Abstract
We conducted monthly bioassay experiments to characterize light and nutrient use efficiency of phytoplankton communities from the chlorophyll-a maximum located in the tidal freshwater region of the James River Estuary. Bioassay results were interpreted in the context of seasonal and inter-annual variation in nutrient delivery and biomass yield using recent and long-term data. Bioassay experiments suggest that nutrient limitation of phytoplankton production has increased over the past 20 years coinciding with reductions in point source inputs and estuarine dissolved nutrient concentrations. Despite increasing nutrient stress, chlorophyll concentrations have not declined due to more efficient nutrient usage. Greater CHLa yield (per unit of N and P) may be due to feedback mechanisms by which the presence of toxin-producing cyanobacteria inhibits grazing by benthic and pelagic filter-feeders. Seasonal patterns in nutrient limitation indicate that phytoplankton in the James respond to variations in inflow concentrations of dissolved nutrients. This association gives rise to an atypical pattern whereby the severity of nutrient limitation diminishes with low discharge in late summer due to minimal dilution of local point sources inputs by riverine discharge. We suggest that this may be a common feature of estuaries located in proximity to urbanized areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Effective nutrient management strategies for eutrophic waterbodies require an understanding of the relationship between nutrient loading and algal production (Cloern 2001; Wagner et al. 2011). Quantitative depictions of the nutrient load-algal biomass relationship are the basis for determining nutrient allocations (caps) to protect designated uses (swimability, fishability, etc.) against impairments arising from eutrophication (Cerco and Cole 1993; Havens and Schelske 2001; Borah et al. 2006; Carstensen et al. 2011). The complexities which influence the relationship between nutrient supply and phytoplankton production pose a challenge to the implementation of this approach. This relationship is influenced by physical forces such as variations in temperature, water residence time, and underwater light availability (Sterner et al. 1997; Sellers and Bukaveckas 2003; Borsuk et al. 2004; Lucas et al. 2009; Ochs et al. 2013), as well as biotic processes such as grazing and nutrient re-generation (Bronk et al. 1998; Vanni et al. 2006; Hall et al. 2007). Limiting resources such as nutrients and light vary spatially and temporally, as do constraints on the ability of phytoplankton to exploit these resources (Monbet 1992; Bukaveckas et al. 2011a). In estuaries, discharge is an important variable that influences both nutrient supply and demand; the former because loading rates are related to riverine inputs, and the latter because advective losses (washout) affect biomass accumulation (Rudek et al. 1991; Borsuk et al. 2004; Murrell et al. 2007; Lucas et al. 2009). The effects of discharge on biomass yield may be viewed as a constraint on resource use efficiency whereby under high discharge conditions, short water residence time limits opportunities for phytoplankton to convert dissolved inorganic nutrients into algal biomass. Our limited knowledge of the interactive effects of light, nutrient and residence time conditions leads to uncertainty in establishing nutrient reduction targets for preventing harmful effects from algal blooms (Strayer et al. 2008; Bukaveckas et al. 2011a; Kratina et al. 2012).
Nutrient limitation arises from the imbalance between supply and demand; this imbalance affects both the severity and form (e.g., N vs. P) of limitation (Conley et al. 2009; Ptacnik et al. 2010). External nutrient supply is dictated by hydrologic loading (the ratio of watershed runoff to the surface area of the receiving water body) as well as anthropogenic activities in the watershed which determine concentrations in runoff (Lewis et al. 1999; Baron et al. 2013). Land use practices affect not only the quantity but also the relative ratios and forms of N and P. Nitrogen is generally thought to be limiting in coastal waters (Howarth and Marino 2006), though co-limitation by N and P is commonly reported (Elser et al. 2007). Forms of N differ in their bioavailability—uptake of dissolved inorganic fractions (e.g., nitrate and ammonia) is well-known, whereas utilization of dissolved organic nitrogen has only recently been appreciated (Mulholland et al. 2009; Bradley et al. 2010; Filippino et al. 2011). Algal nutrient demand is a product of their growth rates and stoichiometry (C/N/P) both of which are affected by light conditions (Sterner et al. 1997; Hall et al. 2004; Brauer et al. 2012). At saturating light intensities, nutrients are more efficiently converted into algal biomass and it has also been shown that production per unit of N or P (e.g., CHLa/P or C/P of particulate matter) is greater under more favorable light conditions (Sterner et al. 1997; Mette et al. 2011). Underwater irradiance is determined by incident solar radiation, light attenuation and the depth of the mixed layer, which in well-mixed estuaries is the overall depth.
The presence and severity of nutrient limitation is often inferred by comparing nutrient ratios in the environment (e.g., DIN/TP) to Redfield values (e.g., Ptacnik et al. 2010). This approach relies on the assumption that measured nutrient concentrations reflect availability which may be problematic given differences in lability among various nutrient fractions (Beardall et al. 2001). Bioassay experiments allow direct measurement of nutrient enrichment on uptake rates, growth rates, and stoichiometry (Tamminen and Andersen 2007; Ren et al. 2009). This approach has long been used to measure nutrient limitation and quantify relationships between nutrient supply and algal biomass yield in diverse settings such as lakes, rivers, and estuaries (Lean and Pick 1981; Elser et al. 1988). In addition to providing qualitative information on which nutrients are limiting, they can be used to measure the severity of nutrient limitation based on the ratio of phytoplankton growth rates at ambient vs. enriched nutrient levels (Tamminen and Andersen 2007). A limitation of this approach is that bioassays isolate phytoplankton from external and certain regenerated sources of nutrients (e.g., sediment nutrient fluxes) such that nutrient deficiency may be artificially enhanced during the experiment. To minimize this, as well as “bottle effects” arising from colonization of surfaces, bioassays are typically run as dilution experiments and over short intervals (e.g., 48–72 h). Being of small scale, these experiments can be replicated to characterize spatial and temporal variation in nutrient limitation (e.g., Fisher et al. 1999) and can be used to assess the effects of light conditions on nutrient use efficiency (e.g., Koch et al. 2004; Whalen and Benson 2007). A third, perhaps under-utilized, value to bioassay experiments is in establishing algal stoichiometric properties to assess growth efficiency (i.e., CHLa or C yield per unit of N and P) under varying nutrient and light conditions (Gowen et al. 1992; Edwards et al. 2003).
