Abstract
We evaluated immunohistochemically the expression profiles of metastasis-associated protein (MTA) 1 and their associations with lymph node metastasis in tonsil cancer. Immunohistochemical analysis of 43 tonsillar neoplasm tissues was performed using antibodies raised to MTA1. Depth of tumor invasion, lymph node metastasis, and clinical outcomes were assessed. Clinical N0 patients were divided into two groups: N0a, negative for MTA1; N0b, positive for MTA1. Occult node metastasis was reevaluated according to the revised clinical N staging system taking account of MTA1 expression. The expression rate of MTA1 was 41.9%. There was a significant correlation between the expression of MTA1 and lymph node metastasis (P = 0.034*). MTA1 had a sensitivity of 53.3% and a specificity of 84.6% for identification of cervical metastases. When cN0b patients were considered to be N+, the recalculated rate of occult metastasis fell from 50% to 7.6% (the false-positive rate remained unchanged). MTA1 was found to be a useful molecular marker to predict lymphatic metastasis in tonsil cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Of the head and neck cancers, squamous cell carcinoma of the oropharynx readily metastasizes to the cervical lymph nodes, often bilaterally, a feature that is related to its invasiveness and one that is suggestive of a poor prognosis [1]. A major cause of treatment failure and death, the metastasis of cancer cells is a multi-step and multi-factorial process that involves interactions between cancerous and normal cells, extracellular matrices, and metastasis-related molecules [2]. Despite extensive molecular investigations, few reliable biochemical markers for predicting lymphatic metastasis in the head and neck cancers have been identified. Metastasis-associated protein (MTA) 1 has been implicated in invasion and lymph node metastasis in a range of human cancers [3–7]. MTA1 is known to be a component of the “nucleosome remodeling and histone deacetylation” (NuRD) complex, which displays histone deacetylase activity. It prevents p53-mediated apoptosis and consequently promotes the metastasis of cancer cells [6]. Moreover, it has been linked with invasion and lymph node metastasis in colon and gastric cancers [4]. Because reliable molecular markers for head and neck cancers have not been identified, this potential maker and its associations with prognostic factors in head neck cancers should be evaluated.
This study was conducted to evaluate the expression of MTA1 in tonsillar cancer, to assess whether MTA1 expression profiles correlated with the depth of histological invasion and with cervical lymph node metastasis, and to assess the usefulness of this potential marker in the prediction of cervical lymph node metastasis and for establishing proper treatment modalities.
Materials and methods
We retrospectively reviewed the medical records of 59 patients who were diagnosed with tonsillar cancer at our clinic between December 1994 and September 2008 and whose tissues were preserved in paraffin. The following exclusion criteria were applied: (1) radiation therapy prior to surgery, (2) lack of neck dissection, (3) sentinel lymph node biopsy, and (4) treatment before hospitalization due to the same condition. Sixteen patients were thus excluded and a total of 43 included.
In most patients, primary resection and en block neck dissection were the surgical treatments performed. Selective neck dissection was performed for prophylactic reasons in clinically negative neck patients and modified radical neck dissection in clinically positive neck patients. All 43 patients underwent radical resection of the primary tumor, 30 of whom simultaneously underwent therapeutic neck dissection and 13 prophylactic neck dissection. Adjuvant treatment was considered when the cancer-free margin was deemed insufficient (<5 mm), when extracapsular invasion was detected, and when multiple nodal metastases were found. Most patients were followed-up every 6 months for the first 2 years following treatment and every 12 months thereafter, and their tumors assessed by computed tomography (CT), magnetic resonance imaging (MRI) and liver/bone scan, or positron emission tomography (PET)/CT scan.
Metastasis-associated protein (MTA) 1 (MTA1) expression was analyzed immunohistochemically on primary tumors. Histological patterns, depth of tumor invasion, and lymph node metastasis were assessed by reviewing available pathology reports and histological slides. Demographic and clinical features including age, gender, TNM stage and clinical stage were assessed using patient medical records and information obtained during follow-up appointments. Primary tumors and cervical lymph node metastases were classified according to the American Joint Committee on Cancer (AJCC) TNM staging system (2002).
The specimens were obtained following the rules of the Helsinki Declaration. The Institutional Review Board of Seoul St Mary’s Hospital (Seoul, Korea) approved the retrospective review of patient medical records and the use of archived tumor specimens.
