Abstract
We combined a mass balance approach with measurements of air–water and sediment–water nitrogen (N) exchange to better understand the mechanisms attenuating N throughputs in a eutrophic coastal lagoon. We were particularly interested in how seasonal shifts in external versus internal N fluxes and the transition from diatom- to cyanobacteria- dominated phytoplankton communities influence N storage and loss to the atmosphere. We found that on an annual basis almost all of the N removed by the lagoon was due to sediment storage following the spring diatom bloom. This period was characterized by high riverine inputs of dissolved inorganic nitrogen, high rates of assimilatory conversion to particulate nitrogen (PN), and net accrual of N in sediments. By contrast, the larger summer bloom was associated with low sediment N storage, which we attribute in part to the presence of positively-buoyant cyanobacteria. Low settling rates during cyanobacteria blooms favored export of PN to the Baltic Sea over sediment accrual in the lagoon. In addition, summer dinitrogen (N2) fixation by cyanobacteria largely offset annual N2 losses via denitrification. These findings show that cyanobacteria blooms diminish N attenuation within the lagoon by altering the balance of N exchange with the atmosphere and by promoting export of particulate N over sediment burial.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Understanding the processes regulating nitrogen (N) fluxes at the landscape scale is critical to management efforts aimed at reducing anthropogenic impacts on inland and coastal waters. Along the flow-path from mountains to the sea, water passes through various hydrologic features (groundwater, streams, wetlands, lakes, estuaries) that differ in their capacity to attenuate throughputs of N (Howarth et al. 1996; Valiela et al. 1997; Vitousek et al. 1997; Boyer et al. 2002; Seitzinger et al. 2002). Their collective effects determine the proportion of N delivered to coastal waters (Seitzinger et al. 2006). The attenuation of N throughputs, commonly referred to as ‘retention’, is typically derived by mass balance from the difference between hydrologic inputs and outputs. The term ‘retention’ is somewhat of a misnomer as it includes both N retained within the system (e.g., via sediment accrual) as well as N lost from the system via atmospheric exchange. To avoid confusion, we use ‘storage’ when referring to accrual within the system, and ‘attenuation’ to describe the overall reduction in N throughputs (i.e., the combined effects of storage and atmospheric losses). Elucidating the fate of N inputs, whether stored in the system or lost to the atmosphere, is important to understanding N attenuation and for forecasting recovery from eutrophication.
Estuaries, being situated at the interface between freshwater and marine environments, are potentially important sites for N attenuation due to high input loads and high rates of biogeochemical activity (Nixon et al. 1996; Boyer and Howarth 2008; Asmala et al. 2017). Numerous studies have investigated estuarine N cycling, often using a mass balance approach to compare inputs and export, and to estimate attenuation by difference (Dettmann 2001; Robson et al. 2008; Bukaveckas et al. 2017a; Vybernaite-Lubiene et al. 2017). Attenuation is governed in part by the rate of assimilation of dissolved inorganic and organic N by primary producers and bacteria. After incorporation into biomass, settling of particulate matter enhances N storage (burial in sediments), and loss to the atmosphere (via denitrification; Nedwell et al. 1999; Sundbäck et al. 2004; Brion et al. 2008; Deek et al. 2013; Eyre et al. 2016). The proportion of the incoming N load that is attenuated is constrained by water residence time and temperature, which in turn regulate autotrophic and heterotrophic assimilation (Voss et al. 2010; Vybernaite-Lubiene et al. 2017). As a result, N attenuation varies seasonally and among estuaries that differ in their hydrogeomorphic settings and trophic state (Sundbäck et al. 2004; Eyre et al. 2013; Loken et al. 2016). Nitrogen cycling in estuaries has received considerable attention, but few studies have quantified the relevant processes (N fixation, denitrification, etc.) in a systematic way such that they can be scaled to the ecosystem level, and evaluated in the context of input and output fluxes (e.g., Brion et al. 2008).
Cyanobacteria often dominate phytoplankton communities in eutrophic waters during summer (Smith et al. 1999; Smith 2006; O’Neil et al. 2012; Paerl and Otten 2013). Their role in the N cycle of lentic waters has received considerable attention, but less is known regarding their presence in fresh-brackish estuaries and coastal lagoons (Howarth et al. 1988a, b; Bianchi et al. 2000; Murrell and Lores 2004). Their ability to form dense blooms, regulate buoyancy and, in some cases, fix N2, has important implications for the N balance of the ecosystem. Bloom events affect redox conditions in the water column due to oxygen demands associated with decomposition of algal biomass, as well as respiration by algae themselves. Our prior work in the Curonian Lagoon has documented the widespread occurrence of transient night-time hypoxia due to high rates of respiration in the water column during cyanobacteria blooms (Zilius et al. 2014). Anoxia may enhance conditions for denitrification fueled by water column nitrates, thereby resulting in greater N loss (Hietanen and Lukkari 2007). N2 fixation by cyanobacteria may partially or fully offset losses via denitrification, and may be particularly important to supporting blooms when external inputs are low (e.g., during low river discharge). In addition to their importance in affecting N2 fixation and denitrification, the ability of cyanobacteria to regulate their buoyancy may influence the fate of algal biomass by altering the balance between sedimentation and export (Walsby 1994; Chu et al. 2007). Positive buoyancy reduces sedimentation of cyanobacteria-dominated communities, thereby resulting in smaller fluxes of phytodetritus to sediments, and potentially greater loss through advective washout. Our prior work has shown that export of phytoplankton and phytodetritus during a cyanobacteria bloom transformed the Curonian Lagoon from an annual sink to a net source of phosphorus (Petkuviene et al. 2016; Vybernaite-Lubiene et al. 2017). The net effect of cyanobacteria dominance on the attenuation of N fluxes will depend on the extent to which they alter the balance between sedimentation and export, and the gain and loss of N via atmospheric exchange.
In this study, we combine a mass balance approach with process-specific measurements of N exchange at the air–water and water–sediment interface to better understand the mechanisms regulating N attenuation in a shallow coastal lagoon. Lagoons are important components of coastal ecosystems. In the Baltic region, they represent 4% of coastal area but account for 40% of total denitrification (Asmala et al. 2017). The Curonian Lagoon is characterized by eutrophic conditions with a succession of algal blooms transitioning from diatoms and green algae in the spring, to cyanobacteria in the summer (Pilkaityte and Razinkovas 2007; Zilius et al. 2014). Our prior work has used mass balance approaches to characterize N and P inputs, export and attenuation (Petkuviene et al. 2016; Vybernaite-Lubiene et al. 2017) and stable isotope analysis to assess N fixation by cyanobacteria (Lesutienė et al. 2014). In this paper we expand on this work by combining mass balance analysis with process-specific measurements of air–water exchange (N2 fixation, denitrification). Our objective was to better understand the processes affecting N attenuation following seasonal transitions from periods of high to low external loads and in conjunction with seasonal changes in phytoplankton abundance and community composition. In addition to characterizing the overall total nitrogen (TN) budget for this system, we derived separate mass balances for particulate and dissolved fractions. These were used to compare predicted and observed changes in water column concentrations and test our ability to depict N transformations during changing seasonal conditions.
Methods
Study site
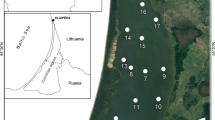
The Curonian Lagoon is a large (1584 km2), shallow (mean depth 3.8 m) waterbody located along the southeast coast of the Baltic Sea (Fig. 1). The lagoon discharges to the Baltic Sea through a narrow strait and occasionally receives inputs from the Baltic during periods of wind-driven tidal forcing (Zemlys et al. 2013). These events are typically of short duration and result in small increases in salinity (typically by 1–2, maximum = 7) in the northern portion of the lagoon. The Nemunas River is the principal tributary (mean annual discharge = 21.8 km3) accounting for 96% of total inputs (Jakimavičius and Kriaučiūnienė 2013). The river has a catchment area of 97,864 km2, which is comprised of 60% cultivated and 40% forested lands (Vaitkuviene and Dagys 2008). The Nemunas is a lowland river with seasonally variable discharge, which is dependent in part on the timing of spring snowmelt. The inflow of the Nemunas divides the lagoon into northern and southern regions that differ in water renewal time, depth and sediment composition (Zilius et al. 2014; Umgiesser et al. 2016). The northern half of the lagoon is characterized by shallower depths (1.5–2 m), shorter water renewal time (annual mean = 76 days; seasonal range = 50–100 days), and sandy sediments (Zilius et al. 2014; Umgiesser et al. 2016). The southern half of the lagoon is deeper (mean = 3.5 m), has a longer water renewal time (annual mean = 190 days; seasonal range = 100–250 days) and organic–rich benthic deposits (predominantly silty sediments). The lagoon is vertically well mixed owing to the shallow depth and weak salinity gradients.
Sampling design
Our sampling scheme was designed to prioritize effort where fluxes were anticipated to be large and variable (e.g., riverine loads during spring runoff, summer N fixation). For the mass balance analysis, water samples were collected at the inflow (Nemunas River) and outflow (Klaipeda Strait) of the lagoon, and from an off-shore site in the Baltic Sea (55°55′13.1″N and 21°02′39.4″E), to estimate riverine inputs, lagoon export, and marine inputs, respectively (Fig. 1). Samples were collected monthly at each of the sites from December 2014 to November 2015, except at the inflow site (Nemunas) where additional samples were obtained (at 1–2 week intervals) during the period of highest discharge (January–April). Our prior work showed that there was greater variability in riverine nutrient concentrations in comparison to lagoon and Baltic Sea waters and therefore we allocated greater effort toward determining riverine loads (Vybernaite-Lubiene et al. 2017). To characterize internal processes, we measured water column production, respiration and N2 fixation, as well as sediment–water N exchange. These processes were measured at two sites: a northern site representing sandy substrates, and a southern site dominated by silty sediments (Fig. 1). Water samples and sediment cores were obtained quarterly (February, April, August and November) to represent Winter, Spring, Summer and Fall. Additional water samples were obtained monthly (and twice per month during July and August) to characterize chlorophyll a (Chl-a), phytoplankton biomass, N fractions and N2 fixation during peak bloom conditions.
