Abstract
Nutrient loads from the Mississippi River watershed are associated with seasonal development of bottom-water hypoxia in the northern Gulf of Mexico. Microbial nitrogen (N) transformations at the sediment-water interface are important in determining system productivity and the development and maintenance of hypoxia. Intact sediment cores were incubated in a continuous-flow system with stable isotope tracers to identify and quantify important N sources (e.g., N fixation), sinks (e.g., denitrification and anammox), and links (e.g., dissimilatory nitrate reduction to ammonium, DNRA). Microbial N sinks on the Louisiana-Texas continental shelf remove up to 68 % of the total N load from the Mississippi River watershed, and up to 29 % of this N removal (mean = 11.8 ± 1.7 %) may be due to anammox. The highest N2 production rates and ammonium effluxes were observed at low bottom-water oxygen concentrations, and sediments were a significant source of ammonium to the water column at all times. DNRA and heterotrophic N fixation were not consistent pathways for total sediment N fluxes, but their potential importance to N balance and productivity in the system warrants further study and inclusion in ecosystem models. Physical disturbance from passage of two hurricanes in 2008 resulted in lower N cycling rates and sediment oxygen consumption, with sediment processes migrating into the water column. Denitrification is the dominant N sink in the northern Gulf of Mexico and provides a valuable ecosystem service by mitigating N loads from the Mississippi River watershed, particularly during seasonal bottom-water hypoxia events.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bottom-water hypoxia has occurred during summer in the northern Gulf of Mexico (NGOMEX) for more than three decades (Boesch et al. 2009). Anthropogenic nutrient loading from the Mississippi River basin drives hypoxia development via increased primary production and subsequent decomposition of autochthonous organic matter. Alternative and/or secondary explanations include oxidation of allochthonous organic matter, physical stratification, and coastal wetland losses (reviewed by Boesch et al. 2009). An oxygen isotope approach supported the nutrient loading paradigm; oxygen dynamics were best explained by algal biomass, with lesser roles for salinity, temperature, depth, and water column stability (Quiñones-Rivera et al. 2010). However, the nutrient loading explanation may over-simplify a complex problem (e.g., Rowe and Chapman 2002). Regardless of the causes and mechanisms, the negative effects of hypoxia on fish and benthic communities, food webs, and habitat are well established (e.g., Breitburg 2002; Middelburg and Levin 2009; O’Connor and Whitall 2007).
Nitrogen (N) is a significant component of nutrient inputs to the NGOMEX, mostly derived from agriculture in the watershed (Dagg and Breed 2003). Over half (53 %) of annual average riverine N inputs into the NGOMEX are composed of nitrate (NO3 −), and the combination of NO3 − and organic N accounts for 96 % of these N inputs (Turner and Rabalais 1991). The size of the mid-summer hypoxic zone is related to the NO3 − concentration in the Mississippi River in the 2 months prior (Turner et al. 2006), and NO3 − loading to the NGOMEX has more than tripled since the 1950s (Turner et al. 2003).
The highest particulate organic matter flux to the sediments occurs during spring, when Mississippi River discharge is highest (Dagg and Breed 2003), and benthic processes control how much settled material is buried or regenerated. Despite the importance of N dynamics in the development and maintenance of hypoxia in the NGOMEX, relatively little is known about internal N transformations relative to sources, sinks, and “links” (c.f., Gardner et al. 2006) and their relationships with seasonal hypoxia. These sources, sinks, and links include heterotrophic N fixation (e.g., Fulweiler et al. 2007), denitrification, and anaerobic ammonium oxidation (anammox; e.g., Mulder et al. 1995), and dissimilatory NO3 − reduction to ammonium (DNRA; e.g., An and Gardner 2002), respectively. While the N sources and links may exacerbate hypoxia development by fueling additional primary production and subsequent respiration (e.g., nitrification), N sinks provide a valuable ecosystem service by removing N from the system as N2 gas (Piehler and Smyth 2011). No studies have evaluated this entire suite of benthic N transformations in the NGOMEX, but previous denitrification estimates were relatively low (e.g., Childs et al. 2002 and erratum). More recently, denitrification was estimated to remove ~39 % of riverine N loads onto the Louisiana continental shelf (Lehrter et al. 2012). Benthic chambers revealed that sediments were always a source of NH4 + and a sink for NOx (Rowe et al. 2002), suggesting remineralization and denitrification, respectively. No significant effects of hypoxia on sediment NH4 + and NOx fluxes were observed, but sediment oxygen consumption (SOC) was depressed during hypoxia (Rowe et al. 2002). These authors suggested that future studies should focus on sediment N recycling using more direct methods.
Sediment denitrification rates, estimated using acetylene inhibition, in the NGOMEX were highest at bottom-water oxygen concentrations of 1–3 mg L−1 (Childs et al. 2002 and erratum). The authors speculated that lower denitrification rates at oxygen concentrations <1 mg L−1 were due to competition for NO3 − from DNRA. Reported denitrification rates using acetylene inhibition were similar to those from stoichiometric evaluation of SOC (Gardner et al. 1993). Acetylene inhibition blocks the final step of denitrification (N2O → N2) to facilitate measurement but also blocks nitrification (NH4 + → NO2 − → NO3 −; e.g., Steingruber et al. 2001). Nitrification often is coupled to denitrification in marine sediments and may represent the dominant source of substrate (e.g., Laursen and Seitzinger 2002). Thus, acetylene inhibition may cause NO3 − limitation in incubations and underestimate denitrification rates. Direct methods for measuring denitrification, such as isotope pairing (Nielsen 1992) and N2/Ar (Kana et al. 1994, 1998), improve accuracy and help identify N2 production pathways.
The biogeochemical pathways and rates contributing to hypoxia development or reducing N eutrophication are thus not constrained in the NGOMEX. Studying these relationships in sediments and overlying water is optimized by techniques that do not disturb the flocculent layer at the sediment-water interface (SWI) or cause re-aeration of surface sediments, while maintaining an intact overlying water column (Gardner et al. 2009). The importance of these issues was demonstrated in the NGOMEX, where methodological differences in sediment nutrient fluxes were observed only during hypoxia (Miller-Way et al. 1994). A “HYPOX” corer, designed to minimize disturbance during core collection (Gardner et al. 2009), was used in our study to collect intact sediment cores and overlying water for continuous-flow incubations.
We characterized SOC, nutrient fluxes, DNRA, heterotrophic N fixation, and denitrification (and/or possible anammox) at several sites in the NGOMEX to determine the relative contributions of microbial N transformations to system N dynamics, as well as to evaluate the ecosystem services provided by N sinks. These sediment N transformations were evaluated in different seasons, at hypoxic and normoxic sites, and at water depth and nutrient gradients with distance from the Mississippi and Atchafalaya River discharges. Specific hypotheses addressed were as follows: (1) Denitrification (and anammox) rates decrease during bottom-water hypoxia via reduced coupling with nitrification, (2) DNRA rates are higher and account for a higher proportion of total NO3 − reduction during hypoxia, and (3) heterotrophic N2 fixation in sediments is not important in the nutrient-rich NGOMEX. Circumstances encountered during the study also allowed consideration of hurricane passage and Deepwater Horizon oil spill effects on SWI N transformation rates.
