Abstract
Over the past three decades, Narragansett Bay has undergone various ecological changes, including significant decreases in water column chlorophyll a concentrations, benthic oxygen uptake, and benthic nutrient regeneration rates. To add to this portrait of change, we measured the net flux of N2 across the sediment–water interface over an annual cycle using the N2/Ar technique at seven sites in the bay for comparison with measurements made decades ago. Net denitrification rates ranged from about 10–90 μmol N2–N m−2 h−1 over the year. Denitrification rates were not significantly different among sites and had no clear correlation with temperature. Net nitrogen fixation (−5 to −650 μmol N2–N m−2 h−1) was measured at three sites and only observed in summer (June–August). Neither denitrification nor nitrogen fixation exhibited a consistent relationship with sediment oxygen demand or with fluxes of nitrite, nitrate, ammonium, total dissolved inorganic nitrogen, or dissolved inorganic phosphate across all stations. In contrast to the mid-bay historical site where denitrification rates have declined, denitrification rates in the Providence River Estuary have not changed significantly over the past 30 years.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Integrated estimates of denitrification in coastal ecosystems are confounded by methodological difficulties, a lack of systematic understanding of the effects of the changing environmental conditions, and inadequate attention to spatial and temporal variability to provide both seasonal and annual rates. Cornwell et al. (1999).
The nitrogen (N) cycle is particularly complex and challenging to study due to the various forms of N in particulate, dissolved, and gaseous phases. An additional complication is that in the marine environment the supply of N is often limiting to primary production yet, when supplied in excess, it can lead to deleterious environmental conditions (Ryther and Dunstan 1971; Kemp et al. 2005). Whether N is the sole limiting nutrient (or if phosphorus also plays a role) in the marine environment and what the ultimate mechanism(s) responsible for N limitation in the marine environment is an area of continuing research and debate (Howarth et al. 1988a; Scott and McCarthy 2010; Howarth et al. 2011). Two opposing processes, denitrification and nitrogen fixation, are probably the main controlling mechanisms. Denitrification is the microbially mediated process that converts nitrate to dinitrogen (N2) gas. Marine denitrification has received much attention in recent years because it can remediate, at least in part, cultural eutrophication (Seitzinger et al. 1984; Seitzinger 1987; Nowicki 1994; Cornwell et al. 1999). In contrast, N-fixation is the microbial conversion of N2 gas to a bioavailable form of N. Until recently, the low rates of N fixation typically found in the marine environment were thought to account for the lack of biologically available (Howarth et al. 1988a, b; Galloway et al. 2003). However, several recent studies have found surprisingly high rates of marine N fixation using an assortment of techniques in a variety of environments (Capone 2001; Davis and McGillicuddy 2006; Gardner et al. 2006; Fulweiler et al. 2007).
As Cornwell et al. (1999), Groffman et al. (2006) and others have made clear, our understanding of denitrification across spatial and temporal scales and the mechanisms that control it are far from understood, at least in part because it is a difficult process to measure. Compared to the well-known Redfield stoichiometry of the open ocean, the low N:P ratios in coastal benthic nutrient fluxes (Nixon et al. 1976; Propp et al. 1980; Boynton and Kemp 1985) and the low N:P ratios in the water column first indicated that denitrification was an important N removal process. However, high background concentrations of N2 in the water made direct flux estimates particularly challenging (Seitzinger et al. 1984; Cornwell et al. 1999; Groffman et al. 2006). Today, direct N2 flux measurements are possible using high precision membrane inlet mass spectrometry (MIMS) and the N2/Ar technique (Sisler and Zobell 1951; Kana et al. 1994, 1998). This approach is not without its problems (e.g., Eyre et al. 2002, 2004; Groffman et al. 2006), but it has the advantage of measuring direct net fluxes of N2 compared with indirect estimates (e.g., stoichiometry or mass balance) and other direct estimates of N exchange (e.g., acetylene inhibition, 15N tracers, etc.). Regardless of the method employed, various environmental factors have been shown to influence denitrification, including sediment organic matter, water column NO3 − concentrations, bottom water oxygen concentrations, benthic microalgae and macrofauna, temperature, and salinity, (Sundback et al. 1991; Weston et al. 1996; Morlock et al. 1997; Cornwell et al. 1999; Cabrita and Brotas 2000).