In this study, we examine seasonal patterns in nutrient and light limitation of phytoplankton communities at an estuarine CHLa maximum. A region of elevated CHLa extends over 40 km within the tidal freshwater segment of the James River Estuary where annual average CHLa concentrations are among the highest in the Chesapeake Bay region (Fig. 1). Longitudinal CHLa maxima have been reported in other estuaries, and in some cases, attributed to hydrodynamic retention whereby particulate matter in surface (seaward) currents is entrained in deeper, landward currents (North and Houde 2001, 2003). This is not the case in James where elevated CHLa is attributed to high growth rates (positive water column NPP) in the region where the channel transitions from a riverine to an estuarine morphometry (Bukaveckas et al. 2011b). It is hypothesized that shallow conditions release phytoplankton from light limitation and allow for greater utilization of nutrients from the upper watershed and local point source inputs. A recent mass balance analyses supports this view by showing high rates of inorganic nutrient assimilation within the CHLa maximum (Bukaveckas and Isenberg 2013). In this paper, we present results from bioassay experiments in which light and nutrient conditions were manipulated to determine the form and severity of resource constraints on phytoplankton growth in the CHLa maximum. We also analyze a 3-year time series of weekly CHLa measurements to make inferences about the relative importance of nutrient availability and water residence time in influencing seasonal patterns of nutrient limitation. Lastly, we consider whether the severity of nutrient limitation has changed in response to nutrient load reductions during the past 20 years by comparing our results to bioassay experiments previously performed at this site (Fisher et al. 1999).
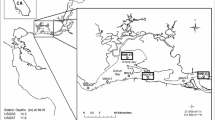
Map of the James River Estuary showing the CHLa maximum in the tidal freshwater segment and the locations of main channel (JMS75) and near-shore (Rice Pier) sampling sites for bioassay experiments. Annual average CHLa concentrations are for 2005–2010 based on monthly measurements by the Virginia Department of Environmental Quality for the Chesapeake Bay Program
Methods
Site Description
The James River Estuary is the southern-most of the five major sub-estuaries of Chesapeake Bay. Its principal tributary, the James River, is the third largest tributary of Chesapeake Bay by discharge and nutrient load. The tidal fresh segment of the estuary (salinity <0.5) extends 115 km from the Fall Line (at Richmond, VA) to the confluence with the Chickahominy River. This segment experiences a large tidal prism (∼60 cm) relative to average depth (∼3.0 m) creating a vertically and laterally well-mixed system. Nutrient loads to this segment are large due to its small surface area (52 km2), large contributing area (watershed = 26,165 km2), and direct point source inputs from the Richmond metropolitan area (Bukaveckas and Isenberg 2013). P loads are principally (∼80 %) from riverine inputs which are transported in particulate form and trapped during high discharge events. For N, watershed and local point sources contribute approximately equally, though the latter dominate with respect to dissolved inorganic fractions and during low discharge periods. Photic depths are typically ∼1 m and are relatively uniform throughout the tidal fresh segment (Bukaveckas et al. 2011b). Despite this, there are large differences in light conditions between the upper, constricted segment, where the deeper channel (>3 m) results in low average underwater irradiance, and the broader channel of the lower segment, where shallow depths (<2 m) result in greater light availability. Water residence time in summer ranges from 5 to 25 day (mean = 15.6 day for May–October 2012). Estimates presented here are based on the date-specific freshwater replacement time (FRT) method (Alber and Sheldon 1999) using river discharge data from USGS gauges located near the Fall Line on the James (no. 2037500) and Appomattox Rivers (no. 2041650).
Monitoring Data
We conducted ∼weekly monitoring of CHLa and nutrient concentrations during July 2010 to December 2012 at a station located within the CHLa maximum (Chesapeake Bay Program designation: JMS75; Fig. 1). The station is located 55 km below the Fall Line in the wide, shallow portion of the tidal fresh segment. Water samples were collected at a depth of 1 m for analysis of particulate matter (CHLa, TSS, POC, PON) and nutrient concentrations (TN, NH4, NO3. TP, PO4). Samples for CHLa, TSS, POC and PON were filtered through Whatman GF/A glass filters (0.5-μm nominal pore size). Filters for CHLa analyses were extracted for 18 h in buffered acetone and analyzed on a Turner Design TD-700 Fluorometer (Arar and Collins 1997). TSS was determined gravimetrically using pre-weighed, pre-combusted filters. Filters for POC and PON analysis were dried at 60 C for 48 h, fumed with HCl to remove inorganic carbon and analyzed on a Perkin–Elmer CHN analyzer. Concentrations of total nitrogen (TN), nitrate (NO3) ammonium (NH4), total phosphorus (TP), and phosphate (PO4) were determined using a Skalar segmented flow analyzer using standard methods (APHA 1998). Urea concentrations (bioassays only) were measured on an Astoria Pacific autoanalyzer using the colorimetric monoxime method of Price and Harrison (1987).
Bioassay Experiments
Experiments were performed monthly from May to October 2012 using water obtained from two locations within the CHLa maximum: a main channel site (JMS75) and the nearby Research Pier at the VCU Rice Center (Fig. 1). The two sites were included in the design to test for differences in the severity of nutrient limitation between near-shore and main channel habitats. Water from these sites was obtained in conjunction with the weekly monitoring program and returned immediately to the lab. Bioassays comprised a 150-mL solution in a 250-mL Erlenmeyer flask containing 50 % raw water and 50 % filtered water (0.5-μm Whatman GF/A glass filter). Bioassays were diluted in order to reduce algal densities below equilibrium to measure algal growth responses (Sterner and Grover 1998). Six nutrient treatments were performed (Control, +NH4, +NO3, +urea, +PO4, +PN) using water obtained from the near-shore site; only the Control (no nutrients added) and +PN treatments were performed at the main channel site. Each treatment was replicated three times. Enrichments entailed the addition of 0.125 mg L−1 of NO3, NH4, or urea and 0.1 mg L−1.of PO4. Combined treatments (+PN) received 0.125 mg L−1 each of NO3 and NH4 and 0.1 mg PO4 L−1. Nutrient additions approximately doubled ambient concentrations increasing DIN from 0.10–0.15 mg L−1 to 0.25–0.3 mg L−1, urea from 0.10–0.20 mg L−1 to 0.20–0.30 mg L−1, and PO4 from 0.05–0.10 mg L−1 to 0.15–0.20 mg L−1.