Preparation of tissue microarray blocks
Duplicate tissue cores per specimen were arrayed on recipient paraffin blocks in order to decrease the error introduced by sampling. Central areas rich in viable tumor cells and near the invasive front were identified by light microscopic examination of hematoxylin–eosin-stained slides and selected for use in the tissue microarrays. Tissue cylinders with a diameter of 2 mm were punched from the previously marked tumor areas of each block (donor block) and then transferred to a recipient paraffin block with 33 wells (30 wells in a 5 × 6 grid, 3 wells in the periphery). Tumor tissue samples were arrayed in the 30 central wells, and control tissues were placed to the three peripheral wells.
Immunohistochemistry
Tissue microarrays were stained using a monoclonal antibody raised to MTA1 (clone A-11, diluted 1:20; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Tissue microarrays were deparaffinized, and then rehydrated with ethanol solutions. To expose inaccessible antigens, they were then treated with buffered citric acid (pH 6.0) at 121°C for 15 min using an RHS-1 microwave vacuum histoprocessor (Milestone, Bergamo, Italy). To block endogenous peroxidase activity, the sections were treated with 3% H2O2 and then rinsed with distilled water. Primary antibodies were diluted in Dako antibody diluent, which contains components that decrease non-specific staining. Tissue microarrays were incubated with primary antibody for 30 min, rinsed with distilled water, and then treated for 30 min with the Envision Plus reagent (Dako). They were then stained with diaminobenzidine (DAB, Dako) for 5 min and counterstained with hematoxylin. Negative controls were performed by replacing the primary antibody with non-immune mouse serum. A positive control sample consisting of stomach cancer known to express MTA1 was included in each tissue microarray. Normal tonsil and liver tissues were used as additional negative control tissue.
Immunohistochemical analysis
Histological slides were analyzed by the staining of the nuclei of the tumor cells twice by a single pathologist who had no access to the patient medical information. If these two readings did not tally, a third reading was made and considered to be the final result. MTA1 staining intensities were graded according to a 4-point scale: 0 no expression; 1 weak; 2 moderate; and 3 marked. Percentages of stained cells were also recorded. Positive immunohistochemical staining was defined by ≥50% cells being stained and by a staining intensity grade of ≥2.
Statistical analyses
The chi-squares test, Fisher’s exact test, and correlation analysis were used, as appropriate to identify significant differences in MTA1 expression and clinical/pathological factors (T stage, cervical metastasis, tumor cell differentiation, extracapsular spread, invasion depth, other molecular markers, perineural invasion, and perivascular invasion). Survival was assessed using the Kaplan–Meier method. A P value <0.05 was deemed to indicate statistical significance. All analyses were performed using the SPSS software (ver. 13.0; SPSS, Chicago, IL, USA).
Results
Patient characteristics
Mean patient age was 56.3 years (range 43–77 years). Of the 43 patients, there were 39 males and four females. Based on the AJCC TNM staging system, the T stage was classified as T1 in nine patients (20.9%), T2 in 26 patients (60.5%), and T3 in eight patients (18.6%); and the N stage as N0 in 13 patients (30.2%), N1 in six patients (14.0%), and N2 in 24 patients (55.8%). The cervical lymph node metastasis rate was thus 69.8% (30/43). The depth of invasion ranged between 1.0 and 35.0 mm (mean ± SD, 13.5 ± 8.4 mm).
Relationship between the expression of MTA1 and the pathological features
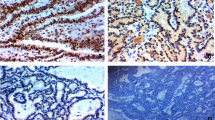
Metastasis-associated protein (MTA) 1 (MTA1) was expressed in the nuclei of tumor cells, whereas no immunoreactivity was found in the normal epithelial cells. MTA1 staining intensity was of grade 2 or 3 in 26 patients (60.5%), in 18 of whom ≥50% cells were stained, yielding a positive immunohistochemical staining rate of 41.9% (18/43; Fig. 1). Positive MTA1 immunohistochemical staining was noted in 16 (53.3%) of the 30 patients with lymph node metastases, but in only two (15.4%) of the 13 patients without lymph node metastases; this difference was statistically significant (P = 0.034*). The specificity, sensitivity, positive predictive value, and negative predictive value of the MTA1 for identification of cervical metastasis are shown in Table 1. MTA1 had a sensitivity of 53.3% and a specificity of 84.6% for identification of cervical metastases. MTA1 expression was not significantly correlated with T stage, depth of invasion, neural invasion, or vascular invasion (Table 2).
Relationship between MTA1 expression and survival rate
The 5-year overall and disease-specific survival rates were 74.4% and 80.0%, respectively. The 5-year disease-specific survival rate for MTA-positive patients was 70.3%, whereas that in the MTA1-negative group was 87.0% (Fig. 2). However, this result did not reach statistical significance (P = 0.196). T stage (P = 0.026*) and nodal metastasis (P = 0.051) were statistically significant prognostic factors in disease-specific survival.