The location of this study, Narragansett Bay, Rhode Island (USA), has had a pivotal role in the history of denitrification research. Nixon et al. (1976) were one of the first to observe that the dissolved inorganic nitrogen (DIN) flux stoichiometrically predicted from sediment oxygen uptake and DIP release was much higher than the measured DIN flux. The missing nitrogen was thought to have been lost as dissolved organic nitrogen (DON) or as N2 gas (Nixon et al. 1976). Sediment DON fluxes were difficult to measure then and have been generally found to be low and into the sediments (Cowan and Boynton 1996; Burdige and Zheng 1998). An exception is a recent study in a subtropical estuary (Moreton Bay, Australia) which reported DON fluxes (16–64 μmol m−2 h−1) comparable to observed DIN fluxes and generally higher than observed sediment N2 fluxes (Ferguson and Eyre 2010). The first direct measurements of N2 production (measured using gas chromatography) over an annual cycle on intact, un-amended marine sediment cores were also made at three stations in Narragansett Bay (Seitzinger et al. 1980, 1984; Seitzinger and Nixon 1985). Combining these early studies with work by Nowicki (1994) and prorating denitrification rates for the fine grained sediment area in the bay, Nixon et al. (1995) estimated that 13–26% of the DIN entering Narragansett Bay from land and atmosphere was removed through sediment denitrification.
Since those first denitrification measurements were made over 30 years ago, Narragansett Bay has undergone some dramatic ecological changes (Sullivan et al. 2001; Nixon et al. 2004, 2009; Oviatt 2004; Li et al. 2009). Most notably, mean annual water column chlorophyll a concentrations in the mid-bay have declined by about 60% and mid-bay benthic nutrient regeneration rates are substantially depressed compared with measurements in the 1970s and 1980s (Fulweiler and Nixon 2009). As part of a larger study (Fulweiler et al. 2007; Fulweiler and Nixon 2009), we returned to two of the sites measured by Seitzinger et al. (1984) and added five additional sites in upper Narragansett Bay and the Providence River Estuary to measure net N2 fluxes across the sediment water interface with the N2/Ar technique. Our purpose was to see if the changing ecology of the bay might also have impacted the major N removal process in the system. We previously reported the shift from net sediment denitrification to net sediment N fixation at some sites in Narragansett Bay (Fulweiler et al. 2007). Our purpose here is to report how net sediment N2 fluxes vary spatially and temporally in Upper Narragansett Bay and the Providence River Estuary and, when appropriate, we include the mid-bay historical site data for comparison. We then relate sediment N2 fluxes to benthic metabolism. The sites were chosen to take advantage of the north south gradient in nutrient concentrations and sediment organic matter found in the bay. We anticipated that denitrification rates would be higher in the more eutrophic portion of the upper bay and would decrease seaward.
Methods
Site description
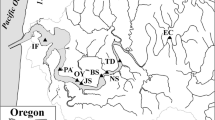
Narragansett Bay and the sites for this study have been described in detail elsewhere (Nixon et al. 1995; Fulweiler et al. 2007; Fulweiler and Nixon 2009). Briefly, Narragansett Bay is a 328 km2 phytoplankton-based temperate ecosystem (latitude 41ºN) with a mean depth of 8.6 m and a mean flushing rate of 26 days (Pilson 1985; Nixon 1995). Unlike many estuaries, freshwater input is low (100 m3 s−1), resulting in a generally well-mixed system with occasional vertical stratification (Nixon et al. 2005). Salinity is fairly uniform and only exhibits a slight down-bay gradient from around 26 psu at the mouth of the Providence River Estuary to around 32 psu at the mouth of Narragansett Bay. Mean annual bottom water concentrations (μM) of DIN and DIP are highest in the eutrophic Providence River Estuary (12.2 ± 8.4; 2.4 ± 11.6, respectively) and Upper Bay (8.8 ± 8.5; 1.6 ± 1.0) and decrease toward the mouth of the estuary (lower bay: 6.4 ± 6.9; 1.0 ± 0.4; (Kremer and Nixon 1978). The annual cycles of all of the major nutrients in near- surface and near- bottom water at a mid bay site are available at http://www.gso.uri.edu/phytoplankton/.