Bioassays were incubated on a shaker table at ambient (river) temperature inside a Conviron growth chamber for 48 h. Control and +PN treatments were incubated at three light levels (3, 6, and 12 E m−2 day−1) to assess light effects on phytoplankton growth, nutrient uptake, and stoichiometry. These values represent the average daily irradiance experienced by phytoplankton circulating through the entire water column over depths ranging to 1, 2, and 4 m taking into account typical summer solar radiation (∼40 E m−2 day−1; Fisher et al. 2003) and underwater light attenuation (mean k d = 3.14 ± 0.33 m−1) measured monthly in conjunction with bioassay experiments (Gosselain et al. 1994). The lowest light level represented ambient conditions in the upper, constricted section of the tidal fresh segment (e.g., near station JMS99; Fig. 1). The higher light levels represented ambient conditions in the broad, shallow reach near JMS75. Light conditions within the incubator were modified by shade cloth and proximity to light sources and verified with a Li-Cor photometer.
Initial and final concentrations of CHLa, POC, and nutrients were determined using the same analytical methods as for monitoring samples. Phytoplankton growth rates (r) were calculated as the slope of the natural logarithms of POC as a function of time. Some other studies have used CHLa to calculate growth rates; however, in preliminary experiments we observed changes in POC/CHLa during incubation in response to varying light exposure. POC-based growth rates were used to calculate effect sizes as the natural logarithm of treatment/control (Koch et al. 2004). An analysis of covariance (ANCOVA) was used to test for interactions between light and nutrient effects. Light saturation effects were assessed by comparing the fit of linear and non-linear (log and tangential) models of phytoplankton growth as a function of irradiance for individual experiments. Nitrogen use efficiency was assessed based on PON production per unit of DIN uptake and from changes in the ratio of C/N in particulate matter. The relationship between DIN uptake and PON production was analyzed using Model II regression analysis since both parameters are measured with error. Statistical analyses were performed using Microsoft Excel and JMP Pro 10.
Results
Seasonal and Inter-annual Variation in CHLa and Nutrients
A 3-year time series of weekly monitoring at JMS75 is presented to place the 2012 bioassay experiments in the broader context of seasonal and inter-annual variation in phytoplankton abundance and nutrient availability. Data from this station located in the CHLa maximum showed well-defined bloom periods in each year corresponding to seasonal patterns in water temperature and residence time (Fig. 2). The periods of elevated CHLa (>20 μg L−1) were associated with water temperature >15 °C and freshwater replacement time >10 day whereas intervening periods (∼November–April) were characterized by low CHLa (<10 μg L−1), low water temperature, and short FRT. Periods with elevated CHLa corresponded to peaks in TP (>0.10 mg L−1) and low DIN (<0.15 mg L−1). This resulted in low DIN/TP ratios during summer months (molar ratio <5). Declines in CHLa during Fall were often associated with storm events which had a pronounced flushing effect (e.g., Tropical Storm Lee, September 2011). Overall, CHLa was positively correlated with FRT in the 3-year time series (N = 100, R 2 = 0.47, p < 0.001). Inter-annual differences in July–August means for CHLa were large (>2-fold) relative to inter-annual differences in TP and TN (CV = 6 and 13 %, respectively). This resulted in a 3-fold range of variation in CHLa yield relative to TP (310–990 w/w) and TN (50–115 w/w). POC concentrations tracked seasonal patterns in CHLa, but like TP and TN, exhibited less inter-annual variability during bloom periods (CV = 12 %). C/N ratios of particulate matter were similar during July–August in all 3 years (means = 6.1–6.3) and comparable to the Redfield ratio (6.6; Redfield 1958).
Bioassay Experiments
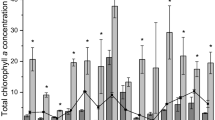
Phytoplankton exhibited statistically significant positive responses to light in 11 of 12 experiments, and to nutrient additions in 11 of 12 experiments performed at the two sites (Table 1). Significant interaction effects were detected for three of six experiments from each site in which there was a synergistic effect from the combination of enhanced light and nutrient enrichment. No significant differences in phytoplankton growth rates were observed between the main channel and near-shore sites in either control or nutrient-enriched bioassays. Nutrient enrichment effects on growth rates were observed throughout the range of light intensities but larger responses were typically observed at the highest light intensity (Fig. 3). Average effect sizes (natural log-transformed ratios of nutrient-enriched to Control growth rates) were 0.26 ± 0.06, 0.31 ± 0.07, and 0.41 ± 0.07 at 3, 6, and 12 E m−2 day−1, respectively. We compared the fit of linear and non-linear models relating phytoplankton growth (as POC) to irradiance and found that the non-linear (saturating) model provided a better fit in three of the six experiments at each site (June, July, and August; Fig. 3).
Phytoplankton responses to light and nutrient amendments (as POC ± SE) during monthly bioassay experiments performed at a near shore site in the tidal fresh James River. Dashed lines represent initial POC levels, hollow symbols represent final concentrations under ambient nutrient conditions (Control) and dark symbols represent final concentrations under nutrient-enriched conditions
Forms of nutrient limitation differed among the monthly experiments (Fig. 4, Table 2). The combined P and N addition resulted in significantly higher growth rates relative to controls in 11 of 12 experiments (excluding September, Main Channel site). Higher growth rates in response to P addition were not observed in any of the six experiments (performed at near-shore site only). Interpretation of N effects was somewhat dependent on the form of N tested. In June, all three forms of N (NO3, NH4, and urea) resulted in significantly higher growth rates relative to Controls. Growth rates were not significantly different among the three N treatments indicating that phytoplankton were capable of exploiting all three forms of N. In August, additions of NH4 and urea stimulated growth rates relative to Controls, whereas NO3 did not. In September, bioassays receiving NO3, exhibited significantly higher growth rates relative to Controls and to those receiving NH4 and urea. Overall, these findings suggest that phytoplankton in the tidal freshwater segment of the James were consistently stimulated by the combined addition of N and P, and in a few cases responded to the addition of N alone.
Initial and final concentrations of DIN, PON and POC in the bioassays were used to derive two metrics of N use efficiency: one which considered the production of particulate N in relation to DIN assimilation, and a second, particulate C production per unit of particulate N production. In enriched bioassays, maximal rates of DIN uptake were ∼0.15–0.20 mg L−1 day−1 such that DIN pools were depleted to ∼20 % of initial (starting) concentrations during the 48-h incubations (Fig. 5). Rates of DIN assimilation were significantly correlated with PON production in each of the monthly experiments (data pooled for both sites; R 2 = 0.51 to 0.82). The slope of this relationship is an indicator of N use efficiency as it represents the proportion of assimilated N which is converted to particulate N. Highest N use efficiency was observed in June (32 %) and August (29 %) corresponding to the months when responses to N addition alone were observed. In other months, N use efficiency was lower (10–19 %) with the exception of October (32 %). Overall, the low proportion of N retained in the particulate fraction indicates that the bulk of assimilated N entered the DON pool. We analyzed variation in algal biomass yield (as C) per unit of N by comparing C/N ratios among bioassays under various light and nutrient conditions (Fig. 6). C/N ratios increased by 2-fold in response to higher light levels in both Control and nutrient-enriched treatments. Highest C/N ratios (∼12) were observed at high light levels (12 E m−2 day−1) and ambient nutrient concentrations. At low light levels (3 E m−2 day−1), C/N ratios were not significantly different between Control and +PN treatments, and were similar to Redfield (∼6).