Methods
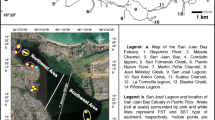
Sediment-water interface N and oxygen dynamics were examined at several sites in the NGOMEX during six cruises aboard the R/V Pelican in July 2008, September (Sept) 2008, January (Jan) 2009, August (Aug) 2009, May 2010, and May 2011 (Fig. 1). The Sept 2008 cruise (Sept 18–23) occurred within 3 weeks of Hurricanes Gustav (Aug 30 to Sept 1) and Ike (Sept 9–13), which passed directly over the study area as Category 3 and 2 storms, respectively. The May 2010 cruise occurred during the early weeks of the Deepwater Horizon oil disaster, which began in late April and affected most of the study area. Details of sites visited on each cruise are given in Table 1. One site (CT2) was selected as a “control” and is located east of the Mississippi River delta, where widespread hypoxia is not expected (but see Brunner et al. 2006). Other sites were located offshore from the Atchafalaya River discharge (F5), at the mouth of the Mississippi River (MRM), and within the area commonly affected by hypoxia (C6 and B7).
Depth profiles of temperature, salinity, and dissolved oxygen (DO) were obtained from a Seabird 911 CTD system aboard the vessel. Bottom-water (1 m above SWI) samples were collected using 20-L Go-Flo bottles attached to the CTD. Samples for ambient nutrient analyses were filtered immediately upon arrival on deck (or upon collection for sediment core incubations; 0.2-μm GE/Osmonics Nylon syringe filters) and frozen for analysis at the University of Texas Marine Science Institute (UTMSI). Ammonium concentration (and isotope ratio) was measured by aqueous NH4 + isotope retention time shift high-performance liquid chromatography (AIRTS-HPLC; Gardner et al. 1995). NO3 −, NO2 −, and o-PO4 3− concentrations for filtered ambient and core incubation samples were determined using an automated, colorimetric Lachat Quikchem 8000 flow injection analysis system.
Intact sediment cores (7.6 cm ID, 15–20-cm depth) and overlying water were collected with the HYPOX corer (Gardner et al. 2009) and sealed immediately with a vinyl cap and electrical tape. Cores were then installed quickly into a continuous-flow system (Gardner and McCarthy 2009; Lavrentyev et al. 2000), which was modified by replacing the inflow reservoir with a Tedlar bag (15 L) to prevent atmospheric contact (McCarthy et al. 2013). The inflow bags were filled with bottom water (1 m above SWI) using tubing attached to the bottom spigot of the Go-Flo bottles. Starting with the Sept 2008 cruise, additional modifications involved replacing Tygon peristaltic pump tubing with gas-impermeable Viton tubing and replacing Teflon inflow and outflow tubing with gas-impermeable PEEK tubing. Dissolved O2 data from July 2008 core incubations were discarded due to atmospheric O2 contamination through the Teflon tubing. For each incubation, duplicate cores were attached to a single Tedlar bag with (1) no enrichment (C cores), (2) ~5 μM 15NH4 + (A cores), or (3) ~50 μM 15NO3 − (N cores). Isotope enrichments represent approximate final concentrations of 15NH4Cl for A cores and Na15NO3 for N cores (Sigma-Aldrich, 98+ % 15 N). Bottom water was pumped into the core overlying water (volume 225 mL) at 1.2 mL min−1 using a Rainin RP-1 peristaltic pump (overlying water residence time ≈ 3.13 h). The cores were pre-incubated with flow for at least 18 h before sampling once daily for 3 days. Inflow and outflow samples were collected nearly simultaneously, and all fluxes (μmol m−2 h−1) were calculated as (C o − C i ) x f/a, where C o is the outflow concentration (μM), C i is the inflow concentration, f is the flow rate, and a is the sediment surface area (0.0045 m2). To facilitate comparison with nutrient fluxes, all dinitrogen fluxes were converted to μmol N m−2 h−1.
Samples were collected from the inflow bags by attaching a 60-mL syringe to the bag valve. Outflow samples for nutrients were collected using a syringe with canula extending to the bottom of a 100-mL plastic beaker collecting the outflow and filtered immediately (0.2-μm GE/Osmonics Nylon syringe filter) into duplicate 14-mL snap-cap tubes (Falcon) for NO3 −, NO2 −, and o-PO4 3− and an 8-mL glass Wheaton vial for NH4 +. Inflow samples for dissolved gases were transferred from the syringe to custom 15-mL ground-glass-stoppered test tubes (Chemglass) and allowed to overflow for the entire volume of the syringe. These vials have a very small surface area/volume (approximately 0.07 cm2 mL−1) to minimize atmospheric contamination during sampling and analysis. Outflow samples were collected by placing the outflow PEEK tubing line to the bottom of the test tubes and allowing overflow for at least 30 min. Dissolved gas samples were preserved immediately with 200 μL 50 % ZnCl2, capped, and stored submerged in a 4-L Nalgene bottle until analysis.
Dissolved gases were analyzed at UTMSI using membrane inlet mass spectrometry (MIMS; Kana et al. 1994) modified for isotope enrichment studies (An et al. 2001). Dissolved gas samples were analyzed within 4 days for O2/Ar (for SOC estimates), 28 N2/Ar, 29 N2, and 30 N2. Monitoring mass 30 in unamended samples allowed evaluation of the O2 effect on N2 (Eyre et al. 2002; Kana and Weiss 2004). The MIMS at UTMSI is not affected by this issue, and no corrections were needed to account for N2 scavenging at low DO. Unamended (C) cores were used to determine SOC, net nutrient fluxes, and net 28 N2 flux. Possible anammox, as 29 N2 production from 15NH4 + (Rysgaard et al. 2004), was determined from “A” cores. Potential DNRA (as 15NH4 + production from 15NO3 − enrichment; An and Gardner 2002), N fixation occurring simultaneously with denitrification (calculated using isotope pairing and measured 28, 29, 30 N2 fluxes, as described in An et al. (2001)), and potential denitrification (DNF; 28+29+30 N2 production plus any N fixation; Gardner and McCarthy 2009) were measured in “N” cores.
Significant relationships between ambient conditions and measured rates were evaluated using linear regression where appropriate. Significant differences between seasons, sampling sites, and treatments were determined using one-way analysis of variance (ANOVA). A repeated measures approach is not suitable for our data because of the inherent time (months between cruises) and space (e.g., vastness of the sampling area relative to core size, vessel movement once on station, and currents) differences between sampling events. Relationships and differences were considered significant at p ≤ 0.05.
Results
Ambient Conditions
Physicochemical characteristics of bottom water, also reported elsewhere (McCarthy et al. 2013), are summarized in Table 1. Bottom-water hypoxia (DO <2 mg L−1) occurred on several occasions at C6 and MRM. Hypoxia was not present at any site in Sept 2008, within 3 weeks of 2 hurricanes passing over the study area, or during winter in Jan 2009. Hypoxia was observed in May 2010 (during the Deepwater Horizon oil spill) at F5, located furthest west, and the control site (CT2) east of the delta—the only time hypoxia was observed at these sites.
Nutrient concentrations varied in the bottom layer (Table 2), but o-PO4 3− and NO3 − concentrations were related to bottom-water DO with negative slopes (Table 3). Bottom-water dissolved inorganic N (DIN = NO3 − + NO2 − + NH4 +) concentrations averaged 7.73 ± 1.21 μM, composed primarily (81 ± 6 %) of oxidized N forms (NO3 − + NO2 − = NOx). The molar DIN/PO4 ranged from 1.85 in July 2008 at C6 to 154 in Aug 2009 at CT2, with a median of 14.1 (mean = 21.4 ± 7.23; Redfield ratio = 16). Bottom-water NH4 + concentrations correlated with bottom-water salinity (Table 3) and were significantly lower (p < 0.05) in Sept 2008 versus other cruises. No other significant differences were observed among the sites and cruises for other nutrients.