Subclassification of N0 stage patients according to MTA1 expression
Of 22 clinically node-negative patients, 11 were classified as being pathologically node-positive. The rate of occult node metastasis (false-negative rate) was 50%. False-positive rate was 9.5%. Occult node metastasis was reevaluated using the clinical N staging system with specific consideration of MTA1 expression. N0 patients were subclassified into two groups: N0a negative for MTA1; N0b positive for MTA1. The cN0b patients were considered to be N+ and thus at high risk of nodal metastasis. Only one of the 13 cN0a patients had positive node, but all of the nine cN0b patients had positive node pathologically. Occult nodal metastasis according to the revised clinical N staging system was 7.6%. The false-positive rate was 6.7% (Fig. 3).
Discussion
Cervical lymph node metastasis is the most important prognostic factor in tonsil cancer [1]. Despite extensive molecular investigations, we still do not have accurate tools to predict the presence of cervical metastasis in patients with clinically negative cervical lymph nodes [2]. If there is a risk of a higher incidence of nodal metastases after considering several factors, such as T stage and tumor depth of invasion, some surgeons are in favor of elective neck dissection or elective neck irradiation, even for clinically N0 patients. However, such elective treatments may often lead to considerable morbidity without therapeutic benefit. Therefore, the investigation of valid biomarkers predicting cervical lymph node metastasis in head and neck cancer has been emphasized.
Metastasis-associated protein (MTA) 1 (MTA1) is an important component of the NuRD complex, which displays histone deacetylase and transcriptional activities [7]. It is also known to be highly expressed in cancer cells with high malignant potential, such as those found in metastatic cancers. It has been reported that MTA1 expression is highest in metastatic prostate cancer, followed by primary prostate cancer and benign prostate tumors [8]. Toh et al. [4] demonstrated that MTA1 is involved in the migration and invasion of immortalized human keratinocytes and that its expression is significantly correlated with invasion and metastasis in colon and stomach cancers. Jang et al. [5], meanwhile, showed that MTA1 overexpression correlated significantly with tumor grade and angiogenesis in human breast cancer. It has separately been documented that reducing MTA1 expression using siRNA inhibits the in vitro invasion and migration of esophageal squamous cell carcinoma cells [9]. It has been reported that MTA1 expression is closely associated with larger tumor size, worse histological differentiation, microvascular invasion, frequent postoperative recurrence, and poor patient survival, in HBV-associated HCCs [10].
Moon et al. [6] reported that metastasis is caused by MTA1-mediated deacetylation of p53 and the consequent inhibition of p53-induced apoptosis. Recent studies have shown that MTA1 induces angiogenesis by activating H1F-la [11], suggesting that MTA1 over-expression in cancer cells is associated with strong metastatic potential. Because MTA-1 inhibits apoptosis while it appears not to inhibit angiogenesis but rather induces angiogenesis, it seems likely to be a molecular marker for gene therapy that inhibits tumor progression and cancer cell metastasis.
Few reported studies have probed the relationships between MTA1 expression and the clinical features of head and neck cancers. Kawasaki et al. [12] proposed that MTA1 expression differs according to T and N stage in patients with oral cavity cancer. From the results of the current study, it was revealed that the expression of MTA1 was significantly higher in node-positive tumors compared to that of node-negative tumors. Positive immunohistochemical staining for MTA1 was noted in 41.9% of tonsil cancer patients. Additionally, it was found in 72.4% of the patients with lymph node metastases, but only 27.6% of patients without lymph node metastasis. N stage was significantly related to MTA1 expression, similar to the findings of Kawasaki et al. [12], but T stage was not.
Roepman et al. investigated gene expression patterns in head and neck squamous cell carcinoma lymph node metastases using DNA microarrays and the gene expression patterns in lymph node metastases were most similar to the corresponding primary tumors. However, MTA1 showed reduced expression in metastases samples compared with matched primary tumors. They concluded that once the primary tumor has gained the metastatic phenotype, few further alteration in gene expression are required for tumor establishment in the lymph nodes, and the reduced expression of MTA1 in the lymph node suggests that MTA1 is only needed at the site of primary tumor [13].
Tang et al. investigated the mRNA expression levels of the MTA1 in laryngeal squamous cell carcinoma and its relationship with metastasis. The frequency of MTA1 mRNA positive expression in 16 patients with cervical lymph node metastasis was 100%, but no expression in patients with patients without nodal metastasis [14].