PN production and remineralization
Production and respiration were measured to derive rates of assimilatory and dissimilatory N fluxes in the water column (Kamarainen et al. 2009; Knoll et al. 2016; Wood et al. 2016). The premise of this approach is that a stoichiometric quotient (e.g., Redfield ratio, or seston C:N) can be used to infer the rate of conversion of dissolved nitrogen (TDN) to particulate (PN) (hereafter, assimilatory uptake) using oxygen- or carbon- based estimates of production, and, that the corresponding dissimilatory process (i.e., conversion of PN to TDN) can be estimated from respiration.
where GPP is the sum of NPP and R, and SestonN:C values are month- and site- specific measured values.
We measured NPP and R in the water column using the light-dark bottle technique (e.g., Strickland 1960). Three replicate 250 ml Winkler glass bottles were filled from each depth layer and incubated in the laboratory under artificial illumination at irradiances representing the mid-point of the each layer (based on PAR profiles). Incubation irradiance varied by site and season (e.g., from 130 to 500 µE s−1 m−2 for 0–1 m layer). Incubation temperatures corresponded to in situ conditions at the time of sample collection. NPP and R were calculated from the change in dissolved oxygen concentrations in light and dark bottles during the 4–6 h incubation. Oxygen concentrations were measured using the Winkler microtitration method (Grasshoff et al. 1983). Volumetric rates were converted to areal rates using average depth and the proportional contribution of each layer. Hourly rates were converted to daily rates taking into account light dosages during the incubation relative to daily in situ values. The latter were determined from incident solar radiation (as monthly average), light attenuation and mean depth (Bukaveckas et al. 2011).
Nitrogen fixation
Rates of N2 fixation in the water column were determined using the 15N2 tracer method. Measurements were made monthly from June to November 2015 (excluding October) and twice per month in July and August. N2 fixation was measured in three depth layers (0–1 m, 1–2.5 m and 2.5–3.5 m) at the deeper, southern station (silty site), and at the surface only at the shallow northern station (sandy site). Measurements of N2 fixation followed methods described in Montoya et al. (1996). The samples were filled without air bubbles into 500 ml transparent HDPE bottles. Through a gas-tight septum, each sample received 0.5 ml 15N2 (98% 15N2, Sigma-Aldrich) injected by syringe. The sample was carefully shaken to allow isotopic equilibration of the N2 tracer. As the isotopic equilibration takes up to several hours (Mohr et al. 2010) we incubated the samples for 24 h (Mulholland et al. 2012). There has been some debate about using the bubble method for N2 fixation measurements (Mohr et al. 2010; Großkopf et al. 2012), but recent work (Wannicke et al. 2018) demonstrated that underestimation of rates is negligible (< 1%) for incubations lasting 12–24 h. Water samples were incubated outdoors at ambient irradiance. Samples from deeper layers (1–2.5 and 2.5–3.5 m) were wrapped in aluminum foil as in situ irradiance was below 1% of surface PAR at these depths. At each site, three controls (without 15N2 additions) were incubated in parallel to correct δ15N for natural abundance. After incubation, the samples were filtered through pre-ashed (8 h at 450 °C) Whatman GF/F filters and stored frozen until analysis with a continuous-flow isotope ratio mass spectrometer (Thermo-Finnigan, Delta S, Bremen) at the Leibniz Institute for Baltic Sea Research Warnemünde (IOW). Volumetric rates of N2 fixation was calculated according to Montoya et al. (1996):
where V (the specific rate of N2 uptake) is derived from:
APN is the 15N atom % enrichment of the PN pool at the beginning (t0) and end (tf) of an incubation, \({{\text{A}}_{{{\text{N}}_{{2}}}}}\) is the 15N atom % enrichment of the dissolved N2 gas in the incubated water, and PN is the concentration of PN at the beginning (t0) and end (tf) of the incubation. Daily volumetric N2 fixation rates were converted to areal rates taking into account the depth of the water column and the thickness of each layer (see above).
Sediment fluxes
Five large (i.d. 8 cm, 30 cm length) and 3 small (i.d. 4.6 cm, 25 cm length) intact sediment cores were collected using a hand corer at each of the two sites. Only undisturbed cores with visually clear overlaying water were used for subsequent analysis. Small cores were used to measure sediment properties including Chl-a, organic carbon (Corg), total N (TNsed), bulk density (dry weight per unit volume) and grain size. Large cores were used to measure net fluxes of NH4+, NO −x , and DON in the dark (and light if in situ irradiance at the sediment surface exceeded 1% of surface PAR) based on changes in concentration in the overlying water (Dalsgaard et al. 2000). The N2:Ar technique was used to estimate net N2 fluxes at the sediment–water interface (i.e., the balance between denitrification and N2 fixation; Fulweiler and Nixon 2012). Cores were transported to the laboratory within 4 h where they were incubated in temperature-controlled (± 0.5 °C) tanks with in situ water. An external magnet rotating at 40 rpm ensured water mixing within each core. After an overnight pre-incubation, a gas-tight lid with optical O2 sensor spots (Pyroscience®) was placed on each core. Incubations lasted from 3 to 13 h in order to keep oxygen within 20% of initial concentration. At the beginning and end of the incubations a 20 ml aliquot was transferred to 12 ml exetainers (Labco®) and fixed with 7 M ZnCl2 for N2:Ar measurements. Thereafter, two more aliquots of 20 ml were immediately filtered (Whatman GF/F filters) and transferred into PE tubes and glass vials for inorganic and organic N analysis. Solute exchange across the water–sediment interface was calculated according to the general flux equation:
where Fx (μmol m−2 h−1) is flux of the x fraction, Ci and Cf (μmol l−1) are the initial and final concentrations, V (l) is volume of the overlying water, A (m2) is the sediment surface area and t (h) is the incubation time. Daily N fluxes (mmol m−2 day−1) across the sediment–water interface were calculated according to the equation:
where hD and hL are respectively the number of dark and light hours corresponding to the date when the core was collected.
Analytical procedures
Water temperature, salinity and dissolved oxygen were measured in situ at the surface (0.5 m depth) and bottom (0.5 m above the sediment) using a YSI 460 multiple probe. Vertical profiles of photosynthetically active radiation (PAR) were measured with a LI-192 underwater quantum sensor (LI-COR®) on dates corresponding to primary production and benthic flux measurements. Water samples (2 l) from surface and bottom layers were collected with a Ruttner bottle, pooled to obtain a vertically-integrated sample, and transported with ice packs. Chl-a concentrations were determined in triplicate. Water samples for Chl-a were filtered within 1–2 h of collection through Whatman GF/F glass fiber filters (nominal pore size 0.7 μm). Pigments were extracted with 90% acetone (24 h at 4 °C) and measured by spectrophotometry (Jeffrey and Humphrey 1975; Parson et al. 1984). Phytoplankton samples were preserved with acetic Lugol’s solution and examined at magnifications of 200× and 400× using a LEICA DMI 3000 inverted microscope (Utermöhl 1958). The abundance of phytoplankton and filamentous diazotrophic cyanobacteria (thousand cells l−1) was calculated according to HELCOM (2015). Phytoplankton biomass (mg l−1) was calculated according methodology described in Olenina et al. (2006).
Water samples were filtered within 1–2 h of collection (GF/F filters), transferred into 10 ml PE tubes for inorganic (NH4+, NO2−, NO3−) analysis, or 25 ml glass vials for organic (DON) analysis, and frozen immediately (− 20 °C). Dissolved inorganic N concentrations (NO2− and NO −x ) were measured with a 4-channel continuous flow analyzer (San++, Skalar) using standard colorimetric methods (Grasshoff et al. 1983). Nitrate (NO3−) was calculated as the difference between NO −x and NO2−. Dissolved ammonium (NH4+) was analyzed manually by means of the salicylate-hypochlorite method, using nitroprussiate as catalyst (Bower and Holm-Hansen 1980). Total dissolved nitrogen (TDN) was analyzed by the high temperature (680 °C) combustion, catalytic oxidation/NDIR method using a Shimadzu TOC 5000 analyzer with a TN module. Dissolved organic nitrogen (DON) was calculated as difference between TDN and DIN (NH4+ + NO2− + NO3−). Particulate matter was collected on pre-ashed (4 h at 550 °C) Whatman GF/F filters for organic carbon (POC) and nitrogen (PN) analysis. These filters were dried at 60 °C for 24 h and stored at 4 °C until analysis. POC and PN were analyzed with an Elementar Vario EL Cube (Elementar Analysen systeme GmbH). Sediment C and N content were analyzed with a mass spectrometer (Thermo Scientific Delta V) coupled with element analyzer (FlashEA 1112, Thermo Electron Corporation) at the Center for Physical Sciences and Technology. Before measurements samples were acidified with 1 N HCl in order to remove carbonates. Median grain size (Md) was determined with laser particle size analyzer (Analysette 22 MicroTec plus, Fritsch GmbH). Dissolved gasses (N2 and Ar) were measured by membrane inlet mass spectrometry (MIMS) at Ferrara University (Bay Instruments; Kana et al. 1994) and corrected for Ar concentration and solubility based on temperature and salinity. N2-scavenging effects of bubbles produced during light incubation were avoided by running a dark incubation prior to the light incubation (Eyre and Ferguson 2002). No bubbles were visible during the incubations.
Lagoon inputs and outputs
Input and output fluxes of N were derived using previously described methods for this site (Vybernaite-Lubiene et al. 2017). Riverine inputs were calculated from monthly measurements of N concentrations and daily discharge of the Nemunas River. Discharge data were provided by the Lithuanian Hydro-meteorological Service. NH4+ and NO3− concentrations were significantly correlated with river discharge (r = 0.71 and r = 0.91, respectively, p < 0.05); these regressions were used to derive concentrations when calculating monthly total loads. N fluxes between the lagoon and the Baltic Sea were derived using estimates of hydrologic exchange obtained from a hydrodynamic model (SHYFEM; http://www.ismar.cnr.it/shyfem) previously calibrated for this site (Zemlys et al. 2013). The model simulates changes in sea surface elevation in response to wind-forcing events and derives estimates of water exchange at 2 h time steps. Fluxes were derived based on the volume of exchange between the lagoon and Baltic Sea and measurements of N concentrations in the outflow (Klaipeda Strait) and inflow (offshore Baltic). For sampling dates when the lagoon outflow sample (Klaipeda Strait) was mixed with seawater (salinity > 0.5), it was necessary to correct N concentrations for dilution by seawater to estimate N fluxes from the lagoon. We calculated N concentrations in the lagoon (CLAGOON) based on simultaneous measurements of salinity and N fractions at the off-shore site and lagoon outlet (Grelowski et al. 2000):
where COUT and CSEA is N concentration at the lagoon outlet and off-shore Baltic Sea. XR was calculated using the following equation:
where SSEA, SOUT and SRIVER is salinity at coastal zone of Baltic Sea, Lagoon outlet and Nemunas River, respectively. Input and output fluxes were expressed on an areal basis (mmol m−2 day−1) for comparison to internal process rates.