Growth rates at ambient nutrient concentrations ranged from 0.1 to 0.4 day−1 (mean = 0.23 day−1) and corresponded to an average doubling time of 3 day (Fig. 7). Nutrient-saturated growth rates were higher (range = 0.2 to 0.6 day−1; mean = 0.44 day−1) and corresponded to an average doubling time of 1.6 day. Incubation temperature ranged from 19 to 30 °C and explained less than 10 % of the variation in growth rates. Strong responses to nutrient enrichment were observed in May and June when phytoplankton growth rates at ambient nutrient concentrations were ∼25 % of nutrient-enriched growth rates. Weaker responses to nutrient enrichment were measured during August-September when growth rates at ambient nutrient concentrations were ∼75 % of nutrient-enriched growth rates. Seasonal patterns in the severity of nutrient limitation followed trends freshwater replacement time. Greater severity of nutrient limitation was associated with short FRT in May and June (5–10 day) with weaker responses to nutrient limitation occurring during periods of longer FRT (15–20 day) in late summer. To assess changes in the nutrient status of the James, we compared current nutrient conditions with historical data from the CBP monthly monitoring. At the site where bioassay experiments were performed (JMS75), average summer values of DIN declined from 0.45 mg L−1 (1990–1996) to 0.25 mg L−1 (1997–2012) while PO4 declined from 0.022 mg L−1 to 0.013 mg L−1 during the same period (Fig. 8). The incidence of very low DIN concentrations (<0.10 mg L−1) has increased in recent years (e.g., >50 % of summer monthly measurements during 2007–2012). Declining nutrient concentrations in the region of the CHLa maximum have led to the development of pronounced longitudinal gradients in nutrient availability as indicated by differences in concentration between stations JMS99 and JMS75 (Fig. 8). Largest differences were observed during 2000–2010 with the exception of 2003, a year with high summer discharge and low CHLa.
Long-term trends in DIN (top panel), PO4 (middle panel) and CHLa (bottom panel) in the tidal freshwater segment of the James River Estuary. Nutrient data are monthly values for June–September at two stations: a low-CHLa site in the upper, constricted section (JMS99) and a high-CHLa site in the broad, shallow section (JMS75). Lines depict summer-average values for each year. CHLa data are year-round monthly values at JMS75 (all data from VA DEQ Chesapeake Bay Monitoring Program)
Discussion
Our most striking finding was the prevalence of nutrient limitation (observed in 11 of 12 experiments), given that a prior study concluded that phytoplankton in the tidal fresh James were exclusively light limited (Fisher et al. 1999). The prior study was conducted at the same station (JMS75) and included monthly experiments performed during the same time of year (May–October 1993) showing no detectable response to nutrient addition. A number of methodological differences complicate direct comparisons including use of different response variables (CHLa vs. POC), incubation length (6–8 day vs. 48 h) and light exposure (8–30 vs. 3–12 E m−2 day−1; Fisher et al. 1999, this study; respectively). Two of these differences (our use of lower irradiances and shorter incubations) would be expected to diminish the likelihood of observing nutrient limitation. Also, our nutrient additions were smaller (DIN = 0.125–0.250 mg L−1, PO4 = 0.1 mg L−1) than those used by Fisher et al. (DIN = 0.35 mg L−1, PO4 = 0.155 mg L−1). Therefore it is unlikely that differences in methodology biased our results in favor of finding nutrient limitation.
The bioassay results suggest that the severity of nutrient limitation has increased during the 20-year interim between the two studies. Two potential mechanisms could account for this: an increase in water clarity and/or a reduction in nutrient availability. An analysis of diffuse attenuation coefficients (k d) measured monthly at this site during May-October of 1994–2010 revealed no long-term trends in water clarity (N = 93, R 2 < 0.10, p = 0.46; data from VA DEQ Chesapeake Bay Program). Average attenuation values measured in conjunction with our bioassay experiments (k d = 3.14 ± 0.33 m−1) were similar to the long-term average at this location (k d = 3.49 ± 0.10 m−1). In contrast, summertime DIN and PO4 concentrations have declined at the site where bioassay experiments were performed with present values being less than half of those measured during the earlier bioassay study. Interpretation of the long-term nutrient data is complicated by changes in analytical methodology after 1994 (Marshall et al. 2009). However, our assessment of nutrient availability is based on a comparison between two sites showing that nutrient concentrations at the station where bioassay experiments were performed (JMS75) have declined relative to an upstream station (JMS99). Concentration differences between the two sites became apparent after 2000. We attribute the lower nutrient concentrations at JMS75 to high rates of assimilatory uptake in the region of the CHLa maximum. The effects of assimilatory uptake in depleting nutrients have likely been enhanced by reductions in point source inputs. Our recent mass balance analyses showed that point source inputs to this segment of the James have declined by one-third (TN) and one-half (TP) since the early 1990s (Bukaveckas and Isenberg 2013). Together, the monitoring and bioassay data suggest that reductions in nutrient loads have fostered a shift toward greater nutrient limitation of phytoplankton while nutrient concentrations have declined to levels not previously seen in the 25-year record.
