05) (Table 1).
Correlation of MVD with clinicopathologic factors
High MVD was significantly correlated with histological differentiation (p < 0.001, Table 1), but not significantly correlated with gender, age, tumor size, and invasion depth (p > 0.05) (Table 1).
Correlation of MTA1 protein expression with prognosis
At the last follow-up, tumor relapse was found in 42 patients. The relapse patterns are listed in Table 2. Tumor relapses occurred in 20 of 40 cases with MTA1 overexpression and in 22 of 71 cases without overexpression (p = 0.047, χ 2 test). Relapse was also seen in 23 of 45 cases with high MVD and 19 of 66 cases with low MVD (p = 0.017, χ 2 test). The rate of hematogenous metastasis among patients with high MVD was 24.4 %, which was higher than that (20.0 %) among patients with MTA1 overexpression—but without a statistically significant difference (p = 0.606, χ 2 test).
The overall survival (OS) was 64.0 %, and the disease-free survival (DFS) was 62.2 %. According to the Kaplan–Meier analysis, the patients with MTA1 overexpression had a significantly poorer OS (42.5 vs. 76.1 %, p < 0.001) (Fig. 2a) and DFS (45.0 vs. 71.8 %, p < 0.001) (Fig. 2b) compared to those without MTA1 overexpression. To determine whether MTA1 overexpression was an independent variable for survival probability prediction, we subsequently performed a multivariate analysis using the Cox model. It showed that MTA1 overexpression is an independent prognostic factor for unfavorable OS and DFS (both p < 0.001) (Table 3).
Correlation of MTA1 protein expression with tumor angiogenesis
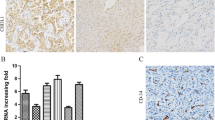
To evaluate the association between MTA1 protein expression and tumor angiogenesis, we examined the MVD using CD105 immunohistochemical staining. As shown in Fig. 3, analysis of MTA1 and CD105 protein expressions in the same serial sections showed that negative or low expression of MTA1 protein was associated with few microvessels, and MTA1 protein overexpression was associated with abundant microvessels (p = 0.001) (Fig. 4).
Discussion
The current study showed that expression of MTA1 protein ranged from undetectable to high levels in pN0 GC tissues. Also, its overexpression was significantly associated with tumor relapse and a poor prognosis. Moreover, MTA1 protein overexpression was significantly associated with tumor angiogenesis. Thus, the findings in the present study are highly significant and relevant in the context of pN0 GC. These results indicate that MTA1 protein plays an important role in tumor angiogenesis and can be considered a new marker of poor prognosis for patients with pN0 GC.
The expression of MTA1 protein has been studied in several human solid tumors and was found to be closely correlated with tumor progression [11–16]. In patients with esophageal squamous cell carcinoma, overexpression of MTA1 protein was significantly associated with T status and tumor relapse. MTA1 overexpression was also an independent prognostic factor of pN0 esophageal squamous cell carcinoma [12]. In another report, 67 of 263 breast cancer patients with high MTA1 protein levels were diagnosed with a tumor of advanced grade [13]. Similar observations have shown that MTA1 protein is closely related to frequent recurrence, microvascular invasion, and poor survival of patients with hepatocellular carcinoma [23].
In the present study, MTA1 protein overexpression was shown to be associated with tumor size in pN0 GC. The survival analysis demonstrated that both OS and DFS in patients overexpressing MTA1 were significantly poorer than those without MTA1 overexpression after the operation. Furthermore, overexpression of MTA1 was closely correlated with tumor angiogenesis, which is consistent with the report on breast cancer [13]. Our results also demonstrated that MTA1 protein plays an important role in the hemodynamics and microcirculation of GC and may represent a potential novel antiangiogenesis therapeutic target. These findings suggest that MTA1 plays an important role in tumor size and angiogenesis and may serve as a significant independent predictor of both relapse and prognosis of patients with pN0 GC. Our results are consistent with previous a report that measurement of MTA1 protein could effectively identify early-stage lung cancers that are more likely to recur after surgery [14]. To the best of our knowledge, this study has, for the first time, demonstrated that MTA1 overexpression has a prognostic role for pN0 GC patients.
Conclusions
The present study has, for the first time, shown that MTA1 protein may have clinical potential to be used as a prognostic factor to identify pN0 GC patients who may be at high risk of relapse and a poor prognosis. MTA1 expression is also closely associated with tumor angiogenesis. These findings suggest that MTA1 protein may be a prognostic marker and a new therapeutic target for pN0 GC.