SWI Nutrient Fluxes
Sediments were an NH4 + source (mean = 77.4 ± 14.6; n = 21; C cores; all rates in μmol N m−2 h−1) to overlying water at all times and stations except C6 in Sept 2008 (Table 4). Net NH4 + fluxes correlated strongly with bottom-water DO (negative slope; p < 0.001; Fig. 2a) and SOC (positive slope; p = 0.001), along with weaker positive relationships (p < 0.05) with o-PO4 3− and NH4 + concentrations (Table 3). Net NH4 + efflux was significantly higher at C6 versus F5 and in May 2010 versus Sept 2008 and Jan 2009. No other significant differences in NH4 + fluxes were observed between sites or cruises. At CT2, mean NH4 + flux in C cores (62.6 ± 19.7) was significantly higher than in A cores (−19.6 ± 42.9). In July and Sept 2008, 15NH4 + addition reversed net NH4 + effluxes in C cores to net uptake by sediments in A cores (p < 0.05) at all sites (and acceleration of net uptake at C6 in Sept 2008; Table 4 and Supplementary Table S1). Other cruises did not have consistent responses to 15NH4 + addition.
Net NH4 + (a) and 28 N2 (b) fluxes relative to bottom-water dissolved oxygen concentration (DO; mg L−1) in unamended (C) cores. Note that the July 2008 data point at C6, where net N fixation was observed, is shown in panel (b) as an open circle (DO = 0.02 mg L−1, net 28 N2 flux = −96.6 μmol N m−2 h−1) and is not included in the regression line
Overall, sediments were a net NO3 − sink (overall mean = −25.0 ± 9.95 μmol N m−2 h−1; n = 21; C cores; Table 4), but variability was high. Net NO3 − fluxes were related to bottom-water NO3 − (negative slope) and DO (positive slope) concentrations (Table 3). No spatially significant differences in net NO3 − fluxes were observed, but mean NO3 − uptake in May 2010 differed (p < 0.05) from NO3 − efflux in Jan 2009. Addition of 15NH4 + often caused a significant positive change (p < 0.05; Supplementary Table S1) in NO3 − flux (11 of 21 cases); i.e., a decrease in NO3 − uptake, an increase in NO3 − efflux, or a reversal from uptake to efflux are all considered positive changes). In most cases, 15NO3 − addition enhanced NO3 − influxes (Supplementary Table S1).
On average, NO2 − was removed from overlying water (C cores; mean = −1.59 ± 1.53 μmol N m−2 h−1; n = 21), but variability was high (Table 4), and net rates were low. Nitrite fluxes in C cores were not related to physicochemical parameters or fluxes, except o-PO4 3− concentration (negative slope) and flux (positive slope; Table 3). Addition of 15NH4 + or 15NO3 − did not affect the overall mean NO2 − flux, but 15NH4 + additions often enhanced sediment NO2 − uptake (Supplementary Table S1). Conversely, NO2 − uptake reversed to NO2 − efflux in A cores in Sept 2008 and Aug 2009 at F5 and Jan 2009 at CT2. The effects of 15NO3 − additions were unidirectional, with enhanced NO2 − fluxes in several cases (Supplementary Table S1).
Sediments in C cores often were a source of o-PO4 3− to overlying water (mean = 3.29 ± 1.66 μmol P m−2 h−1; n = 21), but variability was again high. Sediments were a net P sink in July and Sept 2008 and a P source at all other times (Table 4). Sediments were a consistent P source at CT2, F5, and MRM, but C6 and B7 exhibited both P uptake and efflux at different times. P flux was not related to water column DO or o-PO4 3− concentrations, but a weak positive relationship occurred between P flux and SOC (p = 0.06; Table 4). P efflux was higher in May 2011 (mean = 14.3 ± 3.82 μmol P m−2 h−1; p < 0.05) than all other cruises except May 2010 (mean = 7.17 ± 2.24 μmol P m−2 h−1; p = 0.14).
Sediment Oxygen Consumption (SOC) and net 28 N2 Fluxes
SOC in C cores, reported in detail elsewhere (all rates in μmol O2 m−2 h−1; McCarthy et al. 2013), ranged from 408 to 1800 (mean = 834 ± 83.8). The lowest SOC occurred in Sept 2008, and the highest SOC occurred at lowest DO (McCarthy et al. 2013). No significant differences in SOC were observed between sampling sites. SOC was related positively to NH4 + concentration, NH4 + flux, and 28 N2 efflux in C cores (p < 0.005) as well as potential denitrification (DNF) in N cores (p < 0.05; Table 3). SOC was enhanced in A cores relative to C cores (942 ± 111 vs. 795 ± 91.6, respectively) at sites visited most often (CT2, C6, and F5; n = 5 for each site), but this difference was not statistically robust. Addition of 15NO3 − did not affect SOC in a consistent way.
Net 28 N2 fluxes in C cores were generally positive (i.e., denitrification > N fixation; mean = 129 ± 36.3; rates in μmol N m−2 h−1; n = 21), but negative net 28 N2 fluxes occurred in July 2008 at C6 and Jan 2009 at F5 (Fig. 3). Net 28 N2 fluxes by station (Fig. 3) ranged from 44.4 ± 51.1 at F5 (n = 5) to 258 ± 29.2 at MRM (n = 2), but spatial and between-cruise differences were not significant. Net 28 N2 fluxes by cruise also were not different. Net 28 N2 fluxes related negatively to DO (Fig. 2b) and positively to o-PO4 3− and NH4 + fluxes and SOC (p < 0.01; Table 3). Isotope additions generally did not cause significant changes in net 28 N2 fluxes versus C cores.
Effects of 15 N additions on Isotopic N2 Fluxes
Potential DNRA, as 15NH4 + production from 15NO3 − addition to inflowing water, was observed in 38 % of the incubations (n = 21), with potential rates ranging from 1.12 ± 0.44 μmol N m−2 h−1 at CT2 in May 2011 to 31.4 ± 10.8 μmol N m−2 h−1 at C6 in May 2011 (Table 5). DNRA was not observed at any site in Sept 2008 or Aug 2009 and only at CT2 in Jan 2009. Relative to C cores, significant potential DNRA was observed at CT2, C6, and F5 in May 2010. When observed, DNRA accounted for 16.2 ± 4.61 % of total NH4 + efflux.
Addition of 15NO3 − (N cores; 15 of 21 incubations) and 15NH4 + (A cores; 14 of 21) often stimulated N2 production (i.e., potential DNF = 28+ 29+30 N2 > net 28 N2 flux in C cores). Positive N fixation from isotope calculations in N cores were added to 28+ 29+30 N2 to adjust potential DNF. On average, 29 N2 production was higher than that of 30 N2 in both N (mean 29:30 = 1.13 ± 0.09) and A (mean 29:30 = 4.76 ± 0.54) cores. Mean 29 N2 and 30 N2 productions in A cores (14.8 ± 2.09 and 4.69 ± 1.03 μmol N m−2 h−1, respectively) were lower (p < 0.05; n = 21) than in N cores (31.6 ± 3.40 and 31.5 ± 4.23 μmol N m−2 h−1, respectively; Figs. 4 and 5). The proportion of total N2 production from 15 N additions versus ambient 14 N (15:14) was higher (p < 0.05; n = 21) in N cores (0.84 ± 0.19) than in A cores (0.24 ± 0.07) at all sites and on all cruises.