Nitrogen balance
Nitrogen budgets were developed for winter, spring, summer and fall based on measured input, output and internal fluxes (Nixon et al. 1996; Dettmann 2001). Daily average values of input and output fluxes were derived for 3-month periods representative of each season (Winter = December–February, Spring = March–May, Summer = June–August, and Fall = September–November). These were used in conjunction with lagoon-average estimates of internal fluxes, which were derived from the site-specific measurements and the proportional area comprised of sandy (45%) and silty (55%) sediments (Zilius et al. 2014). The gain or loss of N stored within the lagoon was estimated from riverine inputs, net atmospheric exchange (N2 fixation−denitrification), and net exchange with the Baltic Sea (inputs–outputs).
Storage represents the average daily rate of TN accumulation in the lagoon during each 3-month budget period. We partitioned total (Lagoon) storage into water column and sediment components. As the change in water column concentrations was known, this allowed us to estimate N storage in sediments for each (3-month) budget period:
The change in sediment storage of N reflects the balance between the quantity of N delivered via sedimentation minus the portion lost through remineralization and diffusion into the water column. By estimating sediment storage (above), and accounting for N losses via sediment–water exchange (from core incubations), we obtained a measure of N delivery to sediments:
Quantifying N loss via sedimentation allowed us to develop particulate and dissolved N balances for the water column. The predicted rate of change in water column PN was derived from estimates of PN inputs from internal (production) and external (riverine and marine) sources, and PN losses via export, sedimentation and remineralization (respiration). A similar model was constructed for total dissolved nitrogen (TDN = DIN + DON) taking into account inputs from internal (remineralization) and external (riverine and marine) sources, and losses via assimilatory uptake and net sediment exchange.
Predicted changes in PN and TDN were compared against observed changes in the water column to test our ability to reconcile the sources and fate of N in the lagoon.
Statistical analysis
Linear regression analysis was used to examine the relationships between nutrient concentration and discharge, and between net rates of N2 fixation and the abundance of heterocystous cyanobacteria. Data were tested for normality and homogeneity of variances using Shapiro–Wilk and Cochran’s tests, respectively. If data did not meet these conditions, square root and log transformations were applied. Statistical significance was set at p < 0.05.
Results
Phytoplankton seasonal and spatial patterns
Phytoplankton communities exhibited a transition from diatom-dominated in spring to cyanobacteria-dominated in summer at both sites. Diatoms accounted for up to 76% (northern site) and 82% (southern site) of total phytoplankton biomass during spring. Cyanobacteria accounted for 60% (northern site) and 85% (southern site) of phytoplankton biomass during summer. The shift in community composition occurred in July when Aphanizomenon flos-aquae became abundant (61% of the total biomass). Seasonal patterns in Chl-a, phytoplankton community composition and production were similar at the northern and southern sites with peak values occurring in late summer (August–September; Fig. 2). Peak Chl-a concentrations were higher at the southern site (~ 250 µg l−1) relative to the northern site (~ 100 µg l−1). Heterocystous cyanobacteria comprised 70% (northern site) and 85% (southern site) of cyanobacteria biomass during the bloom. Rates of N2 fixation were higher at the southern site (30–150 μmol m−2 h−1) relative to the northern site (< 30 μmol m−2 h−1). Rates of N2 fixation were strongly correlated with the abundance of heterocysts (R2 = 0.82, p < 0.001). DIN concentrations were in the range from 1.8 to 3.5 µM during this period. Highest GPP (30 μmol m−2 h−1) and R (15 μmol m−2 h−1) were also observed at the southern site in summer. In other months, GPP and R were less than 20 and 10 μmol m−2 h−1, respectively, at both sites.
Seasonal patterns in nitrogen loads and concentrations
Riverine discharge followed expected seasonal patterns with highest flows (> 600 m3 s−1) occurring in January–April, and lowest flows (< 300 m3 s−1) in July–October (Fig. 3a). Riverine N inputs to the lagoon tracked seasonal patterns in discharge (Fig. 3b). The winter–spring period of elevated discharge accounted for 80% of the annual N load. DIN was the dominant fraction accounting for > 80% of TN in riverine inputs. PN was a minor component of the TN load delivered to the lagoon. N concentrations in the lagoon ranged from 100 to 250 μmol l−1 during the period of study (Fig. 3c). Peak concentrations observed in winter–spring were associated with elevated DIN (up to 150 μmol l−1), whereas peak values in late summer were associated with high concentrations of PN (up to 240 μmol l−1). The September peak in PN coincided with highest levels of Chl-a and phytoplankton biomass. N concentrations at the Baltic Sea inflow monitoring location (data not shown) were low (< 75 μmol l−1) in comparison to lagoon and riverine concentrations. N concentrations at the lagoon outflow site (Klaipeda Strait) followed seasonal patterns observed in the lagoon with dominant fractions shifting from DIN (winter–spring) to PN (summer–fall).
Sediment–water fluxes
Differences in sediment properties between the northern and southern stations followed expected patterns based on substrate type. The southern site was dominated by fine silty mud (Md range = 0.033–0.038 mm) with a high Corg (10–14%) and TNsed (1.5–2.0%) content. The northern site was dominated by sandy deposits (Md range = 0.148–0.207 mm) with low Corg (< 0.5%) and TNsed (< 0.1%) content. Chl-a concentrations of the surface sediment layer were 66–218 mg m−2 at the northern site and 62–100 mg m−2 at the southern site.
Seasonal rates in lagoon-scale sediment–water N exchange ranged from − 1.43 to 4.64 mmol m−2 day−1. Sediments were a net source of TDN to the water column in spring and summer (0.79 and 4.64 mmol m−2 day−1, respectively) and a net sink in fall (− 1.43 mmol m−2 day−1). High rates of sediment N release in summer were due to the large efflux of NH4+ at the southern (silty) site (6.62 ± 1.47 mmol m−2 day−1; Fig. 4). In Spring, lagoon-scale estimates of TDN release were lower as high rates of DON efflux at the southern (silty) site (7.55 ± 3.19 mmol m−2 day−1) were partially offset by NO −x loss at this site (− 4.22 ± 0.45 mmol m−2 day−1) and by DON loss at the northern site (− 1.76 ± 0.72 mmol m−2 day−1). In winter, NH4+, NO −x , and DON fluxes were all low (< 1 mmol m−2 day−1), and net exchange at the sediment–water interface was nearly balanced (0.8 mmol m−2 day−1). Seasonal rates of N2 fluxes at the sediment–water interface ranged from − 1.69 to 2.91 mmol m−2 day−1. Positive fluxes, indicative of net denitrification, were observed in winter and summer (2.91 and 1.06 mmol m−2 day−1). Both sites exhibited their highest fluxes in winter (2.61 ± 0.52 and 3.15 ± 0.77 mmol m−2 day−1; northern and southern sites, respectively). Negative net N2 fluxes, indicative of N2 fixation, were observed in spring and fall at the northern site. In Fall, positive fluxes at the southern site (2.94 ± 0.71 mmol m−2 day−1) were offset by negative fluxes at the northern site (− 7.36 ± 2.98 mmol m−2 day−1). Sediment–water fluxes in the light were measured only in April at the sandy site where in situ irradiance reached 168 μmol s−1 m−2, corresponding 10% of surface PAR. Significant differences between light and dark fluxes of N2 showed that in the light there was a prevalence of N2 fixation (negative N2 flux) over denitrification.
Lagoon TN balance
On an annual basis, the Curonian Lagoon was a net sink for TN retaining 28% of inputs (Fig. 5). Average daily inputs from riverine (4 mmol m−2 day−1) and marine (2 mmol m−2 day−1) sources exceeded marine export (4 mmol m−2 day−1) resulting in a net gain of 2 mmol m−2 day−1. Net atmospheric exchange was near zero on an annualized basis indicating that losses from denitrification matched gains from N2 fixation. Thus, attenuation was due to storage within the lagoon, not loss to the atmosphere. As there was no increase in water column TN over the annual cycle, storage was attributed to sediment accrual.
Nitrogen balance for the Curonian Lagoon showing season-specific and annualized daily average fluxes associated with riverine inputs, marine exchange and atmospheric exchange. Attenuation is the reduction in N fluxes during passage through the lagoon (riverine and marine inputs minus marine export). Storage represents the gain in N within the lagoon taking into account riverine inputs, marine inputs and atmospheric exchange
Riverine, marine and atmospheric fluxes were seasonally variable and this resulted in seasonal variation in the TN balance of the lagoon, and in the relative importance of storage to attenuation. The lagoon was a net sink for TN in winter and spring (positive attenuation) and a net source in summer and fall (negative attenuation). Highest attenuation, both by mass and as a proportion of inputs occurred in the spring when riverine inputs were large (6.8 mmol m−2 day−1) and, in combination with marine inputs (2.1 mmol m−2 day−1), exceeded marine export (3.4 mmol m−2 day−1) by 5.6 mmol m−2 day−1 (62% of inputs). Storage during the period was 6.0 mmol m−2 day−1 (66% of inputs); slightly larger than attenuation owing to a small offset from the gain of N via atmospheric exchange (0.4 mmol m−2 day−1). The lagoon was also a net sink for TN in winter, but in contrast to the spring, this occurred largely via loss to the atmosphere, not storage within the lagoon. In winter, riverine inputs (8.0 mmol m−2 day−1) in combination with marine inputs (2.0 mmol m−2 day−1), exceeded marine export (7.0 mmol m−2 day−1) by 3.0 mmol m−2 day−1. The potential gain in N was offset by a net loss to the atmosphere (2.9 mmol m−2 day−1) owing to the predominance of denitrification over N2 fixation. As a result, the gain in N storage within the lagoon was small (2% of inputs) in comparison to attenuation (31% of inputs, inclusive of atmospheric losses).