Despite the increasing severity of nutrient limitation, CHLa levels in the James were unchanged during this period (1990-present; Fig. 8). CHLa yields per unit of N and P are higher now (2010–2012—CHLa/TP = 310–990, CHLa/TN = 50–115) in comparison to data from throughout the 1990s (CHLa/TP ∼200, CHLa/TN ∼30) indicating that increased nutrient use efficiency has compensated for declines in nutrient availability. Carstensen et al. (2011) have similarly reported a doubling of the CHLa/TN ratio in recent decades for estuaries from various regions, including the saline reaches of Chesapeake Bay. They attribute this trend to factors which include rising temperature and CO2 as well as potential reductions in grazing due to increasing abundance of cyanobacteria and other harmful algae. Our results show that this phenomenon extends to the tidal freshwater reaches of the estuary and is evident for both TN and TP. Our data show that CHLa yield is higher during periods of long residence time as indicated by a significant positive relationship between CHLa/TN and FRT in our weekly monitoring data (N = 100, R 2 = 0.34, p < 0.001). However, there were no significant trends in water inputs at this site, either in the long-term data (1899–2011; p = 0.39) or during the period spanning the bioassay studies (1990–2011, p = 0.50) that would suggest that higher CHLa yield could be attributed to reduced flushing. Higher CHLa yield may be indicative of shifts in phytoplankton community composition favoring species with greater nutrient use efficiency. However, the two major groups contributing to phytoplankton biomass in the James (chlorophytes and diatoms) have dominated throughout this period. The abundance of cyanobacteria has been increasing over the past 20 years (Marshall et al. 2009) though their proportional contribution to biomass remains low (e.g., 5 % in 2012; H. Marshall, personal communication) Thus there are no shifts among major phytoplankton groups that could be linked to CHLa yield, though within-group replacement by more nutrient-efficient species could account for this trend. Historical changes in fisheries may also play a role through their effects on zooplankton abundance and consumer-mediated nutrient recycling (Havens et al. 2001; Vanni et al. 2006; Caraco et al. 2006).
A second finding arising from our bioassay experiments is that increasing ratios of ambient to nutrient-enriched growth rates indicate that the severity of nutrient limitation declines with decreasing discharge in late summer. As declining discharge is linked to reduced riverine nutrient loads, a weakening of nutrient limitation appears counter-intuitive. Current paradigms on seasonal patterns of nutrient limitation originate from lake studies where the severity of limitation increases during longer residence time in summer due to diminishing external nutrient inputs and progressive depletion of nutrients from the euphotic epilimnion (Schindler 1977; Wetzel 2001). Similar explanations have been invoked for estuaries. For example, a recent study of the New and Neuse River estuaries reported that CHLa increased over a range of short residence times, but at longer residence times, CHLa declined (Peierls et al. 2012). The shift from positive to negative slope in the CHLa-residence time relationship was attributed to biotic processes exerting a greater influence on phytoplankton biomass during long residence time. These included increases in the severity of nutrient limitation as well as higher losses due to grazing.
For estuaries located in proximity to urban areas, local point source inputs are a key factor influencing nutrient availability. In the James, direct point source inputs account for a large proportion of the external nutrient load, particularly for dissolved inorganic fractions during summer (e.g., 93 % of NH4 and 75 % of NO3 and PO4; Bukaveckas and Isenberg 2013). We contend that phytoplankton in the James are responding to changes in the concentration of nutrients in inflow, rather than to loading rates associated with riverine fluxes. N yields from the James watershed are low among east coast rivers (Boyer et al. 2002; Howarth et al. 2006) such that periods of elevated discharge are characterized by inputs of relatively N-poor waters. Nutrient concentrations in point source inputs are orders of magnitude higher, and during low river discharge, these would be subject to smaller dilution effects from river inflow, thereby resulting in higher inflow concentrations. The influence of point sources on estuarine nutrient concentrations was apparent at our upstream sampling station (JMS99) where summer DIN and PO4 concentrations were consistently higher than those observed at the bioassay site (JMS75; Fig. 8). We suggest that in late summer, the severity of nutrient limitation declines due to higher nutrient concentrations of inflow and that this may be a common feature among estuaries which receive substantial point source inputs. We cannot discount the possibility that acceleration in the rate of internal nutrient recycling could increase nutrient supply in late summer, though we lack data on seasonal variation in grazing rates and sediment nutrient fluxes to test this hypothesis. An important implication of these findings is that nutrient limitation of phytoplankton in the tidal fresh James is principally determined by local point source nutrient inputs, and the extent to which these are diluted by watershed (riverine) runoff. We suggest that in estuaries where local point sources account for a large fraction of inputs, the expected relationship of increasing nutrient stress during low discharge and long water residence time may be reversed.
Lastly we consider the effects of light and nutrient conditions on phytoplankton stoichiometry and nutrient use efficiency. We derived two metrics of efficiency: the proportion of assimilated DIN which was converted to particulate N, and the C biomass yield per unit of particulate N. The proportion of DIN uptake retained in the particulate fraction was variable but low (10–31 %) indicating that 70–90 % of DIN was transferred to the DON pool during the 48-h experiment. This finding is consistent with a number of field and laboratory studies reporting that a large fraction of DIN uptake is subsequently released as DON (e.g., 25–40 % in Bronk et al. 1994). DON release has been attributed to light and salinity effects, phytoplankton physiological condition, and grazing (Hu and Smith 1998; Ward and Bronk 2001; Bradley et al. 2010). Transfer of DIN to the DON pool would potentially shunt more N into the microbial food web (Stepanauskas et al. 1999; Seitzinger et al. 2002a, b; Wiegner et al. 2006). A related study of bacterial communities in the James revealed higher cell densities, greater proportion of live cells and shifts in community composition of active taxa in the region of the CHLa maximum (Franklin et al. 2013). A number of studies have shown that NH4 is the primary source of N for phytoplankton uptake and DON production (e.g., Bronk and Ward 1999; Bradley et al. 2010) though other studies have reported that uptake rates for NO3 exceeded those for NH4 (Parker et al. 2012). In our bioassay experiments we observed similar uptake rates for NH4 (mean = 0.070 ± 0.001 mg L−1 day−1) and NO3 (mean = 0.062 ± 0.003 mg L−1 day−1); urea uptake rates were lower and more variable (mean = 0.045 ± 0.014 mg L−1 day−1). These results suggest that all three forms of N play a role in supporting phytoplankton production and DON release in the tidal fresh segment of the James.
Carbon production per unit of N varied in response to experimental treatments with two-fold higher C/N ratios observed under high light conditions. This finding is consistent with studies in other freshwater systems showing higher C/P ratios of primary producers under more favorable light conditions (e.g., Sterner et al. 1997). Our higher light treatments (6–12 E m−2 day−1) were representative of underwater irradiance in the region where the CHLa maximum occurs and suggest that high phytoplankton production in this zone can be attributed in part to greater biomass yield per unit of N or P. Under these light conditions, C/N was double that of the Redfield ratio – a result which has implications for estimating algal N demand based on C production. In a prior paper we argued that external nitrogen loads could account for only 20 % of phytoplankton demand based on measured production and Redfield ratios (Bukaveckas and Isenberg 2013). Using the higher C/N ratios derived empirically from the bioassay experiments we find that external inputs could account for 40 % of algal demand, with 60 % supported by internal recycling. We observed both linear and non-linear responses to light availability among the monthly bioassay experiments suggesting that phytoplankton are fully or partially released from light limitation at irradiances representative of light conditions in the shallower segment of the estuary (near JMS75). These results are supportive of our earlier hypothesis (Bukaveckas et al. 2011b) that the location of the CHLa maximum is linked to the morphometry of the channel whereby shallow conditions result in greater average water column irradiance and higher nutrient utilization.