References
Parkin DM, Bray F, Ferlay J et al (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Zheng L, Wang L, Ajani J et al (2004) Molecular basis of gastric cancer development and progression. Gastric Cancer 7:61–77
Brennan MF (2005) Current status of surgery for gastric cancer: a review. Gastric Cancer 8:64–70
Alberts SR, Cervantes A, van de Velde CJ (2003) Gastric cancer: epidemiology, pathology and treatment. Ann Oncol 14(Suppl 2):31–36
Ichikura T, Tomimatsu S, Uefuji K et al (1999) Evaluation of the New American Joint Committee on Cancer/International Union Against Cancer classification of lymph node metastasis from gastric carcinoma in comparison with the Japanese classification. Cancer 86:553–558
Nicolson GL, Nawa A, Toh Y et al (2003) Tumor metastasis-associated human MTA1 gene and its MTA1 protein product: role in epithelial cancer cell invasion, proliferation and nuclear regulation. Clin Exp Metastasis 20:19–24
Kumar R, Wang RA, Bagheri-Yarmand R (2003) Emerging roles of MTA family members in human cancers. Semin Oncol 30:30–37
Mazumdar A, Wang RA, Mishra SK et al (2001) Transcriptional repression of oestrogen receptor by metastasis-associated protein 1 corepressor. Nat Cell Biol 3:30–37
Yan C, Wang H, Toh Y et al (2003) Repression of 92-kDa type IV collagenase expression by MTA1 is mediated through direct interactions with the promoter via a mechanism which is both dependent on and independent of histone deacetylation. J Biol Chem 278:2309–2316
Toh Y, Pencil SD, Nicolson GL (1994) A novel candidate metastasis-associated gene, mta1, differentially expressed in highly metastatic mammary adenocarcinoma cell lines: cDNA cloning, expression, and protein analyses. J Biol Chem 269:22958–22963
Toh Y, Pencil SD, Nicolson GL (1995) Analysis of the complete sequence of the novel metastasis-associated candidate gene, mta1, differentially expressed in mammary adenocarcinoma and breast cancer cell lines. Gene 159:97–104
Li SH, Wang Z, Liu XY (2009) Metastasis-associated protein 1 (MTA1) overexpression is closely associated with shorter disease-free interval after complete resection of histologically node-negative esophageal cancer. World J Surg 33:1876–1881
Jang KS, Paik SS, Chung H et al (2006) MTA1 overexpression correlates significantly with tumor grade and angiogenesis in human breast cancers. Cancer Sci 97:374–379
Li SH, Tian H, Yue WM et al (2011) Overexpression of metastasis-associated protein 1 is significantly correlated with tumor angiogenesis and poor survival in patients with early-stage non-small cell lung cancer. Ann Surg Oncol 18:2048–2056
Kai L, Wang J, Ivanovic M et al (2011) Targeting prostate cancer angiogenesis through metastasis-associated protein 1 (MTA1). Prostate 71:268–280
Toh Y, Oki E, Oda S et al (1997) Overexpression of the MTA1 gene in gastrointestinal carcinomas: correlation with invasion and metastasis. Int J Cancer 74:459–463
Arbiser JL (2005) Implications of Epstein-Barr Virus (EBV)-induced carcinogenesis on cutaneous inflammation and carcinogenesis: evidence of recurring patterns of angiogenesis and signal transduction. J Invest Dermatol 124:Xi–xii
Onizuka S, Kawakami S, Taniguchi K et al (2004) Pancreatic carcinogenesis: apoptosis and angiogenesis. Pancreas 28:317–319
World Health Organization (1988) International Histological Classification of Tumours, 2nd edn. Geneva, World Health Organization, 1969–1981. Berlin, Springer
Sobin LH, Wittekind CH (eds) (1997) TNM classification of malignant tumours, 5th edn. Wiley, New York
Yu D, Zhuang L, Sun X et al (2007) Particular distribution and expression pattern of endoglin (CD105) in the liver of patients with hepatocellular carcinoma. BMC Cancer 7:122–131
Weidner N (1995) Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat 36:169–180
Ryu SH, Chung YH, Lee H et al (2008) Metastatic tumor antigen 1 is closely associated with frequent postoperative recurrence and poor survival in patients with hepatocellular carcinoma. Hepatology 47:929–936
Acknowledgments
Grant support for the research came from the National Key Clinical Medical Specialties Foundation, Shandong Province Science Foundation Projects (ZR2009CQ038 and 2007GG 10002012), and China National Natural Science Foundation Projects (30672010).
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deng, X., Du, L., Wang, C. et al. Close Association of Metastasis-Associated Protein 1 Overexpression with Increased Angiogenesis and Poor Survival in Patients with Histologically Node-Negative Gastric Cancer. World J Surg 37, 792–798 (2013). https://doi.org/10.1007/s00268-012-1898-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-012-1898-0