The lagoon was a net source of TN in summer as marine export (3.8 mmol m−2 day−1) exceeded the combined inputs from riverine and marine sources (2.5 mmol m−2 day−1). Net atmospheric exchange was near zero (< 0.1 mmol m−2 day−1) and therefore did not appreciably affect the overall N balance. The lagoon was also a net source of TN in fall, despite a net gain in storage. Marine export (3.0 mmol m−2 day−1) exceeded the combined inputs from riverine and marine sources (2.4 mmol m−2 day−1) resulting in a net loss of 0.6 mmol m−2 day−1. However, factoring in a positive balance from atmospheric exchange (2.3 mmol m−2 day−1), this yielded a positive estimate of N storage (1.8 mmol m−2 day−1), which was equivalent to 72% of riverine and marine inputs. Overall, these results show a high degree of seasonal variability in the N balance of the lagoon with the spring–summer transition marked by a shift from net gain to net loss of N.
Nitrogen transformations within the lagoon
Production and remineralization were the dominant fluxes accounting for the gain and loss of PN from the water column (Fig. 6). Rates of PN production ranged from 2 (Winter) to 41 (summer) mmol m−2 day−1; corresponding values for remineralization ranged from < 1 to 26 mmol m−2 day−1 (respectively). Production exceeded remineralization in all seasons indicating that water column processes were a net source of PN. On an annual basis, production within the water column was almost twofold higher than remineralization (16 and 9 mmol m−2 day−1, respectively). PN inputs from riverine, marine and atmospheric sources were small by comparison (< 1 mmol m−2 day−1) even during periods of elevated river discharge (winter and spring). Inferred rates of PN loss via sediment deposition were larger, with highest rates in spring (8 mmol m−2 day−1). During winter and spring, the combined losses due to sediment deposition, marine export and remineralization were comparable to rates of PN production and other inputs, resulting in small predicted and observed changes in water column PN (< 2 mmol m−2 day−1). In summer and fall, predicted gains in water column PN exceeded observed gains in PN concentration by 11 and 4 mmol m−2 day−1 (respectively). In these seasons, the large imbalance between production and remineralization yielded high estimates of PN production which exceeded the observed changes in water column PN.
Sources and fate of particulate nitrogen (PN) in the water column of the Curonian Lagoon. Positive fluxes are production, marine inputs, riverine inputs and nitrogen fixation. Negative fluxes are losses via remineralization and marine export. Net sediment deposition reflects the balance between sedimentation and N efflux from sediments. Predicted changes in water column PN derived from the mass balance are shown in comparison to observed changes in water column PN
The TDN balance showed that uptake and remineralization were the dominant fluxes, but unlike PN, external inputs were also important in regulating water column concentrations (Fig. 7). On an annualized basis, losses of TDN due to assimilatory uptake and marine export were 16 and 3 mmol m−2 day−1, respectively. TDN sources included remineralization (9 mmol m−2 day−1), riverine and marine inputs (5 mmol m−2 day−1) and a small gain from sediment exchange (1 mmol m−2 day−1). The annualized data indicate that internal processes (water column remineralization and sediment exchange) could account for 62% of assimilatory demand, with external inputs contributing 31%. The relative importance of internal and external sources of TDN varied seasonally. In summer, when assimilatory demand was highest, water column remineralization was equivalent to 60% of demand, and sediment exchange contributed 10%, whereas riverine and marine inputs were small (2%). The mass balance indicated an excess of assimilatory uptake relative to internal and external supply of 12 mmol m−2 day−1, whereas the observed decline in lagoon TDN was smaller (2 mmol m−2 day−1). A similar imbalance was observed in fall (shortfall of TDN supply relative to demand), whereas observed and predicted changes in TDN during winter and spring were smaller (~ 2 mmol m−2 day−1). In fall and winter, the excess of assimilatory demand over remineralization was offset by higher rates of TDN supply from riverine inputs (6–8 mmol m−2 day−1).
Sources and fate of total dissolved nitrogen (TDN) in the water column of the Curonian Lagoon. Positive fluxes are remineralization, marine inputs and riverine inputs. Negative fluxes are assimilatory uptake and marine export. Net sediment fluxes may be positive (release to water column) or negative (flux to sediments). Predicted changes in water column TDN based on the mass balance are shown in comparison to observed water column TDN
Discussion
We combined a mass balance approach with process-specific measurements (sediment fluxes, N2 fixation, etc.) to better understand the role of N storage and atmospheric exchange in governing the attenuation of N fluxes in a shallow eutrophic lagoon. On an annualized basis, the Curonian Lagoon was a net sink for TN as inputs from riverine, marine and atmospheric sources exceeded export losses. Atmospheric exchange via N2 fixation and denitrification was nearly in balance over the annual cycle. Therefore, attenuation of N fluxes can be attributed to storage, and, as there was little change in water column TN over the annual cycle, we conclude that sediment storage was the dominant mechanism. The attenuation of TN fluxes in the Curonian Lagoon (28%) was similar to other lagoons in the Baltic region (12–29% in the Oder Lagoon, Grelowski et al. 2000; 18% in the Vistula Lagoon, Witek et al. 2003), but lower in comparison to some large embayments (82% in Gulf of Riga, Savchuk 2005; 22% in Gulf of Finland, Radtke et al. 2012; 65% in Stockholm Archipelago, Almroth-Rosell et al. 2016).
Our findings show that there was a strong seasonality, not only in rates, but in the dominant processes attenuating N fluxes. Specifically, we distinguish between periods when assimilatory and dissimilatory processes in the water column were dominant (spring and summer), versus when they were not (winter and fall), and for the former, the influence of diatom- versus cyanobacteria-dominated communities (Fig. 8). N storage predominantly occurred during the spring diatom bloom when assimilatory uptake and riverine DIN inputs were high. As external PN inputs were small, our findings indicate that storage, via internal PN production and subsequent deposition of phytodetritus, was the dominant mechanism of N attenuation. The summer cyanobacteria bloom was larger by comparison to the spring diatom bloom, but resulted in negligible storage of N due to (1) a greater proportion of PN being exported to the Baltic Sea, and (2) high rates of sediment N remineralization (see below). We speculate that lower settling velocities of cyanobacteria relative to diatoms favors export of algal N over sedimentation and storage (Poister and Armstrong 2004). Positive buoyancy may be advantageous in microtidal systems where lentic conditions allow cyanobacteria to gain more favorable light conditions near the surface (Chu et al. 2007; Visser et al. 2016). The development of surface scums comprised of cyanobacteria is not uncommon, particularly in the southern portion of the lagoon during low-wind conditions (Bresciani et al. 2014).
Nitrogen fluxes [total dissolved (TDN), particulate (PN) and dinitrogen (N2)] in the Curonian Lagoon show the seasonal transition from dominance by external loads and denitrification (winter) to a dominance by internal recycling during diatom (Spring) and cyanobacteria (Summer–Fall) blooms (units are mmol m−2 day−1)
Cyanobacteria were present in measurable abundance by mid-spring, but accounted for a small proportion of the diatom-dominated community. During this period, the dominant forms were non-N2-fixing cyanobacteria such as Planktothrix agardhii and Microcystis spp. Increasing temperature and decreasing DIN were accompanied by the appearance of N2 fixing cyanobacteria (e.g., A. flos-aquae) during summer. Our prior work showed that the establishment of these N2 fixing cyanobacteria resulted in a large drop (~ 5‰) in the δ15N values of PON, indicating an important contribution from N2 fixation (Lesutienė et al. 2014). Areal rates of N2 fixation reported here (~ 1.1 mmol m−2 day−1 in summer) were lower than those in the Baltic proper (2.5 mmol m−2 day−1; Wasmund et al. 2001), but larger than other coastal sites (e.g., Roskilde fjord < 0.1 mmol m−2 day−1; Bentzon-Tilia et al. 2015). Large colonies of filamentous A. flos-aquae were likely the dominant N2 fixers in the Curonian Lagoon though we cannot discount the importance of unicellular cyanobacteria (< 10 μm), which are the main contributors to N2 fixation in the Baltic Sea (Wasmund et al. 2001; Bentzon-Tilia et al. 2015). Prior work in the Aphanizomenon-dominated Lake 227 showed that rates of N2 fixation could be reliably predicted from the abundance of heterocysts (Findlay et al. 1994). Our results scale appropriately, in that mid-range values for Lake 227 (10,000,000 heterocysts L−1 and 20 mg N2 m−3 day−1, respectively; Higgins et al. 2017) were approximately fourfold higher than corresponding values for the lagoon (2640,000 heterocysts L−1 and 3.8 mg N2 m−3 day−1).
With the increasing dominance of cyanobacteria in eutrophic waters there is considerable interest in the importance of N2 fixation to biogeochemical cycles (Bentzon-Tilia et al. 2015; Adam et al. 2016; Benavides et al. 2018) and for higher trophic levels (Lesutiene et al. 2014; Motwani et al. 2018). There is a need for further studies that depict spatial and temporal variability in N2 fixation, and to contextualize these within ecosystem N budgets. In the lagoon, the spatial distribution of buoyant cyanobacteria is affected by changes in wind speed and direction (Pilkaityte and Razinkovas 2007; Bresciani et al. 2014; Zilius et al. 2014). Changes in the distribution of heterocystous cyanobacteria in turn affects N2 fixation (Wasmund et al. 2001; Bertos-Fortis et al. 2016). For example, during two consecutive samplings in July, rates of N2 fixation declined by ~ 80% due to changing weather conditions, which transported cyanobacteria downwind of our sampling location. The patchy and dynamic distribution of cyanobacteria poses a significant challenge to reliably extrapolating results from site-specific measurements to the ecosystem scale. Further work at this site will benefit from the use of continuous ship-board data to map Chl-a and assess hot spots for N2 fixation.