Net 28, 29, and 30 N2 fluxes averaged by cruise in cores amended with (a) 15NH4 + (A cores) and (b) 15NO3 − (N cores). Error bars represent 1 standard error. Note that three stations were sampled (n = 3) in July and September 2008 and May 2011, and four stations (n = 4) were sampled in January and August 2009 and May 2010
Possible anammox, as significant net 29 N2 production in A cores (vs. C cores; p < 0.05; all rates in μmol N m−2 h−1), occurred in all cores and ranged from 4.28 ± 1.04 at C6 in May 2010 to 42.8 ± 15.5 at MRM in Aug 2009. Mean 29 N2 production in A cores ranged from 8.89 ± 1.94 at B7 (n = 2) to 25.2 ± 17.6 at MRM (n = 2), with no significant differences between stations. Net 29 N2 fluxes in A cores were related negatively to DO (p = 0.05) and positively to NH4 + flux (p < 0.05; Table 3). Possible anammox was 1.7–29.1 % of potential DNF, as defined above, with generally lower proportions in May 2010 and 2011 (Fig. 6).
Calculated N fixation co-occurring with denitrification (An et al. 2001) was observed at all sites except MRM and all cruises except May (Table 6). Calculated N fixation rates exceeded potential DNF in N cores two times (C6 in July 2008 and F5 in Jan 2009).
Discussion
Nitrogen Removal in the NGOMEX
Nitrogen removal from aquatic systems, via denitrification and other mechanisms (e.g., anammox), can help mitigate eutrophication from excess N loading (e.g., Seitzinger 1988). However, DNRA and N fixation could support additional production and exacerbate hypoxia (Gardner et al. 2006). The ecosystem services provided by denitrification as a sink for bioreactive N have been monetized for other coastal systems (Piehler and Smyth 2011), with “values” of N removal via denitrification ranging from US$100,000 to 750,000 km−2 year−1, depending on the type of habitat. In the NGOMEX, the extent of bottom-water hypoxia in mid-summer relates to NO3 − discharged by the Mississippi and Atchafalaya Rivers in the previous 2 months (Turner et al. 2006). Therefore, the potential importance of denitrification (and anammox) as an ecosystem service is substantial for the NGOMEX. To our knowledge, no previous studies have evaluated anammox, N fixation, or DNRA in these sediments.
Denitrification rate measurements are limited for this area and involve several different methodologies (Table 7). Despite the methodological, temporal, and spatial differences, denitrification estimates are similar for the NGOMEX, but the range of reported rate minima and maxima varies considerably. Our denitrification rates have the largest range and include the lowest minimum and highest maximum rates (Table 7). However, our study also incorporated the largest seasonal component; it is the only study to include the November through February period (Jan 2009). The passage of two hurricanes and the Deepwater Horizon oil spill represent additional sources of variability.
Our techniques provide a “best estimate” for in situ denitrification by adding calculated N fixation (if any) to net 28 N2 flux in C cores. Our median denitrification estimate (88.1 μmol N m−2 h−1), which includes any anammox, is within the ranges from most other studies (Table 7). Scaled to the Louisiana-upper Texas continental shelf, within the 100-m isobath and using an eastern boundary of Mobile Bay (area = 94,560 km2; Justić and Wang 2013), annual denitrification is 1.023 × 106 metric tons of N per year. The 5-year (2007–2011) average annual N load from the Mississippi River watershed is 1.500 × 106 metric tons of N per year, which is similar to the 1980–1996 average N load (USGS; http://water.usgs.gov/nasqan/nitrogen_phosphorus_gulf.html). Using these data, we estimate that denitrification removes approximately 68 % of the annual N load from the Mississippi River watershed. Using the smaller shelf area estimate of Kim and Min (2013; 32,400 km2), which is confined to west of the river delta, our median denitrification rate accounts for 23 % of the Mississippi/Atchafalaya N load. Another recent study used a shelf area of 65,910 km2 and estimated that denitrification removes 39 % of the N load (Lehrter et al. 2012). Our denitrification rate and the shelf area estimate from Lehrter et al. (2012) gives a similar N removal estimate of 47 %. The N load value is likely an under-estimate, since N load from other river systems affecting the area was not included (e.g., the Mobile River system; Harned et al. 2004). Likewise, our denitrification estimates are not ideal, since only rates from January were measured within the October through April period.
A previous study using acetylene inhibition in the NGOMEX found significantly lower denitrification rates at DO <1 mg L−1 (Childs et al. 2002). In contrast, our results showed a significant negative relationship between bottom-water DO and net 28 N2 flux (Fig. 2b), with the highest single rate measurement (492 μmol N m−2 h−1; C6 in Aug 2009; Fig. 3) occurring at 0.11 mg L−1 DO. Interestingly, net N fixation (−96.6 μmol N m−2 h−1) and high sediment NH4 + efflux were associated with the lowest DO concentration (0.02 mg L−1; C6 in July 2008) (Table 4; Fig. 2a). Exclusion of this data point results in a stronger negative relationship between DO and net 28 N2 flux (Fig. 2b). One problem with acetylene inhibition is that nitrification also is blocked (Steingruber et al. 2001), and nitrifiers remain active at very low DO (e.g., Carlucci and McNally 1969). In May 2010 (all sites hypoxic), we evaluated bottom-water (<1 m) denitrification in gastight bags and found significant N2 production at B7 only in the layer immediately above the SWI (<1 cm; 2.92 ± 0.03 nmol N L−1 day−1; see “Methods” in Supplementary Material). The lack of denitrification in overlying water supports the idea that sediment denitrification remained coupled to nitrification during hypoxia. In our incubations, N2 produced from ambient 14 N was usually higher than from 15 N, especially in A cores, suggesting that coupled nitrification-denitrification was also coupled to organic matter decomposition. Strong positive relationships between SOC and net 28 N2 flux and potential DNF (Table 3) support this interpretation, and the highest SOC also occurred at the lowest DO (McCarthy et al. 2013). Together, these results show that sediment denitrification remains a significant N sink when the bottom-water becomes hypoxic.
Anammox, DNRA, and N Fixation
Potential anammox relative to total N removal in coastal systems is generally low, with denitrification the dominant N removal pathway (Dalsgaard et al. 2005; Dong et al. 2011). Our results support these observations, but the isotope method used here cannot confirm anammox. 29 N2 production from 15NH4 + and 15NO3 − may be due to anammox, but other combinations of nitrification, denitrification, and DNRA also can explain 29 N2 production. For example, one 15NO3 − generated via nitrification of added 15NH4 + combined with one 14NO3 − would produce 29 N2 via denitrification. Our incubations do not exclude nitrification or select for a specific pathway. We used a functional gene microarray to evaluate denitrification and anammox nirS at stations CT2 and C6 (see “Methods” in Supplementary Material). Anammox nirS from Kuenenia stuttgartiensis (Supplementary Fig. S1) was confirmed at both sites from all cruises except August 2009 (not detected at CT2, no sample from C6). Anammox nirS accounted for 0.10 % or less of total nirS at C6 and up to 0.83 % at CT2 (Supplementary Fig. S1), but K. stuttgartiensis often is not the most abundant anammox organism in estuarine sediments (Hou et al. 2013). The microarray confirmed anammox nirS but cannot confirm activity, and only one anammox organism was represented. Therefore, anammox nirS was perhaps underestimated. However, the anammox estimate in A cores, if it is actually anammox, is a potential rate, since one of the substrates was provided.