Silt and medium/coarse sand sediments characterized the majority of our sites (Table 1). From here forward we define Narragansett Bay proper as the main section of the estuary below Conimicut Point and excluding Mount Hope Bay and the Sakonnet Passage; Providence River Estuary is the northern most stem of the bay above Conimicut Point, and Greenwich Bay is the western arm (Fig. 1).
Sediment collection and incubation
We collected sediment samples for net N2 flux analysis at seven stations in Narragansett Bay between June 2005 and September 2006. Each station was sampled at least four times during this period and incubated in the dark at in situ temperatures ranging from 6 to 23°C, approximately the annual temperature range. Intact triplicate cores (10 cm inner diameter and 30.5 cm long) were taken at each site using either a 4.5 m long pull corer or SCUBA divers. In either case, the overlying water and delicate flocculent surface layer were retained during core collection. We took care to maintain sediment structure whenever cores were collected or moved. We transported the capped cores in ambient water-filled coolers to an environmental chamber at the Graduate School of Oceanography. The sediment cores were placed in a water bath in the dark and air was gently bubbled through the surface water overnight or until the incubation began (8–16 h). Before each incubation we carefully replaced the water overlying each core with filtered (1 μM) lower Narragansett Bay (~32 psu) water collected concomitantly with the sediment cores and maintained in the same environmental chamber. Thus, the replacement water was at the same temperature and conditions as the sediment cores. The cores were then sealed with a gas tight lid (no headspace) and gently stirred at approximately 40 rpm using magnetic stir bars attached to the core lids. Replicate water samples for N2/Ar analysis were collected at five points over the course of an incubation and preserved in exetainers with 20 μl of saturated HgCl2 solution. The exetainers were then stored under water at incubation temperature until analysis. Typically analysis was completed with 4–6 weeks of sample collection. Initial and final water samples were collected for Winkler analysis of dissolved oxygen (Carrit and Carpenter 1966). An additional core container with filtered water and no sediments was incubated as a “control”. If necessary, corrections were made for any changes that occurred in the control core, but generally these changes were negligible. Oxygen concentrations declined by at least 2 mg l−1, but incubations were stopped before oxygen dropped below 2 mg l−1. Incubations lasted between 4 and 31 h.
After the N2/Ar incubation, sediment cores were incubated for the dissolved inorganic nutrient fluxes. A complete description of these incubations has been published elsewhere (Fulweiler et al. 2010). Briefly, the overlying water was carefully removed and replaced with filtered (1 μM) lower Narragansett Bay water. The cores were then sealed with the gas-tight lid used in the N2/Ar incubations. Over the course of an incubation we collected water samples for DIN (ammonium (NH4 +), nitrate (NO3 −), nitrite (NO2 −)) and DIP (PO4 3−). We collected water samples at three time points (initial, mid, and final) using acid washed 60-ml polypropylene syringes and glass fiber filters (Whatman GF/F 0.70 μm). The filtrate was captured and stored in acid-washed polyethylene bottles and stored at −15°C until analysis on a Lachat Instrument QuikChem 8000 flow injection analyzer using standard techniques (Grasshoff 1976). Nutrient incubations lasted between 5 and 34 h depending on the season and were stopped after O2 concentrations had dropped by at least 2 mg l−1 (Fulweiler et al. 2010).
Sample and data analysis
Dissolved gas samples were analyzed with a quadrupole membrane inlet mass spectrometer that requires a small sample size (<10 ml), and no sample preparation. The instrument allows rapid throughput (~20–30 samples h−1) and provides a precision of ±0.03% (Sisler and Zobell 1951; Kana et al. 1994, 1998). For the N2/Ar method, the change in N2 concentration was determined from the change in the measured N2/Ar multiplied by the predicted water Ar concentration at air saturation (Colt 1984). N2 change for each of the triplicate cores was determined from a five-point linear regression. Rates were then prorated for the volume of water overlying the core and the sediment area of the core. The N2/Ar technique is actually a measure of net N2 flux (gross denitrification—gross N fixation). From here forward, positive N2 fluxes are considered to be a net denitrification rate, while negative N2 fluxes indicate net N fixation.