Seasonal measurements revealed that sediment–water N fluxes varied in both direction (switching from sink to source) and in the dominant forms of N exchange. During summer base flow conditions, sediments were the main source of dissolved N to the water column, with efflux of NH4+ being the dominant form. NH4+ efflux from silty sediments (southern site = 6.6 mmol m−2 day−1) was higher than that reported for shallow coastal waters in the Baltic region (e.g.; 2.1 mmol m−2 day−1, Bonaglia et al. 2014) and for temperate river embayments (5.0 mmol m−2 day−1, McCarthy et al. 2016), but lower than in temperate river estuaries (8.2 mmol m−2 day−1, Deek et al. 2013; 10.5 mmol m−2 day−1, Gao et al. 2014). NH4+ release occurred following the cyanobacteria bloom, which likely provided fresh organic matter to stimulate re-mineralization (Zilius et al. 2014; Gao et al. 2014; Song and Burgin 2017). NH4+ release at the silty site corresponded to higher Chl-a content of surficial sediments, indicative of greater phytodetritus deposition (growth of microphytobenthos being unlikely to due to < 1% PAR). We assume that organic matter mineralization was the main source of NH4+ because its production via dissimilative NO3− reduction (DNRA) seems to be negligible (Zilius et al. 2016). Although NH4+ release dominated in summer, DON accounted for > 90% of sediment–water N exchange in spring. High DON release from sediments may represent an intermediate phase of N regeneration as hydrolysis, fermentation and cell lysis liberate DON, while conversion to NH4+ lags (Hansen and Blackburn 1992; Enoksson 1993; Lomstein et al. 1998; Zilius et al. 2016). We do not have a causal explanation as to why the diatom bloom was associated with greater DON release from sediments (southern site only), whereas the cyanobacteria bloom was followed by NH4+ release (both sites). The springtime DON release was small (< 1 mmol m−2 day−1), particularly in comparison to external inputs at this time (riverine inputs = 6 mmol m−2 day−1), whereas NH4+ release in summer was larger (4 mmol m−2 day−1), and coincided with low external inputs (riverine inputs = 0.6 mmol m−2 day−1). Nitrification rates have not been measured in the Curonian Lagoon, but we hypothesize that in summer, the shallow oxygen penetration depth (< 0.5 mm, Zilius et al. 2012) constrains nitrifiers to a narrow sediment layer.
Net N2 fluxes at the sediment–water interface ranged from − 7.4 to +3.2 mmol m−2 day−1, indicating that the sediments acted as both a source and sink of atmospheric N. Our measured values fell within the range reported for other estuarine systems (− 18 to +6 mmol m−2 day−1; Bruesewitz et al. 2013; Deek et al. 2013; Smyth et al. 2013). Assuming negligible NO3− uptake by microphytobenthos and that 1 mmol N2 flux is equal to 2 mmol of denitrified NO3−, mean denitrification would be 1.14 mmol m−2 day−1. These rates are similar to those previously measured with the isotope pairing technique (1.2 mmol N m−2 day−1, Zilius 2011) and are among the highest reported for shallow coastal sites in the Baltic region (e.g. Silvennoinen et al. 2007; Hietanen and Kuoparinen 2008; Bonaglia et al. 2014; Asmala et al. 2017). Recent studies have shown that benthic N2 fixation in estuarine environments is mostly carried out by sulfate reducers, sulfur oxidizers and denitrifiers (Fulweiler and Nixon 2012, Andersson et al. 2014; Newell et al. 2016). Seasonality in rates of denitrification has been reported for a number of estuaries; with maximum rates typically occurring in spring, summer or fall (Pina-Ochoa and Álvarez-Cobelas 2006; Hietanen and Kuoparinen 2008; Zilius 2011). In the Curonian Lagoon, we observed highest rates of N2 efflux from sediments in winter. Highest rates of sediment N2 efflux corresponded to periods of high input loads and water column concentrations of NO3−. This is consistent with recent work showing that denitrification in freshwater systems is controlled by NO3− concentration and by temperature (Cavaliere and Baulch 2018). Our data suggest that organic matter availability is also an important constraint on denitrification. Negative values, indicative of a predominance of N2 fixation over denitrification, were observed only at the northern (sandy) site, whereas the more organic- and N- rich (silty) site always exhibited positive values (net denitrification). We cannot exclude the possibility that microphytobenthos may contribute to N2 fixation. However, light conditions at the sediment–water interface are generally too low, except during the brief (spring) clear-water phase. Comparisons of sediment–water nutrient fluxes between the sandy and silty sites are complicated by differences in sediment permeability (Huettel et al. 2014), and our limited ability to replicate in situ hydrodynamics during core incubations. We used a standard approach to provide gentle mixing for the cores, but recognize the need to better link experimental and in situ conditions for variables such as benthic sheer stress and turbulence velocity (Porter et al. 2018).
Assimilatory and dissimilatory processes dominated N fluxes in the Curonian Lagoon as these internal transformations were large relative to fluxes associated with riverine inputs, marine exchange, atmospheric exchange and sediment–water exchange. The low rates of sediment–water exchange relative to water column processes were somewhat surprising given the shallow nature of the lagoon. This may be a legacy of eutrophication which results in a shift from benthic to pelagic production, and therefore favors water column over sediment-based nutrient cycling. Some studies in shallow eutrophic lakes have shown that water column regeneration supports N demands for primary production (Présing et al. 2008; Ferber et al. 2004; McCarthy et al. 2013, 2016). In the Curonian Lagoon, this was most apparent in summer and Fall, which overlapped the period of highest cyanobacteria abundance. By contrast, winter was a period of high throughput of N due to high riverine loads and marine export, and low assimilatory uptake. These findings are in contrast to some estuarine systems, where N attenuation is highest during peak N loads in winter (Bukaveckas et al. 2017a). In systems where PN is a large component of the N load, sediment trapping results in high N storage, whereas in the Curonian Lagoon, N inputs were predominantly in the form of DIN. The main tributary to the lagoon (Nemunas River) is a lowland river, which carries a small sediment load (Daunys et al. 2006; Remekaite-Nikiene et al. 2017). Low rates of assimilatory uptake during cold periods, coupled with small PN loads, resulted in low storage, though attenuation was bolstered by high rates of denitrification in winter.
Our primary goal was to develop a TN mass balance that would allow us to better understand the mechanisms attenuating N throughputs in shallow eutrophic waters. A secondary goal was to use the mass balance and process-specific measurements to align the processes regulating the transformation of N between particulate and dissolved fractions. We found that we were not able to adequately reconcile predicted and observed changes in water column PN and TDN by this approach. Assessments of uncertainty are difficult where aggregate properties (e.g., changes in concentration) are derived from many constituent processes (internal and external inputs). Potential explanations for this disparity include (1) a missing or underestimated external loading component, and (2) overestimation of assimilatory demand relative to supply via remineralization. We think the first is unlikely because (a) neglected components of our budget (point source and groundwater inputs) have been shown to be negligible for this system (Petkuviene et al. 2016; Vybernaite-Lubiene et al. 2017), and (b) measured components (riverine inputs, marine exchange, sediment–water exchange) would need to be an order of magnitude higher than current estimates to balance TDN supply and demand.
We note that the lack of agreement between observed and predicted values was most apparent in summer and fall when assimilatory and dissimilatory fluxes dominated over all other internal and external fluxes. We applied the common approach of estimating assimilatory and dissimilatory fluxes using measurements of production and respiration converted to N equivalents based on O2, C and N stoichiometry. Seston C:N ratios are commonly used for this conversion, which may be problematic if allochthonous contributions of high C:N material are large. This would lead to underestimation of assimilatory demand, and potentially underestimate supply, if the allochthonous fraction is re-mineralized at a lower rate than the autochthonous component. However, allochthonous inputs of particulate matter are low in the Curonian Lagoon. Suspended matter is dominated by phytoplankton and phytodetritus as indicated by the low C:N ratios observed in this and prior studies (Bukaveckas et al. 2017b; Remekaite-Nikiene et al. 2017). Furthermore, our findings were not appreciably affected by substituting the Redfield ratio for seston C:N values, which ranged from 15 to 20 (molar ratio) in February, and from 5 to 10 in other months. Our derivations benefitted from the fact that production and respiration were measured directly, rather than inferred from open water diel O2 changes (Knoll et al. 2016), but we recognize the limitation of having few observations (1 per season).
We used GPP, not NPP, to estimate gross TDN demand via autotrophic and heterotrophic assimilation, which resulted in an excess of demand over supply. The shortfall in TDN supply was apparent even when sediment remineralization (based on sediment–water N fluxes) was taken into account, as the combined TDN inputs (remineralization, riverine, marine, etc.) accounted for only 73% of the estimated assimilatory demand. A potential explanation for this disparity is that recycling of N is enhanced by rapid losses following assimilation. Our prior work in the James River Estuary has shown that the proportion of DIN uptake retained in the particulate fraction was low (10–30%) indicating that 70–90% of DIN was transferred to the DON pool within 48 h (Wood and Bukaveckas 2014). This finding is consistent with a number of field and laboratory studies reporting that a large fraction of DIN uptake was subsequently released by exudation or due to virus attack (e.g., 25–40% in Bronk et al. 1994; see also Ohlendieck et al. 2007; Adam et al. 2016; Cairns et al. 2016). Recent work in the Baltic region has shown that half of the N fixed by cyanobacteria was released as NH4+ and subsequently taken up by autotrophic and heterotrophic microbes (Adam et al. 2016). Resolving uncertainties regarding the recycling of N is an important aspect of forecasting recovery in response to external load reductions.
A number of assumptions inherent to estimating N supply and demand could also account for the observed disparity. For example, our estimation of external N supply is based on DIN. We recognize that DON may be re-mineralized, or assimilated directly (McCarthy et al. 2013; Bruesewitz et al. 2015; Hampel et al. 2018). However, external DON inputs are too small (Fig. 3) to balance the disparity between supply and demand. Our method of estimating N remineralization also assumes that all respiration is conducted with oxygen as the terminal electron acceptor. We feel that this is appropriate for the lagoon because anoxic conditions in the shallow water column are infrequent and transient (night-time; Zilius et al. 2014). Anaerobic conditions predominate in the sediments, but our estimates of N supply from the sediments were based on measured fluxes, not inferred from sediment respiration. We feel it is unlikely that these factors could account for the apparent disparity between N supply and demand.