DNRA dominates benthic NO3 − reduction in some coastal systems (e.g., Dong et al. 2011; Gardner and McCarthy 2009) but is unimportant in others (e.g., Hou et al. 2012). DNRA was suggested as a potentially important mechanism in the NGOMEX when bottom-water DO was <1 mg L−1 and NH4 + accumulated in overlying water (Childs et al. 2002). We observed potential DNRA in eight of 21 incubations, four occurring at CT2 (Table 6). The highest potential DNRA rates, however, were observed in May 2010 at C6, CT2, and F5 and 2011 at C6, when DO was 1–2 mg L−1. Bottom-water DO was <1 mg L−1 on four occasions, but DNRA was not observed at these times. Thus, our data do not support our second hypothesis, proposed by Childs et al. (2002), that DNRA competes more effectively for NO3 − at low DO. These potential rates reflect the presence and relative magnitude of DNRA but do not necessarily represent actual rates. While potential DNRA rates may be enhanced by 15NO3 −, they are also underestimated because they do not include DNRA from unlabeled NO3 − or produced 15NH4 + lost to adsorption onto particles via cation exchange mechanisms (Gardner and Seitzinger 1991). The relative magnitudes of these processes vary temporally and spatially (Gardner and McCarthy 2009). Thus, potential DNRA rates reported here are relative rather than absolute rates.
Fermentative DNRA may be more important in NO3 −-limited, carbon-rich sediments (Burgin and Hamilton 2007). When observed, DNRA occurred at relatively high bottom-water NO3 − concentrations for this study, ranging from 1.87 to 10.9 μM. This observation supports work in Australia, where low NO3 − inhibited DNRA during hypoxia (Roberts et al. 2012). DNRA is often associated with sulfur cycling (e.g., Brunet and Garcia-Gil 1996), and H2S was observed in a parallel microelectrode study in May 2010 (K.S. McNeal, unpublished data). DNRA rates, when observed, were low relative to denitrification (DNRA/DNF = 0.01–0.46) and accounted for 1–43 % of total sediment NH4 + efflux (Table 5). DNRA was not a dominant NO3 − reduction pathway, but its significance for N recycling should be considered in ecosystem models, particularly where NH4 + limits microbial activity (Lin et al. 2011).
In most cases, calculated N fixation occurring simultaneously with denitrification (An et al. 2001) was not evident (Table 6). However, calculated N fixation exceeded denitrification two times. Bottom-water DIN concentrations were >1.3 μM at both C6 in July 2008 and F5 in Jan 2009, DO conditions were hypoxic and normoxic, respectively, and NH4 + effluxed in both cases. Thus, there is no obvious explanation for N fixation exceeding denitrification at these times. We did not measure N fixation directly or evaluate the genetic basis (nifH), which provides an opportunity for future studies to evaluate sediment N fixation in the NGOMEX. As noted, H2S was present in May 2010, and sulfate reduction has been observed in this area (Lin and Morse 1991). Many sulfate reducers can fix N, which may be enhanced by bioturbation (Bertics et al. 2010). Therefore, N fixation in the NGOMEX is not unexpected, regardless of bottom-water oxygen conditions. More direct approaches for evaluating N fixation, such as 30 N2 uptake and nifH quantification, would enhance the value of our isotope methods in future studies.
Effects of Isotope Additions on SWI Nutrient Fluxes
Nitrite is an intermediate product of nitrification, denitrification, and DNRA, while anammox combines NO2 − with NH4 + to form N2 (Burgin and Hamilton 2007). Tracking NO2 − fluxes with and without isotope additions may provide insights into the N transformation pathways. For example, increased NO2 − flux in N versus C cores may indicate incomplete denitrification or DNRA (Gardner and McCarthy 2009). Comparing the SWI fluxes in Table 4 and Supplementary Table S1, 15NO3 − addition stimulated net NO2 − efflux in most cases. Sulfide can inhibit the final step (N2O → N2) of denitrification (Sørensen et al. 1980), which may cause N2O efflux from sediments and underestimation of denitrification by N2-based methods. However, N2O fluxes in our Jan and Aug 2009 incubations were low relative to N2 (mean N2O/N2 = 0.002 ± 0.001; n = 8; H.M. Baulch, unpublished data), and this ratio does not distinguish between N2O from denitrification versus nitrification. The lability and C/N of organic matter also affects NO2 − reduction during denitrification in wastewater treatment, often leading to NO2 − accumulation (e.g., Sun et al. 2009). Thus, organic matter quality and quantity may be an alternative explanation for incomplete denitrification/DNRA than sulfide inhibition in this case, since N2O production was minimal. If denitrification is not completed to N2, released NO2 − could instead fuel additional production and subsequent hypoxia, representing another mechanism where high N loads can impair the system.
Addition of 15NH4 + stimulated NO2 − efflux in seven incubations (suggesting nitrification), while NO2 − uptake (possibly suggesting anammox) was stimulated in seven others (Table 4 and Supplementary Table S1). Increased NO2 − flux in A cores suggests that the first step of nitrification was NH4 +-limited, in agreement with previous studies showing apparent NH4 + limitation in the NGOMEX (Lin et al. 2011). The second nitrification step, however, may be inhibited by sulfide (Ortiz et al. 2013). Incomplete nitrification, combined with 15NH4 +, may have enhanced anammox, as in the seven cases where NO2 − uptake was stimulated in A cores. The proportion of possible anammox to total N2 production (29A/DNF) was higher (ANOVA; p = 0.03) when NO2 − uptake was stimulated (mean = 12 ± 1.5 %) versus when NO2 − efflux was stimulated (mean = 7.4 ± 1.2 %). Despite the low abundance of anammox nirS (from one organism), anammox may be a significant N sink in the NGOMEX, but denitrification is likely the dominant pathway.
Do the Results Support Our Hypotheses?
We hypothesized that microbial N sinks are inhibited during hypoxia due to lower nitrification rates (Childs et al. 2002). On the contrary, actual and potential denitrification (DNF, including possible anammox) was enhanced at low DO (Table 3 and Fig. 2b), despite evidence of sulfide inhibition of nitrification. The strong negative relationship between NH4 + flux and DO (Fig. 2a), and the positive relationship between NO3 − flux and DO (Table 3) support this finding. The positive relationship between SOC and denitrification, as both potential and net 28 N2 flux (Table 3), suggests that organic matter degradation and redox conditions help regulate the denitrification rate. Possible anammox was not related to any nutrient concentrations or fluxes except for a positive relationship with NH4 + flux. Despite its apparently limited role, anammox warrants consideration in ecosystem models for the NGOMEX due to its effects on net heterotrophy/autotrophy (Koeve and Kähler 2010). However, anammox activity in the NGOMEX should be confirmed using genetic approaches.