In summary, our prior work has shown that the tidal freshwater segment of the James Estuary is a hot spot for phytoplankton production and nutrient retention owing to favorable conditions of light, residence time and nutrient supply. In the present study we report that reductions in point sources inputs have resulted in lower concentrations of dissolved inorganic nutrients and a strengthening of longitudinal nutrient gradients in this segment of the estuary. Seasonal patterns in nutrient limitation suggest that phytoplankton in the James are responsive to local point sources inputs and the extent to which these are diluted by riverine discharge. Reduced riverine inputs during late summer result in a weakening of nutrient stress due to higher inflow concentrations. Increasing nutrient limitation in the James is a promising first step toward oligotrophication. However, the increase in biomass yield, as indicated by higher CHLa/TP and CHLa/TN, has outweighed the effects of declining nutrient availability such that phytoplankton abundance remains unchanged. Achieving reductions in the magnitude and duration of blooms may depend on internal nutrient cycles and their influence on the nutrient load-algal biomass relationship.
References
Alber, M., and J.E. Sheldon. 1999. Use of a date-specific method to examine variability in the flushing times of Georgia Estuaries. Estuarine, Coastal and Shelf Science 49: 469–482.
APHA Standard Methods for the Examination of Water and Wastewater. 1998. American Public Health Association. 20th edition. Washington, DC, USA.
Arar, E.J., and G.B. Collins. 1997. In vitro determination of chlorophyll a and phaeophytin a in marine and freshwater algae by fluorescence. Cincinnati: National Exposure Research Laboratory, US Environmental Protection Agency.
Baron, J.S., E.K. Hall, B.T. Nolan, J.C. Finlay, E.S. Bernhardt, J.A. Harrison, F. Chan, and E.W. Boyer. 2013. The interactive effects of excess reactive nitrogen and climate change on aquatic ecosystems and water resources of the United States. Biogeochemistry 114: 71–92.
Beardall, J., E. Young, and S. Roberts. 2001. Approaches for determining phytoplankton nutrient limitation. Aquatic Sciences 63: 44–69.
Borah, D.K., G. Yagow, A. Saleh, P.L. Barnes, W. Rosenthal, E.C. Krug, and L.M. Hauck. 2006. Sediment and nutrient modeling for TMDL development and implementation. Transactions of the Asabe 49: 967–986.
Borsuk, M.E., C.A. Stow, and K.H. Reckhow. 2004. Confounding effect of flow on estuarine response to nitrogen loading. Journal of Environmental Engineering 130: 605–614.
Boyer, E.W., C.L. Goodale, N.A. Jaworsk, and R.W. Howarth. 2002. Anthropogenic nitrogen sources and relationships to riverine nitrogen export in the northeastern USA. Biogeochemistry 57: 137–169.
Bradley, P.B., M.W. Lomas, and D.A. Bronk. 2010. Inorganic and organic nitrogen use by phytoplankton along Chesapeake Bay measured using a flow cytometric approach. Estuaries and Coasts 33: 971–984.
Brauer, V.S., Stomp, and J. Huisman. 2012. The nutrient-load hypothesis: patterns of resource limitation and community structure driven by competition for nutrients and light. The American Naturalist 179: 721–740.
Bronk, D.A., P.M. Gilbert, and B.B. Ward. 1994. Nitrogen uptake, dissolved organic nitrogen release, and new production. Science 265: 1843–1846.
Bronk, D.A., P.M. Glibert, T.C. Malone, S. Banahan, and E. Sahlsten. 1998. Inorganic and organic nitrogen cycling in Chesapeake Bay: autotrophic versus heterotrophic processes and relationships to carbon flux. Aquatic Microbial Ecology 15: 177–189.
Bronk, D. A., and B. B. Ward 1999. Gross and net nitrogen uptake and DON release in the euphotic zone of Monterey Bay, California. Limnology and Oceanography 44: 573–585.
Bukaveckas, P.A., and W.N. Isenberg. 2013. Loading, transformation and retention of nitrogen and phosphorus in the tidal freshwater James River (Virginia). Estuaries and Coasts 36: 1219–1236.
Bukaveckas, P.A., A. Macdonald, A.K. Aufdenkampe, J.H. Chick, J.E. Havel, R.E. Schultz, T. Angradi, D.W. Bolgrien, T.M. Jicha, and D. Taylor. 2011a. Phytoplankton abundance and contributions to suspended particulate matter in the Ohio, Upper Mississippi and Missouri Rivers. Aquatic Sciences 73: 419–436.
Bukaveckas, P.A., L.E. Barry, M.J. Beckwith, V. David, and B. Lederer. 2011b. Factors determining the location of the chlorophyll maximum and the fate of algal production within the tidal freshwater James River. Estuaries and Coasts 34: 569–582.
Caraco, N.F., J.J. Cole, and D.L. Strayer. 2006. Top-down control from the bottom: Regulation of eutrophication in a large river by benthic grazing. Limnology and Oceanography 51: 664–670.
Carstensen, J., M. Sánchez-Camacho, C.M. Duarte, D. Krause-Jensen, and N. Marbà. 2011. Connecting the dots: responses of coastal ecosystems to changing nutrient concentrations. Environmental Science and Technology 45: 9122–9132.
Cerco, C., and T. Cole. 1993. 3-dimensional eutrophication model of Chesapeake Bay. Journal of Environmental Engineering-Asce 119: 1006–1025.
Cloern, J.E. 2001. Our evolving conceptual model of the coastal eutrophication problem. Marine Ecology Progress Series 210: 223–253.
Conley, D.J., H.W. Paerl, R.W. Howarth, D.F. Boesch, S.P. Seitzinger, K.E. Havens, C. Lancelot, and G.E. Likens. 2009. Controlling eutrophication: nitrogen and phosphorus. Science 323: 1014–1015.
Edwards, V.R., P. Tett, and K.J. Jones. 2003. Changes in the yield of chlorophyll a from dissolved available inorganic nitrogen after an enrichment event—applications for predicting eutrophication in coastal waters. Continental Shelf Research 23: 1771–1785.