We tested for differences among sites and relationships with temperature with two-way ANOVA and analysis of co-variance using the statistical program SAS. If differences existed, we used a least significant difference (LSD) post hoc test to determine which stations were different from each other. We used an alpha level of 0.05 for all statistical tests unless noted.
Results and discussion
Spatial and temporal variability
Denitrification rates ranged from about 10–90 μmol N2–N m−2 h−1 (Table 2), with the highest mean rate observed at the inner Greenwich Bay site (Fig. 1 site 4). Rates were not significantly different among stations, confirming the surprising finding of Seitzinger et al. (1984) who also reported similar denitrification rates throughout the bay despite the more organic rich sediments in the Providence River Estuary versus Narragansett Bay proper [>4% and 2–3% C LOI, respectively; (Murray et al. 2007)] (Table 3). Low rates of N-fixation were measured at the outer Greenwich Bay site in August of 2005 and high rates of N fixation at four other sites in the summer of 2006, ranging from −5 to −650 μmol N2–N m−2 h−1 (Fig. 2; Table 2). N fixation rates were also not significantly different among sites except for Barrington (p < 0.01). The lack of any clear spatial pattern in net sediment N2 fluxes in this system is consistent with the lack of significant spatial gradients in benthic metabolism in Narragansett Bay proper as measured by oxygen uptake or dissolved inorganic nutrient regeneration (Hale 1975, Nixon et al. 1976).
While significant differences (p < 0.05) were found among sites at each temperature, no clear pattern emerged (Fig. 2; Table 4). Temperature only had a significant effect on the most anthropogenically impacted sites in the upper bay (Fig. 1 sites 1–3) and inner Greenwich Bay (Fig. 1 site 4; Table 5). No clear correlation of sediment denitrification with temperature in this system was also reported by Seitzinger et al. (Seitzinger et al. 1984).
Various studies in other systems have shown a strong seasonal pattern in denitrification, where higher temperatures increased denitrification (Jorgensen 1989; Cabrita and Brotas 2000). In addition, a variety of studies have observed a strong sediment N2 flux response to changes in organic matter deposition and/or content (Seitzinger and Giblin 1996; Caffrey et al. 1998; Cornwell et al. 1999; Fulweiler et al. 2008). Increases in sediment denitrification have also been found in mid Narragansett Bay sediments in mesocosm nutrient addition experiments (Seitzinger and Nixon 1985; Nowicki and Oviatt 1990). Thus, the lack of a significant difference in N2 fluxes between the Providence River Estuary with higher water column nutrient concentrations, primary production, and sediment organic matter and the mid bay site is puzzling. The Providence River Estuary also exhibited the highest rates of sediment oxygen demand (SOD) (Fulweiler et al. 2010). We only observed N fixation in these sediments during summer at ambient water temperatures of 17 and 22°C. This is consistent with other studies in a variety of environments which have observed a strong correlation between rates of N fixation and ambient temperature (Chapin et al. 1991; Marcarelli and Wurtsbaugh 2006; Boyd et al. 2010).
Sediment net N2 flux and benthic metabolism
Various studies have reported a strong positive relationship between SOD and denitrification rates (Seitzinger and Giblin 1996; Laursen and Seitzinger 2002; Piehler and Smyth 2011). However, we found no significant relationship between net sediment N2 fluxes and SOD for any of the stations except Barrington (Table 6). Sediment net N2 fluxes varied strongly as a function of sediment nitrite flux at Field’s Point and Barrington (R 2 = 0.77, 0.62, respectively). Sediment net N2 fluxes were not a function of sediment nitrate flux at any of the stations except Conimicut Point (Fig. 1 site 2; Table 6). At Conimicut Point, sediment nitrate uptake was positively correlated (R 2 = 0.65) with net N2 production (i.e., denitrification). This correlation between sediment nitrate uptake and net N2 production is indicative of direct denitrification.