Second, we hypothesized that DNRA is enhanced during hypoxia. The highest potential DNRA rates were observed during hypoxia (Table 5), but this pattern was not predictable using DO. Significant DNRA was not usually observed, and when it was, the DO was as likely to be >2 mg L−1 as <2 mg L−1 (Table 5). However, DNRA rates accounted for a significant proportion of total NH4 + efflux, especially at C6. Ammonium produced via DNRA can fuel additional production and respiration via nitrification (Carini et al. 2010; Gardner et al. 2006). Therefore, DNRA may exacerbate hypoxia, while N sinks would be a beneficial NO3 − reduction pathway (i.e., an ecosystem service). As for anammox, genetic studies combined with isotopic tracers are needed to confirm DNRA in these and other sediments.
Our final hypothesis was that heterotrophic N fixation in sediments is not an important N source in the NGOMEX, based on proximity to nutrient inputs from the Mississippi River. Our results generally support this hypothesis, but N fixation two times exceeded denitrification—once during hypoxia, and once during normoxia. Both instances occurred in the presence of >1.4 μM DIN. These N fixation > denitrification events also occurred during summer (July 2008) and winter (Jan 2009), so temperature likely was not a factor. Recent discoveries have linked N fixation to the sulfur cycle (e.g., Bertics et al. 2010) and deep-sea, methane-oxidizing archaea (Dekas et al. 2009). Sulfate reduction and sulfide have both been observed in NGOMEX sediments, and the archaeal community in the study area shifted during and after the Deepwater Horizon oil spill (Newell et al. 2013). Our results and the referenced studies, combined with method limitations for quantifying N fixation, suggest that heterotrophic N fixation remains an unknown component of the N cycle in these sediments, regardless of oxygen and nutrients.
Possible Effects of the Deepwater Horizon Oil Spill and Hurricanes
Our May 2010 cruise coincided with the Deepwater Horizon oil spill, which affected the study area beginning in late April 2010. A floating oil mousse was visible on the water surface at stations CT2, C6, and MRM. All stations had hypoxia in May 2010; the only time hypoxia was observed at CT2 and F5 during our study. One response to the oil spill was freshwater release to push oil away from shorelines (Rabalais 2011). As a result, we observed low surface salinities at CT2 (~23) and MRM (~8), which, combined with nutrient inputs and stratification, likely contributed to hypoxia. No significant differences were observed in the SWI N transformation rates in May 2010 versus other cruises. Thus, we have no evidence that the oil spill influenced hypoxia or N transformations at the SWI at our stations.
The NGOMEX was impacted by two hurricanes between our July and Sept 2008 cruises. Hurricane Gustav affected the study area from Aug 30 to Sept 1, making landfall just west of the delta. Hurricane Ike traversed the study area over several days before making landfall on Sept 13 at Galveston Island, Texas. Our sampling began 5 days later, and the water column at all sites was completely mixed. We measured lower SOC after the hurricanes (McCarthy et al. 2013), and bottom-water NH4 + concentrations also were lower (Table 2). Combined, these results suggest that organic matter decomposition was inhibited after the hurricanes, also supported by the reversal of SWI NH4 + efflux to influx at C6. Possible anammox was lower in Sept 2008 than in some other cruises, and no potential DNRA was observed. Thus, the SWI N cycle may simplify upon physical disturbance and sediment resuspension, which has been linked to short-term increases in water column nutrient concentrations and primary productivity (Fogel et al. 1999). Water column respiration rates also were highest in Sept 2008 (McCarthy et al. 2013). Together, these results suggest that hurricanes may have shifted benthic biogeochemical processes into the pelagic realm. Other studies have evaluated long-term and broad-scale impacts of hurricanes (e.g., Paerl et al. 2001; Williams et al. 2008), but we are not aware of other studies evaluating SWI N transformations so soon after storm passage.
Summary
We describe a suite of N transformation rates and fluxes in the NGOMEX hypoxic zone using continuous-flow incubations of intact sediment cores. The advantages and limitations of this technique are discussed elsewhere (McCarthy et al. 2013); briefly, these incubations maintain bottom-water (up to 1 m above the SWI) oxygen conditions for several days. This experimental duration allows use of isotope tracers, which provide insights into important pathways. Denitrification, mostly coupled to nitrification and organic matter remineralization, is the dominant pathway, but anammox may also contribute as an N sink. Denitrification (including anammox) can remove 23–68 % of the total N load from the Mississippi/Atchafalaya Rivers, depending on the shelf area used for extrapolation. In any case, denitrification/anammox removes a substantial proportion of the total N load to the shelf, providing a valuable ecosystem service. Our results do not support previous speculation that denitrification in NGOMEX sediments is inhibited at low bottom-water DO. DNRA and N fixation were observed, but neither were consistent factors in the SWI N cycle. However, method limitations inhibit our ability to constrain the importance of these mechanisms, both of which may be related to sulfur cycling. Additional studies using direct measurements and genetic verifications are needed, but these pathways should be incorporated into ecosystem models used to evaluate nutrient loading and climate changes. We cannot attribute any patterns or anomalous results to the Deepwater Horizon oil spill, but the passage of two hurricanes resulted in an apparent relocation of some benthic processes into the water column.
References
An, S., and W.S. Gardner. 2002. Dissimilatory nitrate reduction to ammonium (DNRA) as a nitrogen link versus denitrification as a sink in a shallow estuary (Laguna Madre/Baffin Bay, Texas). Marine Ecology Progress Series 237: 41–50.
An, S., T.M. Kana, and W.S. Gardner. 2001. Simultaneous measurement of denitrification and nitrogen fixation using isotope pairing with membrane inlet mass spectrometry analysis. Applied and Environmental Microbiology 67(3): 1171–1178.
Bertics, V.J., J.A. Sohm, T. Treude, C.-E.T. Chow, D.G. Capone, J.A. Fuhrman, and W. Ziebis. 2010. Burrowing deeper into benthic nitrogen cycling: the impact of bioturbation on nitrogen fixation coupled to sulfate reduction. Marine Ecology Progress Series 409: 1–15.
Boesch, D.F., W.R. Boynton, L.B. Crowder, R.J. Diaz, R.W. Howarth, L.D. Mee, S.W. Nixon, N.N. Rabalais, R. Rosenberg, J.G. Sanders, D. Scavia, and R.E. Turner. 2009. Nutrient enrichment drives Gulf of Mexico hypoxia. Eos 90(14): 117–119.
Breitburg, D. 2002. Effects of hypoxia, and the balance between hypoxia and enrichment, on coastal fish and fisheries. Estuaries 25(4B): 767–781.
Brunet, R.C., and L.J. Garcia-Gil. 1996. Sulfide-induced dissimilatory nitrate reduction to ammonia in anaerobic freshwater sediments. FEMS Microbiology Ecology 21: 131–138.
Brunner, C.A., J.M. Beall, S.J. Bentley, and Y. Furukawa. 2006. Hypoxia hotspots in the Mississippi Bight. Journal of Foraminiferal Research 36(2): 95–107.
Burgin, A.J., and S.K. Hamilton. 2007. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Frontiers in Ecology and the Environment 5(2): 89–96.
Carini, S.A., M.J. McCarthy, and W.S. Gardner. 2010. An isotope dilution method to measure nitrification rates in the northern Gulf of Mexico and other eutrophic waters. Continental Shelf Research 30: 1795–1801.
Carlucci, A.F., and P.M. McNally. 1969. Nitrification in marine bacteria in low concentrations of substrate and oxygen. Limnology and Oceanography 14(5): 736–739.