Elser, J.J., M.M. Elser, N.A. MacKay, and S.R. Carpenter. 1988. Zooplankton-mediated transitions between N- and P-limited algal growth. Limnology and Oceanography 33: 1–14.
Elser, J.J., M.E.S. Bracken, E.E. Cleland, D.S. Gruner, W.S. Harpole, H. Hillebrand, J.T. Ngai, E.W. Seabloom, J.B. Shurin, and J.E. Smith. 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters 10: 1135–1142.
Filippino, K.C., M.R. Mulholland, P. Bernhardt, G. Boneillo, R. Morse, M. Semcheski, H.G. Marshall, N. Love, Q. Roberts, and D. Bronk. 2011. The bioavailability of effluent-derived organic nitrogen along an estuarine salinity gradient. Estuaries and Coasts 34: 269–280.
Fisher, T., A. Gustafson, K. Sellner, R. Lacouture, L. Haas, R. Wetzel, R. Magnie, D. Everitt, B. Michaels, and R. Karrh. 1999. Spatial and temporal variation of resource limitation in Chesapeake Bay. Marine Biology 133: 763–778.
Fisher, T., A. Gustafson, G. Radcliffe, K. Sundberg, and J. Stevenson. 2003. A long-term record of photosynthetically available radiation (PAR) and total solar energy at 38.60 degrees N, 78.2 degrees W. Estuaries 26: 1450–1460.
Franklin, R.B., C. Luria, L.S. Ozaki, and P.A. Bukaveckas. 2013. Community composition and activity state of estuarine bacterioplankton assessed using differential staining and metagenomic analysis of 16S rDNA and rRNA. Aquatic Microbial Ecology 69: 247–261.
Gosselain, V., J.-P. Descy, and E. Everbecq. 1994. The phytoplankton community of the River Meuse, Belgium: Seasonal dynamics (year 1992) and the possible incidence of zooplankton grazing. Hydrobiologia 289: 179–191.
Gowen, R.J., P. Tett, and K.J. Jones. 1992. Predicting marine eutrophication: the yield of chlorophyll from nitrogen in Scottish coastal waters. Marine Ecology Progress Series 85: 153–161.
Hall, S.R., M.A. Leibold, D.A. Lytle, and V.H. Smith. 2004. Stoichiometry and planktonic grazer composition over gradients of light, nutrients, and predation risk. Ecology 85: 2291–2301.
Hall, S.R., M.A. Leibold, D.A. Lytle, and V.H. Smith. 2007. Grazers, producer stoichiometry, and the light: nutrient hypothesis revisited. Ecology 88: 1142–1152.
Havens, K.E., and C.L. Schelske. 2001. The importance of considering biological processes when setting total maximum daily loads (TMDL) for phosphorus in shallow lakes and reservoirs. Environmental Pollution 113: 1–9.
Havens, K.E., J. Hauxwell, A.C. Tyler, S. Thomas, K.J. McGlathery, J. Cebrian, I. Valiela, A.D. Steinman, and S.J. Hwang. 2001. Complex interactions between autotrophs in shallow marine and freshwater ecosystems: implications for community responses to nutrient stress. Environmental Pollution 113: 95–107.
Howarth, R.W., and R. Marino. 2006. Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: evolving views over three decades. Limnology and Oceanography 51: 364–376.
Howarth, R.W., D.P. Swaney, E.W. Boyer, R. Marino, N. Jaworski, and C. Goodale. 2006. The influence of climate on average nitrogen export from large watersheds in the Northeastern United States. Biogeochemistry 79: 163–186.
Hu, S., and W.O. Smith Jr. 1998. The effects of irradiance on nitrate uptake and dissolved organic nitrogen release by phytoplankton in the Ross Sea. Continental Shelf Research 18: 971–990.
Koch, R.W., D.L. Guelda, and P.A. Bukaveckas. 2004. Phytoplankton growth in the Ohio, Cumberland and Tennessee rivers, USA: inter-site differences in light and nutrient limitation. Aquatic Ecology 38: 17–26.
Kratina, P., H.S. Greig, P.L. Thompson, T.S.A. Carvalho-Pereira, and J.B. Shurin. 2012. Warming modifies trophic cascades and eutrophication in experimental freshwater communities. Ecology 93: 1421–1430.
Lean, D.R.S., and F.R. Pick. 1981. Photosynthetic response of lake plankton to nutrient enrichment: a test for nutrient limitation. Limnology and Oceanography 26: 1001–1019.
Lewis, W.M., J.M. Melack, W.H. McDowell, M. McClain, and J.E. Richey. 1999. Nitrogen yields from undisturbed watersheds in the Americas. Biogeochemistry 46: 149–162.
Lucas, L.V., J.K. Thompson, and L.R. Brown. 2009. Why are diverse relationships observed between phytoplankton biomass and transport time? Limnology and Oceanography 54: 381–390.
Marshall, H.G., M.F. Lane, K.K. Nesius, and L. Burchardt. 2009. Assessment and significance of phytoplankton species composition within Chesapeake Bay and Virginia tributaries through a long-term monitoring program. Environmental Monitoring and Assessment 150: 143–155.
Mette, E.M., M.J. Vanni, J.M. Newell, and M.J. González. 2011. Phytoplankton communities and stoichiometry are interactively affected by light, nutrients, and fish. Limnology and Oceanography 56: 1959–1975.
Monbet, Y. 1992. Control of phytoplankton biomass in estuaries: a comparative analysis of microtidal and macrotidal estuaries. Estuaries 15: 563–571.
Mulholland, M.R., R.E. Morse, G.E. Boneillo, P.W. Bernhardt, K.C. Filippino, L.A. Procise, J.L. Blanco-Garcia, H.G. Marshall, T.A. Egerton, W.S. Hunley, K.A. Moore, D.L. Berry, and C.J. Gobler. 2009. Understanding causes and impacts of the Dinoflagellate, Cochlodinium polykrikoides, blooms in the Chesapeake Bay. Estuaries and Coasts 32: 734–747.
Murrell, M., J. Hagy, E. Lores, and R. Greene. 2007. Phytoplankton production and nutrient distributions in a subtropical estuary: importance of freshwater flow. Estuaries and Coasts 30: 390–402.
North, E.W., and E.D. Houde. 2001. Retention of White Perch and Striped Bass larvae: biological-physical interactions in Chesapeake Bay estuarine turbidity maximum. Estuaries 24: 756–769.