Sediment net N2 fluxes varied significantly as a function of ammonium for each of the sites (Fig. 3, Table 6) except Inner Greenwich Bay (Fig. 1 site 4). Only in some cases were high ammonium fluxes out of the sediment accompanied by high N fixation rates (Site 1: R 2 = 0.82) and low denitrification rates by high ammonium uptake rates (Site 6: R 2 = 0.50). Net sediment N2 flux did not vary consistently with sediment DIN flux, but at Sites 1 and 3, increasing DIN efflux corresponded with high rates of N fixation (R 2 = 0.80, 0.51, respectively). Finally, net sediment N2 flux did not vary as a function of DIP flux at any of the stations except in the upper Providence River (Fig. 1 site 1), where an increase in DIP flux out of the sediment was accompanied by an increase in N fixation (Table 6).
Nitrate for denitrification can come directly from the water column and diffuse into the sediments (direct denitrification) or it can be produced by the microbial oxidation of ammonium during nitrification (coupled nitrification–denitrification) (Jenkins and Kemp 1984; Seitzinger 1988; Risgaard-Petersen 2003). In many coastal marine systems coupled nitrification–denitrification dominates and can account for 60–100% of the total denitrification (Laursen and Seitzinger 2002). Denitrification was positively correlated with nitrate uptake only at Conimicut Point (Fig. 1 site 2) suggesting that this estuary is dominated by coupled nitrification–denitrification. This is consistent with earlier work in Narragansett Bay that observed that the mean net flux of NO3 −+NO2 − was out of, rather than into, the sediments and therefore concluded direct denitrification was not important (Seitzinger et al. 1984). In addition, low summer water column nitrate concentrations make direct denitrification particularly unlikely for sediments in the mid and lower bay (Fulweiler and Nixon 2009). In fact, experimental additions of 50 μM nitrate water to sediment cores from the mid-bay did not change the net sediment N2 flux (Fulweiler et al. 2008).
Net N2 flux historical trends in Narragansett Bay
Denitrification was measured in 1978 and 1979 at two sites in Narragansett Bay with what was then a novel direct N2 method (Seitzinger et al. 1984). Briefly, this method involved the gas tight incubation of sediment cores at ambient temperature. The overlying water was repeatedly replaced until all background N2 from porewater was removed and N2 production could be measured overtime, from head space samples collected for measuring N2 concentrations on a gas chromatograph (Seitzinger et al. 1984). The historical denitrification measurements from Site 2 (Conimicut Point) and those from this study are not statistically different (Fig. 4). SOD and sediment fluxes of ammonium, nitrate, and nitrite at this site and in the upper Providence River Estuary have not changed significantly since earlier measurements in 1975 and 1976 by Elderfield et al. (1981) or in 1983 and 1984 by Nixon et al. (1990a, b) (Fulweiler et al. 2010). This is contrary to our findings from a historic site in mid Narragansett Bay (Fig. 1 site 7), where the mean annual denitrification rate significantly declined (p < 0.01) from 74 μmol N2–N m−2 h−1 in 1979 to 40 μmol N2–N m−2 h−1 in 2005 and the spring of 2006; oxygen uptake and nutrient regeneration rates had also declined markedly in 2005 compared with the 1970s (Fulweiler and Nixon 2009). This suggests that the ecological changes affecting the mid-bay site [e.g., declining chlorophyll and productivity, shifting phenology of blooms; (Fulweiler and Nixon 2009; Nixon and Fulweiler 2009)] may not be altering the more anthropogenically impacted Upper Bay and Providence River Estuary.