Overall, our findings show that phytoplankton assimilation of DIN during the spring diatom bloom and subsequent sediment deposition was the most important mechanism attenuating N fluxes through the Curonian Lagoon. Efflux of N2 from sediments indicates that denitrification was the dominant mechanism of attenuation in winter. These losses were offset by N2 fixation in summer, resulting in a net balance for atmospheric N2 exchange. In summer and fall, the Curonian Lagoon functioned predominantly as a transformer of N with high rates of TDN uptake and PN production. During this period, pelagic N2 fixation was large (exceeding riverine and marine DIN inputs), and the bulk of PN was re-mineralized or exported to the Baltic, with little sediment storage. Our findings suggest that cyanobacteria blooms in the Curonian Lagoon influence the attenuation of N fluxes in two ways: N2 fixation in summer offsets denitrification in winter, thus negating atmospheric loss as a mechanism of attenuation. Secondly, positive buoyancy of cyanobacteria favors export of algal N to the Baltic during bloom periods, which, in combination with high sediment remineralization, results in minimal storage of N during peak phytoplankton production in summer. These findings suggest that factors favoring cyanobacteria dominance, alter the fate of algal N and thereby reduce attenuation of N by transitional waters while enhancing export to marine coastal environments.
References
Adam B, Klawonn I, Svedén JB, Bergkvist J, Nahar N, Walve J, Littmann S, Whitehouse MJ, Lavik G, Kuypers MMM, Ploug H (2016) Significant N2-fixation, ammonium release and N-transfer to the microbial and classical food web within a plankton community. ISME 10:450–459. https://doi.org/10.1038/ismej.2015.126
Almroth-Rosell E, Edman M, Eilola K, Markus Meier HE, Sahlberg J (2016) Modelling nutrient retention in the coastal zone of an eutrophic sea. Biogeosciences 13:5753–5769. https://doi.org/10.5194/bg-13-5753-2016
Andersson B, Sundbäck K, Hellman M, Hallin S, Alsterberg C (2014) Nitrogen fixation in shallow-water sediments: spatial distribution and controlling factors. Limnol Oceanogr 59:1932–1944. https://doi.org/10.4319/lo.2014.59.6.1932
Asmala E, Carstensen J, Conley DJ, Slomp CP, Stadmark J, Voss M (2017) Efficiency of the coastal filter: nitrogen and phosphorus removal in the Baltic Sea. Limnol Oceanogr 62:S222–S238. https://doi.org/10.1002/lno.10644
Benavides M, Martias C, Elifantz H, Berman-Frank I, Dupouy C, Bonnet S (2018) Dissolved organic matter influences N2 fixation in the New Caledonian Lagoon (western tropical south pacific). Front Mar Sci 5:89. https://doi.org/10.3389/fmars.2018.00089
Bentzon-Tilia M, Traving SJ, Mantikci M, Knudsen-Leerbeck H, Hansen JLS, Markager S, Riemann L (2015) Significant N-2 fixation by heterotrophs, photoheterotrophs and heterocystous cyanobacteria in two temperate estuaries. ISME J 9:273–285. https://doi.org/10.1038/ismej.2014.119
Bertos-Fortis M, Farnelid HM, Lindh MV, Casini M, Andersson A, Pinhassi J, Legrand C (2016) Unscrambling cyanobacteria community dynamics related to environmental factors. Front Microbiol 7:625. https://doi.org/10.3389/fmicb.2016.00625
Bianchi TS, Engelhaupt E, Westman P, Andren T, Rolff C, Elmgren R (2000) Cyanobacterial blooms in the Baltic Sea: natural or human-induced? Limnol Oceanogr 45:716–726. https://doi.org/10.4319/lo.2000.45.3.0716
Bonaglia S, Deutsch B, Bartoli M, Marchant HK, Brüchert V (2014) Seasonal oxygen, nitrogen and phosphorus benthic cycling along an impacted Baltic Sea estuary: regulation and spatial patterns. Biogeochemistry 119(1–3):139–160. https://doi.org/10.1007/s10533-014-9953-6
Bower CE, Holm-Hansen T (1980) A salicylate-hypochlorite method for determining ammonia in seawater. Can J Fish Aquat Sci 37(5):794–798
Boyer EW, Howarth RW (2008) Nitrogen fluxes from rivers to the coastal oceans. In: Nitrogen in the marine environment, 2nd edn. Academic Press, San Diego, pp 1565–1587
Boyer EW, Goodale CL, Jaworski NA, Howarth RW (2002) Effects of anthropogenic nitrogen loading on riverine nitrogen export in the northeastern US. Biogeochemistry 57(58):137–169. https://doi.org/10.1023/A:1015709302073
Bresciani M, Adamo M, De Carolis G, Matta E, Pasquariello G, Vaičiūtė D, Giardino C (2014) Monitoring blooms and surface accumulation of cyanobacteria in the Curonian Lagoon by combining MERIS and ASAR data. Remote Sens Environ 146:124–135. https://doi.org/10.1016/j.rse.2013.07.040
Brion N, Andersson MGI, Elskens M, Diaconu C, Baeyens W, Dehairs F, Middelburg JJ (2008) Nitrogen cycling, retention and export in a eutrophic temperate macrotidal estuary. Mar Ecol Prog Ser 357:87–99. https://doi.org/10.3354/meps07249
Bronk DA, Glibert PM, Ward BB (1994) Nitrogen uptake, dissolved organic nitrogen release, and new production. Science 265(5180):1843–1846. https://doi.org/10.1126/science.265.5180.1843
Bruesewitz DA, Gardner WS, Mooney RF, Pollard L, Buskey EJ (2013) Estuarine ecosystem function response to flood and drought in a shallow, semiarid estuary: nitrogen cycling and ecosystem metabolism. Limnol Oceanogr 58:2293–2309. https://doi.org/10.4319/lo.2013.58.6.2293
Bruesewitz DA, Gardner WS, Mooney RF, Buskey EJ (2015) Seasonal water column NH4 cycling along a semi-arid sub-tropical river-estuary continuum: responses to episodic events and drought conditions. Ecosystems 18:792–812
Bukaveckas PA, Barry LE, Beckwith MJ, David V, Lederer B (2011) Factors determining the location of the chlorophyll maximum and the fate of algal production within the tidal freshwater James River. Estuaries Coasts 34:569–582. https://doi.org/10.1007/s12237-010-9372-4
Bukaveckas PA, Beck M, Devore D, Lee WM (2017a) Climatic variability and its role in regulating C, N and P retention in the James River Estuary. Estuar Coast Shelf Sci 100:101. https://doi.org/10.1016/j.ecss.2017.10.004
Bukaveckas PA, Lesutiene J, Gasiunaite ZR, Lozys L, Olenina I, Pilkaityte R, Putys Z, Tassone S, Wood JD (2017b) Microcystin in aquatic food webs of the Baltic and Chesapeake Bay regions. Estuar Coast Shelf Sci 191:50–59. https://doi.org/10.1016/j.ecss.2017.04.016
Cairns J, Coloma S, Sivonen K, Hiltunen T (2016) Evolving interactions between diazotrophic cyanobacterium and phage mediate nitrogen release and host competitive ability. R Soc Open Sci 3:160839. https://doi.org/10.1098/rsos.160839
Cavaliere E, Baulch HM (2018) Denitrification under lake ice. Biogeochemistry. https://doi.org/10.1007/s10533-018-0419-0
Chu Z, Jin X, Yang B, Zeng Q (2007) Buoyancy regulation of Microcystis flos-aquae during phosphorus-limited and nitrogen-limited growth. J Plankton Res 29(9):739–745. https://doi.org/10.1093/plankt/fbm054
Dalsgaard T, Nielsen LP, Brotas V, Viaroli P, Underwood G, Nedwell D, Sundbäck K, Rysgaard S, Miles A, Bartoli M, Dong L, Thornton DCO, Otossen LDM, Castaldelli G, Risgaard-Petersen N (2000) Protocol handbook for NICE—nitrogen cycling in estuaries: a project under the EU research program: Marine Science and Technology (MAST III). National Environmental Research Institute, Silkeborg
Daunys D, Zemlys P, Olenin S, Zaiko A, Ferrarin C (2006) Impact of the zebra mussel Dreissena polymorpha invasion on the budget of suspended material in a shallow lagoon ecosystem. Helgoland Mar Res 60:113–120. https://doi.org/10.1007/s10152-006-0028-5
Deek A, Dähnke K, van Beusekom J, Meyer S, Voss M, Emeis K (2013) N2 fluxes in sediments of the Elbe Estuary and adjacent coastal zones. Mar Ecol Prog Ser 493:9–21. https://doi.org/10.3354/meps10514
Dettmann EH (2001) Effect of water residence time on annual export and denitrification of nitrogen in estuaries: a model analysis. Estuaries 24(4):481–490. https://doi.org/10.2307/1353250
Enoksson V (1993) Nutrient recycling by coastal sediments: effects of added algal material. Mar Ecol Prog Ser 92:245–254
Eyre BD, Ferguson AJP (2002) Comparison of carbon production and decomposition, benthic nutrient fluxes and denitrification in seagrass, phytoplankton, benthic microalgae- and macroalgae-dominated warm-temperate Australian lagoons. Mar Ecol Prog Ser 229:43–59
Eyre BD, Maher DT, Squire P (2013) Quantity and quality of organic matter (detritus) drives N2 effluxes (net denitrification) across seasons, benthic habitats, and estuaries. Glob Biogeochem Cycles 27:1083–1095. https://doi.org/10.1002/2013GB004631
Eyre BD, Maher DT, Sanders C (2016) The contribution of denitrification and burial to the nitrogen budgets of three geomorphically distinct Australian estuaries: importance of seagrass habitats. Limnol Oceanogr 61(3):1144–1156. https://doi.org/10.1002/lno.10280
Ferber LR, Levine SN, Lini A, Livingston GP (2004) Do cyanobacteria dominate in eutrophic lakes because they fix atmospheric nitrogen? Freshw Biol 49:690–708. https://doi.org/10.1111/j.1365-2427.2004.01218.x