Childs, C.R., N.N. Rabalais, R.E. Turner, and L.M. Proctor. 2002. Sediment denitrification in the Gulf of Mexico zone of hypoxia. Marine Ecology Progress Series 240: 285–290 (Erratum 247: 310).
Dagg, M.J., and G.A. Breed. 2003. Biological effects of Mississippi River nitrogen on the northern Gulf of Mexico – a review and synthesis. Journal of Marine Systems 43: 133–152.
Dalsgaard, T., B. Thamdrup, and D.E. Canfield. 2005. Anaerobic ammonium oxidation (anammox) in the marine environment. Research in Microbiology 156: 457–464.
Dekas, A.E., R.S. Poretsky, and V.J. Orphan. 2009. Deep-sea archaea fix and share nitrogen in methane-consuming microbial consortia. Science 326: 422–426.
Delaune, R.D., A. Jugsujinda, I. Devai, and A.X. Hou. 2005. Denitrification in bottom sediment near oil production facilities off the Louisiana Gulf Coast. Chemistry and Ecology 21(2): 101–108.
Dong, L.F., M.N. Sobey, C.J. Smith, I. Rusmana, W. Phillips, A. Stott, A.M. Osborn, and D.B. Nedwell. 2011. Dissimilatory reduction of nitrate to ammonium, not denitrification or anammox, dominates benthic nitrate reduction in tropical estuaries. Limnology and Oceanography 56(1): 279–291.
Eyre, B.D., S. Rysgaard, T. Dalsgaard, and P.B. Christensen. 2002. Comparison of isotope pairing and N2:Ar methods for measuring sediment denitrification – assumptions, modifications, and implications. Estuaries 25: 1077–1087.
Fennel, K., D. Brady, D. DiToro, R.W. Fulweiler, W.S. Gardner, A. Giblin, M.J. McCarthy, A. Rao, S. Seitzinger, M. Thouvenot-Korppoo, and C. Tobias. 2009. Modeling denitrification in aquatic sediments. Biogeochemistry 93(1–2):159–178.
Fogel, M.L., C. Aguilar, R. Cuhel, D.J. Hollander, J.D. Willey, and H.W. Paerl. 1999. Biological and isotopic changes in coastal waters induced by Hurricane Gordon. Limnology and Oceanography 44(6): 1359–1369.
Fulweiler, R.W., S.W. Nixon, B.A. Buckley, and S.L. Granger. 2007. Reversal of the net dinitrogen gas flux in coastal marine sediments. Nature 448: 180–182.
Gardner, W.S., H.A. Bootsma, C. Evans, and P.A. St. John. 1995. Improved chromatographic analysis of 15N:14N ratios in ammonium or nitrate for isotope addition experiments. Marine Chemistry 48: 271–282.
Gardner, W.S., E.E. Briones, E.C. Kaegi, and G.T. Rowe. 1993. Ammonium excretion by benthic invertebrates and sediment-water nitrogen flux in the Gulf of Mexico near the Mississippi River outflow. Estuaries 16(4): 799–808.
Gardner, W.S., and M.J. McCarthy. 2009. Nitrogen dynamics at the sediment-water interface in shallow, sub-tropical Florida Bay: why denitrification efficiency may decrease with increased eutrophication. Biogeochemistry 95: 185–198.
Gardner, W.S., M.J. McCarthy, S. An, D. Sobolev, K.S. Sell, and D. Brock. 2006. Nitrogen fixation and dissimilatory nitrate reduction to ammonium (DNRA) support nitrogen dynamics in Texas estuaries. Limnology and Oceanography 51: 558–568.
Gardner, W.S., M.J. McCarthy, S.A. Carini, A.C. Souza, H. Lijun, K.S. McNeal, M.K. Puckett, and J. Pennington. 2009. Collection of intact sediment cores with overlying water to study nitrogen- and oxygen-dynamics in regions with seasonal hypoxia. Continental Shelf Research 29: 2207–2213.
Gardner, W.S., and S.P. Seitzinger. 1991. The effects of sea salts on the forms of nitrogen released from estuarine and freshwater sediments: does ion pairing affect ammonium flux? Estuaries 14(2): 157–166.
Harned, D.A., J.B. Atkins, and J.S. Harvill. 2004. Nutrient mass balance and trends, Mobile River basin, Alabama, Georgia, and Mississippi. Journal of the American Water Resources Association 40(3): 765–793.
Hou, L., M. Liu, S.A. Carini, and W.S. Gardner. 2012. Transformation and fate of nitrate near the sediment-water interface of Copano Bay. Continental Shelf Research 35: 86–94.
Hou, L., Y. Zheng, M. Liu, J. Gong, X. Zhang, G. Yin, and L. You. 2013. Anaerobic ammonium oxidation (anammox) bacterial diversity, abundance, and activity in marsh sediments of the Yangtze Estuary. Journal of Geophysical Research, Biogeosciences 118: 1237–1246.
Justić, D., and L. Wang. 2013. Assessing temporal and spatial variability of hypoxia over the inner Louisiana-upper Texas shelf: application of an unstructured-grid three-dimensional coupled hydrodynamic-water quality model. Continental Shelf Research 72: 163–179.
Kana, T.M., C. Darkangelo, M.D. Hunt, J.B. Oldham, G.E. Bennett, and J.C. Cornwell. 1994. Membrane inlet mass spectrometer for rapid high-precision determination of N2, O2, and Ar in environmental water samples. Analytical Chemistry 66: 4166–4170.
Kana, T.M., M.B. Sullivan, J.C. Cornwell, and K.M. Groszkowski. 1998. Denitrification in estuarine sediments determined by membrane inlet mass spectrometry. Limnology and Oceanography 43(2): 334–339.
Kana, T.M., and D.L. Weiss. 2004. Comment on “Comparison of isotope pairing and N2:Ar methods for measuring sediment denitrification” by B.D. Eyre, S. Rysgaard, T. Dalsgaard, & P.B. Christensen. 2002. Estuaries 25: 1077–1087. Estuaries 27: 173–176.
Kim, I.-N., and D.-H. Min. 2013. Temporal variation of summertime denitrification rates in the Texas-Louisiana inner shelf region in the Gulf of Mexico: a modeling approach using the extended OMP analysis. Continental Shelf Research 66: 49–57.
Koeve, W., and P. Kähler. 2010. Heterotrophic denitrification vs. autotrophic anammox – quantifying collateral effects on the oceanic carbon cycle. Biogeosciences 7: 2327–2337.
Laursen, A.E., and S.P. Seitzinger. 2002. The role of denitrification in nitrogen removal and carbon mineralization in Mid-Atlantic Bight sediments. Continental Shelf Research 22: 1397–1416.
Lavrentyev, P.J., W.S. Gardner, and L. Yang. 2000. Effects of the zebra mussel on nitrogen dynamics and the microbial community at the sediment-water interface. Aquatic Microbial Ecology 21: 187–194.
Lehrter, J.C., D.L. Beddick Jr., R. Devereux, D.F. Yates, and M.C. Murrell. 2012. Sediment-water fluxes of dissolved inorganic carbon, O2, nutrients, and N2 from the hypoxic region of the Louisiana continental shelf. Biogeochemistry 109: 233–252.
Lin, S., and J.W. Morse. 1991. Sulfate reduction and iron sulfide mineral formation in Gulf of Mexico anoxic sediments. American Journal Sciences 291: 55–89.