North, E.W., and E.D. Houde. 2003. Linking ETM physics, zooplankton prey, and fish early-life histories to striped bass Morone saxatilis and white perch M. americana recruitment. Marine Ecology Progress Series 260: 219–236.
Ochs, C.A., O. Pongruktham, and P.V. Zimba. 2013. Darkness at the break of noon: phytoplankton production in the Lower Mississippi River. Limnology and Oceanography 58: 555–568.
Parker, A.E., V.E. Hogue, F.B. Wilkerson, and R.C. Dugdale. 2012. The effect of inorganic nitrogen speciation on primary production in the San Francisco Estuary. Estuarine, Coastal and Shelf Science 104–105: 91–101.
Peierls, B.L., N.S. Hall, and H.W. Paerl. 2012. Non-monotonic responses of phytoplankton biomass accumulation to hydrologic variability: a comparison of two coastal plain North Carolina estuaries. Estuaries and Coasts 35: 1376–1392.
Price, N.M., and P.J. Harrison. 1987. Comparison of methods for the analysis of dissolved urea in seawater. Marine Biology 94: 307–317.
Ptacnik, R., T. Andersen, and T. Tamminen. 2010. Performance of the Redfield Ratio and a family of nutrient limitation indicators as thresholds for phytoplankton N vs. P limitation. Ecosystems 13: 1201–1214.
Redfield, A.C. 1958. The biological control of chemical factors in the environment. American Scientist 46: 205–221.
Ren, L., N. Rabalais, R. Turner, W. Morrison, and W. Mendenhall. 2009. Nutrient limitation on phytoplankton growth in the Upper Barataria Basin, Louisiana: microcosm bioassays. Estuaries and Coasts 32: 958–974.
Rudek, J., H.W. Paerl, M.A. Mallin, and P.W. Bates. 1991. Seasonal and hydrological control of phytoplankton nutrient limitation in the lower Neuse River Estuary, North Carolina. Marine Ecology Progress Series 75: 133–142.
Schindler, D.W. 1977. Evolution of phosphorus limitation in Lakes. Science 195: 260–262.
Seitzinger, S., C. Kroeze, A. Bouwman, N. Caraco, F. Dentener, and R. Styles. 2002a. Global patterns of dissolved inorganic and particulate nitrogen inputs to coastal systems: recent conditions and future projections. Estuaries and Coasts 25: 640–655.
Seitzinger, S.P., R.W. Sanders, and R. Styles. 2002b. Bioavailability of DON from natural and anthropogenic sources to estuarine plankton. Limnology and Oceanography 47: 353–366.
Sellers, T., and P.A. Bukaveckas. 2003. Phytoplankton production in a large, regulated river: a modeling and mass balance assessment. Limnology and Oceanography 48: 1476–1487.
Stepanauskas, R., L. Leonardson, and L.J. Tranvik. 1999. Bioavailability of wetland-derived DON to freshwater and marine bacterioplankton. Limnology and Oceanography 44: 1477–1485.
Sterner, R.W., and J.P. Grover. 1998. Algal growth in warm temperate reservoirs: kinetic examination of nitrogen, temperature, light and other nutrients. Water Research 32: 3539–3548.
Sterner, R.W., J.J. Elser, E.J. Fee, S.J. Guildford, and T.H. Chrzanowski. 1997. The light:nutrient ratio in lakes: the balance of energy and materials affects ecosystem structure and process. The American Naturalist 150: 663–684.
Strayer, D.L., M.L. Pace, N.F. Caraco, J.J. Cole, and S.E.G. Findlay. 2008. Hydrology and grazing jointly control a large-river food web. Ecology 89: 12–18.
Tamminen, T., and T. Andersen. 2007. Seasonal phytoplankton nutrient limitation patterns as revealed by bioassays over Baltic Sea gradients of salinity and eutrophication. Marine Ecology Progress Series 340: 121–138.
Vanni, M.J., A.M. Bowling, E.M. Dickman, R.S. Hale, K.A. Higgins, M.J. Horgan, L.B. Knoll, W.H. Renwick, and R.A. Stein. 2006. Nutrient cycling by fish supports relatively more primary production as lake productivity increases. Ecology 87: 1696–1709.
Wagner, T., P.A. Soranno, K.E. Webster, and D.S. Cheruvelil. 2011. Landscape drivers of regional variation in the relationship between total phosphorus and chlorophyll in lakes. Freshwater Biology 56: 1811–1824.
Ward, B.B., and D.A. Bronk. 2001. Net nitrogen uptake and DON release in surface waters: importance of trophic interactions implied from size fractionation experiments. Marine Ecology Progress Series 219: 11–24.
Wetzel, R.G. 2001. Limnology, 3rd ed, 220–224. New York: Academic.
Whalen, S.C., and P.M. Benson. 2007. Influence of nutrient reduction, light and light-nutrient interactions on phytoplankton biomass, primary production and community composition in the Neuse River, USA. Fundamental and Applied Limnology 168: 257–270.
Wiegner, T.N., S.P. Seitzinger, P.M. Gilbert, and D.A. Bronk. 2006. Bioavailability of dissolved organic nitrogen and carbon from nine rivers in the eastern United States. Aquatic Microbial Ecology 43: 277–287.
Acknowledgements
We are grateful to our field crew (Dave Hopler, William Isenberg, and Aaron Porter) for their diligence and dedication in carrying out the weekly monitoring. We also thank Ryan Weaver for assisting with the bioassay experiments, William Mac Lee who performed the nutrient analyses and KC Filippino and Margie Mulholland (ODU) who provided the urea data. We thank our colleagues at DEQ who conduct the CBP long-term monitoring program (Louis D. Seivard and his crew at the Piedmont Office) and Arthur Butt and Anne Schlegel for comments on an early draft of this manuscript. Joe Wood was supported by a fellowship through VCU’s Integrated Life Sciences PhD program. This study was supported in part by the DEQ and the City of Richmond. The views expressed herein are those of the authors and do not necessarily reflect those of the funding agencies. This paper is contribution no. 41 to the VCU Rice Center.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jacob Carstensen
Rights and permissions
About this article
Cite this article
Wood, J.D., Bukaveckas, P.A. Increasing Severity of Phytoplankton Nutrient Limitation Following Reductions in Point Source Inputs to the Tidal Freshwater Segment of the James River Estuary. Estuaries and Coasts 37, 1188–1201 (2014). https://doi.org/10.1007/s12237-013-9756-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-013-9756-3