Findlay DL, Hecky RE, Hendzel LL, Stainton MP, Regehr GW (1994) Relationship between N2 fixation and heterocyst abundance and its relevance to the nitrogen budget of Lake 227. Can J Fish Aquat Sci 51:2254–2266
Fulweiler RW, Nixon SW (2012) Net sediment N2 fluxes in a southern New England estuary: variations in space and time. Biogeochemistry 111:111–124. https://doi.org/10.1007/s10533-011-9660-5
Gao Y, Cornwell JC, Stoecker DK, Owens MS (2014) Influence of cyanobacteria blooms on sediment biogeochemistry and nutrient fluxes. Limnol Oceanogr 59(3):959–971. https://doi.org/10.4319/lo.2014.59.3.0959
Grasshoff K, Ehrhardt M, Kremling K (1983) Methods of seawater analysis, 2nd edn. Verlag Berlin Chemie, Berlin
Grelowski A, Pastuszak M, Sitek S, Witek Z (2000) Budget calculations of nitrogen, phosphorus and BOD 5 passing through the Oder estuary. J Mar Syst 25(3):221–237. https://doi.org/10.1016/S0924-7963(00)00017-8
Großkopf T, Mohr W, Baustian T, Schunck H, Gill D, Kuypers MMM, Lavik G, Schmitz RA, Wallace DWR, LaRoche J (2012) Doubling of marine dinitrogen-fixation rates based on direct measurements. Nature 488:361–364. https://doi.org/10.1038/nature11338
Hampel JJ, McCarthy MJ, Gardner WS, Zhang L, Xu H, Zhu G, Newell SE (2018) Nitrification and ammonium dynamics in Taihu Lake, China: seasonal competition for ammonium between nitrifiers and cyanobacteria. Biogeosciences 15:733–748
Hansen LS, Blackburn TH (1992) Effect of algal bloom deposition on sediment respiration and fluxes. Mar Biol 112:147–152
HELCOM (2015) Guidelines for the Baltic Monitoring Programme for the third stage, Part D. Biological determinands. In: Baltic Sea Environment Proceedings No. 27 D. Baltic Marine Environment Protection Commission, Helsinki Commission, p 164
Hietanen S, Kuoparinen J (2008) Seasonal and short-term variation in denitrification and anammox at a coastal station on the Gulf of Finland, Baltic Sea. Hydrobiologia 596(1):67–77. https://doi.org/10.1007/s10750-007-9058-5
Hietanen S, Lukkari K (2007) Effects of short-term anoxia on benthic denitrification, nutrient fluxes and phosphorus forms in coastal Baltic sediment. Aquat Microb Ecol 49:293–302. https://doi.org/10.3354/ame01146
Higgins SN, Paterson MJ, Hecky RE, Schindler DW, Venkiteswaran JJ, Findlay DL (2017) Biological nitrogen fixation prevents the response of a eutrophic lake to reduced loading of nitrogen: evidence from a 46-year whole-lake experiment. Ecosystems. https://doi.org/10.1007/s10021-017-0204-2
Howarth RW, Marino R, Lane J, Cole JJ (1988a) Nitrogen fixation in freshwater, estuarine and marine ecosystems. 1. Rates and importance. Limnol Oceanogr 33:669–687
Howarth RW, Marino R, Cole JJ (1988b) Nitrogen fixation in freshwater, estuarine and marine ecosystems. 2. Biogeochemical controls. Limnol Oceanogr 33:688–701
Howarth RW, Billen G, Swaney D, Townsend A, Jaworski NA, Lajtha K, Downing JA, Elmgren R, Caraco NF, Jordan T, Berendse F, Freney J, Kudeyarov V, Murdoch PS, Zhao-liang Z (1996) Regional nitrogen budgets and riverine N and P fluxes for the drainages to the North Atlantic Ocean: natural and human influences. Biogeochemistry 35:181–226. https://doi.org/10.1007/BF02179825
Huettel M, Berg P, Kostka JE (2014) Benthic exchange and biogeochemical cycling in permeable sediments. Annu Rev Mar Sci 6:23–51
Jakimavičius D, Kriaučiūnienė J (2013) The climate change impact on the water balance of the Curonian Lagoon. Water Resour 40(2):120–132. https://doi.org/10.1134/S0097807813020097
Jeffrey ST, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz (BPP) 167:191–194
Kamarainen AM, Penczykowski RM, Van de Bogert MC, Hanson PC, Carpenter SR (2009) Phosphorus sources and demand during summer in a eutrophic lake. Aquat Sci 71:214–227. https://doi.org/10.1007/s00027-009-9165-7
Kana TM, Darkangelo C, Hunt MD, Oldham JB, Bennett GE, Cornwell JC (1994) Membrane inlet mass spectrometer for rapid high-precision determination of nitrogen, oxygen, and argon in environmental water samples. Anal Chem 66:4166–4170. https://doi.org/10.1021/ac00095a009
Knoll LB, Morgan A, Vanni MJ, Leach TH, Williamson TJ, Brentrup JA (2016) Quantifying pelagic phosphorus regeneration using three methods in lakes of varying productivity. Inland Waters 6:509–522. https://doi.org/10.1080/IW-6.4.86
Lesutienė J, Bukaveckas PA, Gasiūnaitė ZR, Pilkaitytė R, Razinkovas-Baziukas A (2014) Tracing the isotopic signal of a cyanobacteria bloom through the food web of a Baltic Sea coastal lagoon. Estuar Coast Shelf Sci 138:47–56. https://doi.org/10.1016/j.ecss.2013.12.017
Loken L, Small GE, Finlay JC, Sterner R, Stanley EH (2016) Nitrogen cycling in a freshwater estuary. Biogeochemistry 127(2):1–18. https://doi.org/10.1007/s10533-015-0175-3
Lomstein BA, Jensen AGU, Hansen JW, Andreasen JB, Hansen LS, Berntsen J, Kunzendorf H (1998) Budgets of sediment nitrogen and carbon cycling in the shallow water of Knebel Vig, Denmark. Aquat Microb Ecol 14:69–80
McCarthy MJ, Gardner WS, Lehmann MF, Bird DF (2013) Implications of water column ammonium uptake and regeneration for the nitrogen budget in temperate, eutrophic Missisquoi Bay, Lake Champlain (Canada/USA). Hydrobiologia 718(1):173–188. https://doi.org/10.1007/s10750-013-1614-6
McCarthy MJ, Gardner WS, Lehmann MF, Guindon A, Bird DF (2016) Benthic nitrogen regeneration, fixation, and denitrification in a temperate, eutrophic lake: effects on the nitrogen budget and cyanobacteria blooms. Limnol Oceanogr 61:1406–1423. https://doi.org/10.1002/lno.10306
Mohr W, Großkopf T, Wallace DWR, LaRoche J (2010) Methodological underestimation of oceanic nitrogen fixation rates. PLoS ONE 5(9):e12583. https://doi.org/10.1371/journal.pone.0012583
Montoya PJ, Voss M, Kähler P, Capone DG (1996) A simple, high-precision, high-sensitivity tracer assay for N2 fixation. Appl Environ Microbiol 62(3):986–993
Motwani N, Duberg J, Sved JB, Gorokhova E (2018) Grazing on cyanobacteria and transfer of diazotrophic nitrogen to zooplankton in the Baltic Sea. Limnol Oceanogr 63:672–686. https://doi.org/10.1002/lno.10659
Mulholland MR, Bernhardt PW, Blanco-Garcia JL, Mannino A, Hyde K, Mondragon E, Turk K, Moisander PH, Zehr JP (2012) Rates of dinitrogen fixation and the abundance of diazotrophs in North American coastal waters between Cape Hatteras and Georges Bank. Limnol Oceanogr 57:1067–1083. https://doi.org/10.4319/lo.2012.57.4.1067
Murrell MC, Lores EM (2004) Phytoplankton and zooplankton seasonal dynamics in a subtropical estuary: importance of cyanobacteria. J Plankton Res 26:371–382. https://doi.org/10.1093/plankt/fbh038
Nedwell DB, Jickells TD, Trimmer M, Sanders R (1999) Nutrients in estuaries. Adv Ecol Res 29:43–92
Newell SE, McCarthy MJ, Gardner WS, Fulweiler RW (2016) Sediment nitrogen fixation: a call for re-evaluating coastal N budgets. Estuaries Coasts 39(6):1626–1638. https://doi.org/10.1007/s12237-016-0116-y
Nixon SW, Ammerman JW, Atkinson LP, Berounsky VM, Billen G, Boicourt WC, Boynton WR, Church TM, Ditoro DM, Elmgren R, Garber JH, Giblin AE, Jahnke RA, Owens PJ, Pilson MEQ, Seitzinger SP (1996) The fate of nitrogen and phosphorus at the land-sea margin of the North Atlantic Ocean. Biogeochemistry 35:141–180. https://doi.org/10.1007/BF02179826
Ohlendieck U, Gundersen K, Meyerhöfer M, Fritsche P, Nachtigall K, Bergmann B (2007) The significance of nitrogen fixation to new production during early summer in the Baltic Sea. Biogeosciences 4:63–73. https://doi.org/10.5194/bg-4-63-2007
Olenina I, Hajdu S, Edler L, Andersson A, Wasmund N, Busch S, Göbel J, Gromisz S, Huseby S, Huttunen M, Jaanus A, Kokkonen P, Ledaine I, Niemkiewicz E (2006) Biovolumes and size-classes of phytoplankton in the Baltic Sea. In: HELCOM Baltic Sea environmental proceedings, No. 106, p 144
O’Neil JM, Davis TW, Burford MA, Gobler CJ (2012) The rise of harmful cyanobacteria blooms: the potential roles of eutrophication and climate change. Harmful Algae 14:313–334. https://doi.org/10.1016/j.hal.2011.10.027
Paerl HW, Otten TG (2013) Harmful cyanobacterial blooms: causes, consequences and controls. Microb Ecol 65:995–1010. https://doi.org/10.1007/s00248-012-0159-y
Parson TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, New York
Petkuviene J, Zilius M, Lubiene I, Ruginis T, Giordani G, Razinkovas-Baziukas A, Bartoli M (2016) Phosphorus cycling in a freshwater estuary impacted by cyanobacterial blooms. Estuaries Coasts 39(5):1386–1402. https://doi.org/10.1007/s12237-016-0078-0
Pilkaitytė R, Razinkovas A (2007) Seasonal changes in phytoplankton composition and nutrient limitation in a shallow Baltic lagoon. Boreal Environ Res 12(5):551–559
Pina-Ochoa E, Álvarez-Cobelas M (2006) Denitrification in aquatic environments: a cross-system analysis. Biogeochemistry 81:111–130. https://doi.org/10.1007/s10533-006-9033-7