Lin, X., M.J. McCarthy, S.A. Carini, and W.S. Gardner. 2011. Net, actual, and potential sediment-water interface NH4 + fluxes in the northern Gulf of Mexico (NGOMEX): evidence for NH4 + limitation of microbial dynamics. Continental Shelf Research 31: 120–128.
McCarthy, M.J., S.A. Carini, Z. Liu, N.E. Ostrom, and W.S. Gardner. 2013. Oxygen consumption in the water column and sediments of the northern Gulf of Mexico hypoxic zone. Estuarine, Coastal and Shelf Science 123: 46–53.
Middelburg, J.J., and L.A. Levin. 2009. Coastal hypoxia and sediment biogeochemistry. Biogeosciences 6: 1273–1293.
Miller-Way, T., G.S. Boland, G.T. Rowe, and R.R. Twilley. 1994. Sediment oxygen consumption and benthic nutrient fluxes on the Louisiana continental shelf: a methodological comparison. Estuaries 17(4): 809–815.
Mulder, A., A.A. van de Graaf, L.A. Robertson, and J.G. Kuenen. 1995. Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiology Ecology 16: 177–184.
Newell, S.E., D. Eveillard, M.J. McCarthy, W.S. Gardner, Z. Liu, and B.B. Ward. 2013. A shift in the archaeal nitrifier community in response to natural and anthropogenic disturbances in the northern Gulf of Mexico. Environmental Microbiology Reports. doi:10.1111/1758-2229.12114.
Nielsen, L.P. 1992. Denitrification in sediment determined from nitrogen isotope pairing. FEMS Microbiology Ecology 86: 357–362.
O’Connor, T., and D. Whitall. 2007. Linking hypoxia to shrimp catch in the northern Gulf of Mexico. Marine Pollution Bulletin 54: 460–463.
Ortiz, D.I.B., F. Thalasso, F. de M.C. López, and A.-C. Texier. 2013. Inhibitory effect of sulfide on the nitrifying respiratory process. Journal of Chemical Technology and Biotechnology 88(7): 344–1349.
Paerl, H.W., J.D. Bales, L.W. Ausley, C.P. Buzzelli, L.B. Crowder, L.A. Eby, J.M. Fear, M. Go, B.L. Peierls, T.L. Richardson, and J.S. Ramus. 2001. Ecosystem impacts of three sequential hurricanes (Dennis, Floyd, and Irene) on the United States’ largest lagoonal estuary, Pamlico Sound, NC. PNAS 98(10): 5655–5660.
Piehler, M.F., & A.R. Smyth. 2011. Habitat-specific distinctions in estuarine denitrification affect both ecosystem function and services. Ecosphere 2(1): 16pp.
Quiñones-Rivera, Z.J., B. Wissel, N.N. Rabalais, and D. Justić. 2010. Effects of biological and physical factors on seasonal oxygen dynamics in a stratified, eutrophic coastal ecosystem. Limnology and Oceanography 55(1): 289–304.
Rabalais, N.N. 2011. Troubled waters of the Gulf of Mexico. Oceanography 24(2): 200–211.
Roberts, K.L., V.M. Eate, B.D. Eyre, D.P. Holland, and P.L.M. Cook. 2012. Hypoxic events stimulate nitrogen recycling in a shallow salt-wedge estuary: The Yarra River estuary, Australia. Limnology and Oceanography 57(5): 1427–1442.
Rowe, G.T., and P. Chapman. 2002. Continental shelf hypoxia: some nagging questions. Gulf of Mexico Science 2002(2): 153–160.
Rowe, G.T., M.E. Cruz Kaegi, J.W. Morse, G.S. Boland, and E.G. Escobar Briones. 2002. Sediment community metabolism associated with continental shelf hypoxia, northern Gulf of Mexico. Estuaries 25(6A): 1097–1106.
Rysgaard, S., R.N. Glud, N. Risgaard-Petersen, and T. Dalsgaard. 2004. Denitrification and anammox activity in Arctic marine sediments. Limnology and Oceanography 49(5): 1493–1502.
Seitzinger, S.P. 1988. Denitrification in freshwater and coastal marine systems: ecological and geochemical significance. Limnology and Oceanography 33(4, Part 2): 702–724.
Sørensen, J., J.M. Tiedje, and R.B. Firestone. 1980. Inhibition by sulfide of nitric and nitrous oxide reduction by denitrifying Pseudomonas fluorescens. Applied and Environmental Microbiology 39(1): 105–108.
Steingruber, S.M., J. Friedrich, R. Gachter, and B. Wehrli. 2001. Measurement of denitrification in sediments with the 15N isotope pairing technique. Applied and Environmental Microbiology 67(9): 3771–3778.
Sun, H., Q. Yang, Y. Peng, H. Shi, S. Wang, and S. Zhang. 2009. Nitrite accumulation during the denitrification process in SBR for the treatment of pre-treated landfill leachate. Chinese Journal of Chemical Engineering 17(6): 1027–1031.
Turner, R.E., and N.N. Rabalais. 1991. Changes in the Mississippi River water quality this century – implications for coastal food webs. Bioscience 41: 140–147.
Turner, R.E., N.N. Rabalias, and D. Justić. 2006. Predicting summer hypoxia in the northern Gulf of Mexico: riverine N, P, and Si loading. Marine Pollution Bulletin 52: 139–148.
Turner, R.E., N.N. Rabalais, D. Justić, and Q. Dortch. 2003. Global patterns of dissolved N, P and Si in large rivers. Biogeochemistry 64: 297–317.
Williams, C.J., J.N. Boyer, and F.J. Jochem. 2008. Indirect hurricane effects on resource availability and microbial communities in a subtropical wetland-estuary transition zone. Estuaries and Coasts 31: 204–214.
Acknowledgments
We thank the captain and crew of the R/V Pelican for assistance with sampling and cruise logistics, Peter Thomas and Ed Buskey for sharing ship time, Afonso Souza, Jiqing Liu, and Lin Xiao for assistance with core and overlying water sampling, Hou Lijun for the PEEK retrofitting, Rae Mooney for the site map, Bess Ward for microarray access and support to SEN, Zhanfei Liu and Nathaniel Ostrom for helpful discussions, Helen Baulch and Roxane Maranger for sharing N2O flux data, and Karen S. McNeal and her students for sharing oxygen and sulfide microelectrode data. We also thank Dubravko Justić and Il-Nam Kim for the surface area estimates of the continental shelf. This work was supported by Grant #NA07NOS4780225 from the National Oceanic and Atmospheric Administration-Center for Sponsored Coastal Ocean Research to WSG, Grant #1240798 from the National Science Foundation-Dimensions in Biodiversity to WSG, and a National Science Foundation Postdoctoral Fellowship to SEN.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Alf Norkko
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig S1
(DOC 68 kb)
Supplementary Table S1
(DOC 65 kb)
ESM 3
(DOC 35 kb)
Rights and permissions
About this article
Cite this article
McCarthy, M.J., Newell, S.E., Carini, S.A. et al. Denitrification Dominates Sediment Nitrogen Removal and Is Enhanced by Bottom-Water Hypoxia in the Northern Gulf of Mexico. Estuaries and Coasts 38, 2279–2294 (2015). https://doi.org/10.1007/s12237-015-9964-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-015-9964-0