The occult nodal metastasis rate in our patients was 50%, which is relatively high. Clinical data, including radiological image of the patients with occult nodal metastasis, were reevaluated: clinical stage classifications remained unchanged. We regarded the N0b patients as being at high risk of nodal metastasis. Occult nodal metastasis according to the revised clinical N staging system decreased to 7.6%. Additionally, the false-positive rate remained unchanged. Thus, we considered revised nodal staging taking account of MTA1 expression to be effective at decreasing occult nodal metastasis. Although the cervical metastases of tumor cells are complex, dynamic sequences of biological events, we investigated whether the expression of MTA1 in tonsil cancer reflect their propensity for cervical lymph node metastases and invasion.
In conclusion, we identified MTA1 expression as a potentially useful molecular marker for predicting lymphatic metastasis in tonsil cancer and suggest that it may be helpful in decreasing the occult nodal metastasis rate.
References
Talamini R, La Vecchia C, Levi F, Conti E, Favero A, Franceschi S, Franceschi S (1998) Cancer of the oral cavity and pharynx in nonsmokers who drink alcohol and in nondrinkers who smoke tobacco. J Natl Cancer Inst 90:1901–1903
Yokozaki H, Tahara E (1994) Metastasis-related genes. Gan To Kagaku 21:2541–2548
Parker C, Whittaker PA, Usmani BA, Lakshmi MS, Sherbet GV (1994) Induction of 18A2/mts1 gene expression and its effects on metastasis and cell cycle control. DNA Cell Biol 13:1021–1028
Toh Y, Oki E, Oda S, Tokunaga E, Ohno S, Maehara Y, Nicolson GL, Sugimachi K (1997) Overexpression of the MTA1 gene in gastrointestinal carcinomas: correlation with invasion and metastasis. Int J Cancer 74:459–463
Jang KS, Paik SS, Chung H, Kong G (2006) MTA1 overexpression correlates significantly with tumor grade and angiogenesis in human breast cancers. Cancer Sci 97:374–379
Moon H, Cheon H, Lee M (2007) Metastasis-associated protein 1 inhibits p53-induced apoptosis. Oncol Rep 18:1311–1314
Xue Y, Wong J, Moreno GT, Young MK, Cote J, Wang W (1998) NURD, a novel complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities. Mol Cell 2:851–861
Hofer MD, Kuefer R, Varambally S, Li H, Ma J, Shapiro GI, Gschwend JE, Hautmann RE, Sanda MG, Giehl K, Menke A, Chinnaiyan AM, Rubin MA (2004) The role of metastasis-associated protein 1 in prostate cancer progression. Cancer Res 64:825–829
Qian H, Lu N, Xue L, Zhang X, Fu M, Xie Y, Zhan Q, Liu Z, Lin C (2005) Reduced MTA1 expression by RNAi inhibits in vitro invasion and migration of esophageal squamous cell carcinoma cell line. Clin Exp Metastasis 22:652–662
Ryu SH, Chung Y, Lee H, Kum JA, Shin HD, Min HJ, Seo DD, Jang MK, Yu E, Kim KW (2008) Metastatic tumor antigen 1 is closely associated with frequent postoperative recurrence and poor survival in patients with hepatocellular carcinoma. Hepatology 47:929–936
Moon HE, Cheon H, Chun KH, Lee SK, Kim YS, Jung BK, Park JA, Jeong JW, Lee MS (2006) Metastasis-associated protein 1 enhances angiogenesis by stabilization of HIF-1alpha. Oncol Rep 16:929–935
Kawasaki G, Yanamoto S, Yoshitomi I, Yamada S, Mizuno A (2008) Overexpression of metastasis-associated MTA1 in oral squamous cell carcinomas: correlation with metastasis and invasion. Int J Oral Maxillofac Surg 37:1039–1046
Roepman P, De Jager A, GrootKerkamp MJ, Kummer JA, Slootweg PJ, Holstege FC (2006) Maintenance of head and neck tumor gene expression profiles upon lymph node metastasis. Cancer Res 66:11110–11114
Tang QF, Ji WY, Pan ZM, Zheng Y, Guan C (2003) Expression of the metastasis-associated gene 1 in laryngeal squamous cell carcinoma: correlation with cervical lymph node matastasis. Zhong hua er bi yan hou ke za zhi 38:213–216
Acknowledgment
This work was supported by grants from the alumni associated of the Department of Otolaryngology Head and Neck Surgery, The Catholic University of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, JO., Jung, CK., Sun, DI. et al. Relationships between metastasis-associated protein (MTA) 1 and lymphatic metastasis in tonsil cancer. Eur Arch Otorhinolaryngol 268, 1329–1334 (2011). https://doi.org/10.1007/s00405-010-1478-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-010-1478-6