Poister D, Armstrong DE (2004) Seasonal sedimentation trends in a mesotrophic lake: influence of diatoms and implications for phosphorus dynamics. Biogeochemistry 65:1–13
Porter ET, Sanford LP, Porter FS, Mason RP (2018) STURM: resuspension mesocosms with realistic bottom shear stress and water column turbulence for benthic-pelagic coupling studies: design and applications. J Exp Mar Biol Ecol 499:35–50
Présing M, Preston T, Takátsy A, Spróber P, Kovács AW, Vörös L, Kenesi G, Kóbor I (2008) Phytoplankton nitrogen demand and the significance of internal and external nitrogen sources in large shallow lake (Lake Balaton, Hungary). Hydrobiologia 599:95–97. https://doi.org/10.1007/s10750-007-9191-1
Radtke H, Neumann T, Voss M, Fennel W (2012) Modeling pathways of riverine nitrogen and phosphorus in the Baltic Sea. J Geophys Res 117:C09024. https://doi.org/10.1029/2012JC008119
Remekaite-Nikiene N, Lujaniene G, Malejevas V, Bariseviciute R, Zilius M, Vybernaite-Lubiene I, Garnaga-Budre G, Stankevicius A (2017) Assessing nature and dynamics of POM in transitional environment (the Curonian Lagoon, SE Baltic Sea) using a stable isotope approach. Ecol Indic 82:217–226. https://doi.org/10.1016/j.ecolind.2017.06.035
Robson BJ, Bukaveckas PA, Hamiltond DP (2008) Modelling and mass balance assessments of nutrient retention in a seasonally-flowing estuary (Swan River Estuary, Western Australia). Estuar Coast Shelf Sci 76(2):282–292. https://doi.org/10.1016/j.ecss.2007.07.009
Savchuk OP (2005) Resolving the Baltic Sea into seven subbasins: N and P budgets for 1991–1999. J Mar Syst 56(1–2):1–15. https://doi.org/10.1016/j.jmarsys.2004.08.005
Seitzinger SP, Styles R, Boyer EW, Alexander RB, Billen G, Howarth RW, Mayer B, van Breeman N (2002) Nitrogen retention in rivers: model development and application to watersheds in the northeastern USA. Biogeochemistry 57(68):199–237. https://doi.org/10.1023/A:1015745629794
Seitzinger S, Harrison JA, Böhlke JK, Bouwman AF, Lowrance R, Peterson B, Tobias C, Van Drecht G (2006) Denitrification across landscapes and waterscapes: a synthesis. Ecol Appl 16(6):2064–2090
Silvennoinen H, Hietanen S, Liikanen A, Stange CF, Russow R, Kuparinen J, Martikainen PJ (2007) Denitrification in the river estuaries of the northern Baltic Sea. Ambio 36(2–3):134–140. https://doi.org/10.1579/0044-7447(2007)36%5b134:DITREO%5d2.0.CO;2
Smith VH (2006) Responses of estuarine and coastal marine phytoplankton to nitrogen and phosphorus enrichment. Limnol Oceanogr 51:377–384. https://doi.org/10.4319/lo.2006.51.1part_2.0377
Smith VH, Tilman DG, Nekola JC (1999) Eutrophication: impacts of excess nutrient inputs on freshwater, marine and terrestrial ecosystems. Environ Pollut 100:179–196. https://doi.org/10.1016/S0269-7491(99)00091-3
Smyth AR, Geraldi NR, Piehler MF (2013) Oyster-mediated benthic-pelagic coupling modifies nitrogen pools and processes. Mar Ecol Prog Ser 493:23–30. https://doi.org/10.3354/meps10516
Song K, Burgin AJ (2017) Perpetual phosphorus cycling: eutrophication amplifies biological control on internal phosphorus loading in agricultural reservoirs. Ecosystems 20:1483–1493. https://doi.org/10.1007/s10021-017-0126-z
Strickland JDH (1960) Measuring the production of marine phytoplankton. Bull Fish Res Board Can 122:172
Sundbäck K, Linares F, Larson F, Wulff A, Engelsen A (2004) Benthic nitrogen fluxes along a depth gradient in a microtidal fjord: the role of denitrification and microphytobenthos. Limnol Oceanogr 49(4):1095–1107. https://doi.org/10.4319/lo.2004.49.4.1095
Umgiesser G, Zemlys P, Erturk A, Razinkova-Baziukas A, Mežinė J, Ferrarin Ch (2016) Seasonal renewal time variability in the Curonian Lagoon caused by atmospheric and hydrographical forcing. Ocean Sci 12:391–402. https://doi.org/10.5194/os-12-391-2016
Utermöhl H (1958) Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Int Assoc Theor Appl Limnol 9:1–38
Vaitkuviene D, Dagys M (2008) CORINE 2006 land cover, Lithuania. Implementing CLC2006 project in Lithuania, Report. Institute of Ecology of Vilnius University, Vilnius (in Lithuanian)
Valiela I, Collins G, Kremer J, Lajtha K, Geist M, Seely B, Brawley J, Sham CH (1997) Nitrogen loading from coastal watersheds to receiving estuaries: new method and application. Ecol Appl 7:358–380. https://doi.org/10.1890/1051-0761(1997)007%5b0358:NLFCWT%5d2.0.CO;2
Visser PM, Ibelings BM, Bormans M, Huisman J (2016) Artificial mixing to control cyanobacterial blooms: a review. Aquat Ecol 50(3):423–441. https://doi.org/10.1007/s10452-015-9537-0
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
Voss M, Deutsch B, Liskow I, Pastuszak M, Schulte U, Sitek S (2010) Nitrogen retention in the Szczecin lagoon, Baltic Sea. Isot Environ Health Stud 46(3):355–369. https://doi.org/10.1080/10256016.2010.503895
Vybernaite-Lubiene I, Zilius M, Giordani G, Petkuviene J, Vaiciute D, Bukaveckas PA, Bartoli M (2017) Effect of algal blooms on retention of N, Si and P in Europe’s largest coastal lagoon. Estuar Coast Shelf Sci 194:217–228. https://doi.org/10.1016/j.ecss.2017.06.020
Walsby AE (1994) Gas vesicles. Microbiol Rev 58:94–144
Wannicke N, Benavides M, Dalsgaard T, Dippner JW, Montoya JP, Voss M (2018) New perspectives on nitrogen fixation measurements using 15N2 gas. Front Mar Sci 5:120. https://doi.org/10.3389/fmars.2018.00120
Wasmund N, Voss M, Lochte K (2001) Evidence of nitrogen fixation by non-heterocystous cyanobacteria in the Baltic Sea and re-calculation of a budget of nitrogen fixation. Mar Ecol Prog Ser 214:1–14. https://doi.org/10.3354/meps214001
Witek Z, Humborg C, Savchuk O, Grelowski A, Lysiak-Pastuszak E (2003) Nitrogen and phosphorus budgets of the Gulf of Gdansk (Baltic Sea). Estuar Coast Shelf Sci 57(1–2):239–248. https://doi.org/10.1016/S0272-7714(02)00348-7
Wood JD, Bukaveckas PA (2014) Increasing severity of phytoplankton nutrient limitation following reductions in point source inputs to the tidal freshwater segment of the James River Estuary. Estuaries Coasts 37:1188–1201. https://doi.org/10.1007/s12237-013-9756-3
Wood JD, Elliott D, Garman G, Hopler D, Lee W, McIninch S, Porter AJ, Bukaveckas PA (2016) Autochthony, allochthony and the role of consumers in influencing the sensitivity of aquatic systems to nutrient enrichment. Food Webs 7:1–12. https://doi.org/10.1016/j.fooweb.2016.03.001
Zemlys P, Ferrarin Ch, Umgiesser G, Gulbinskas S, Bellafiore D (2013) Investigation of saline water intrusions into the Curonian Lagoon (Lithuania) and two-layer flow in the Klaipėda Strait using finite element hydrodynamic model. Ocean Sci 9(3):573–584. https://doi.org/10.5194/os-9-573-2013
Zilius M (2011) Oxygen and nutrient exchange at the sediment–water interface in the eutrophic boreal lagoon (Baltic Sea). Dissertation, University of Klaipeda
Zilius M, Bartoli M, Daunys D, Pilkaityte R, Razinkovas A (2012) Patterns of benthic oxygen uptake in a hypertrophic lagoon: spatial variability and controlling factors. Hydrobiologia 699:85–98. https://doi.org/10.1007/s10750-012-1155-4
Zilius M, Bartoli M, Bresciani M, Katarzyte M, Ruginis T, Petkuviene J, Lubiene I, Giardino C, Bukaveckas PA, de Wit R, Razinkovas-Baziukas A (2014) Feedback mechanisms between cyanobacterial blooms, transient hypoxia, and benthic phosphorus regeneration in shallow coastal environments. Estuaries Coasts 37(3):680–694. https://doi.org/10.1007/s12237-013-9717-x
Zilius M, de Wit R, Bartoli M (2016) Response of sedimentary processes to cyanobacteria loading. J Limnol 75(2):236–247. https://doi.org/10.4081/jlimnol.2015.1296
Acknowledgements
We kindly thank the Coast Guard District of the State Border Guard Service for logistic support, our boat crews for outstanding field campaigns between drifting ices sheets, and Tomas Ruginis for assistance in field sampling. Manuscript preparation was supported by the BONUS project “Nutrient Cocktails in Coastal zones of the Baltic Sea (COCOA)” (No. BONUS-2/2014 and BONUS- COCOA BMBF funded under 03F0683A). The study was partly supported by the Lithuanian EPA project (No. 28TP-2015-19 SUT-15P-13). We gratefully thank the Lithuanian Marine Research Department of the Ministry of Environment for providing meteorological and Nemunas River discharge data. We are indebted to the associated editor of journal and two anonymous reviewers for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jacques C. Finlay.
Rights and permissions
About this article
Cite this article
Zilius, M., Vybernaite-Lubiene, I., Vaiciute, D. et al. The influence of cyanobacteria blooms on the attenuation of nitrogen throughputs in a Baltic coastal lagoon. Biogeochemistry 141, 143–165 (2018). https://doi.org/10.1007/s10533-018-0508-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-018-0508-0