N2–N fluxes (mean ± SD) as a function of temperature measured in 1979 by Seitzinger (1984) (shaded circle) and those of this study (open circle) for Conimicut Point
Nitrogen fixation
At this time, we do not know what organisms were responsible for the sediment nitrogen fixation in the bay. It is unlikely that the N fixation was driven by cyanobacteria because the sediment cores were maintained in darkness and all of our incubations were conducted in the dark. Sediments from several sites (Fig. 1 sites 1, 3, 5, and 7) were also analyzed for the presence or absence of the cyanobacteria pigment, Zeaxanthin (Pinckney et al. 2001; Paerl et al. 2003). Only Site 1 at the head of the Providence River Estuary tested positive (K. Rossignol, University of North Carolina, Chapel Hill, pers. commun.). While chlorophyll a was present in the sediment at each station, we don’t know if this was epibenthic chlorophyll or settled chlorophyll from the water column (Table 1). The lack of cyanobacteria pigment at the other sites implies that they are likely not the main contributor to N fixation in Narragansett Bay. If we discount Site 1, then the observed sediment N fixation was probably primarily carried out by heterotrophic bacteria. We also measured nitrogen fixation in surface sediment (0–2 cm) at three sites (Fig. 1 sites 1, 3, and 7) with the acetylene reduction assay (ARA). Measured in this way, nitrogen fixation rates followed a similar pattern to that observed using the N2/Ar technique, with the highest rates at Site 3. The addition of molybdate, a specific inhibitor of sulphate-reducing bacteria, inhibited acetylene reduction by about 60% for Site 1 (Fulweiler et al. 2007). This suggests that the bacteria responsible for N fixation are probably sulfate reducers and that at Site 1 fixation may be due to both autotrophic and heterotrophic nitrogen fixers.
The question remains as to why bacteria in sediments presumably rich in nitrogen would need fix their own nitrogen. After all, nitrogen fixation is an energetically expensive process consuming approximately 16 mol ATP per mol of N2 fixed (Postgate et al. 1987). Traditionally, organisms that fix N tend to have lower growth rates than those who use ammonium (Postgate 1982; Yoch and Whiting 1986). In addition, it is widely assumed that ammonium concentrations control rates of N fixation through regulation of the enzyme nitrogenase. Unfortunately, we did not measure porewater ammonium concentrations in this study. However, we did measure ammonium fluxes and at the outer Greenwich Bay site and mid-Narragansett Bay site (Fig. 1 site 6 and 7, respectively) we found significant decreases ammonium flux from the sediments over the last 30 years (Fulweiler et al. 2010). This suggests that lower ammonium concentrations would also be found in the sediments, thus increasing the potential for N fixation. In addition, it is well known that O2 quickly and permanently inactivates nitrogenase, the enzyme responsible for N fixation (Paerl and Carlton 1988; Tibbles et al. 1994; Coyer et al. 1996). Estuarine sediments, such as these, maybe provide hypoxic or anoxic conditions that preserve the nitrogenase enzyme thus making N fixation possible.
Greenwich Bay and Narragansett Bay nitrogen budgets
Before this study, denitrification had not been measured in Greenwich Bay, an embayment in the northwest corner of Narragansett Bay (Fig. 1). Various nitrogen input inventories for Greenwich Bay have been developed (DiMilla et al. 2011). Granger et al. (2000) and the Rhode Island Coastal Resources Management Council (CRMC) Special Area Management Plan (SAMP) independently estimated a range of N inputs of about 10–18 million moles per year. And a recent isotopic study by DiMilla et al. (2011) reported that Narragansett Bay proper contributes approximately 54% of the DIN input to Greenwich Bay. Recent mapping has shown a variety of sediment types in Greenwich Bay, ranging from sand in the eastern Bay to fine-grained silt and silty sand in the western portion (Oakley and Boothyroyd 2006). The surface sediments (0–6 cm) from our Greenwich Bay sites were predominantly (>70%) medium sand (Table 1). Because mean site denitrification rates were not significantly different from each other we applied the average denitrification rate to the entire Greenwich Bay. On an annual basis, the mean denitrification rate for all of Greenwich Bay, about 4 × 106 mol year, could remove 20–40% of the total nitrogen input. This is similar to denitrification removal rates found in other estuaries (Seitzinger 1988) and roughly equal to the combined amount of N entering Greenwich Bay directly from the local wastewater treatment plant and from groundwater (DiMilla et al. 2011).
Determining N removal by denitrification for Narragansett Bay proper is not as straightforward as it was for Greenwich Bay. Nixon et al. (1995) previously reported an annual input of 650 million moles of N to the Bay. If we apply the mean denitrification rates of Seitzinger et al. (1984) and Nowicki (1994) to the area of fine-grained sediment in the bay as a whole (217 km2 or ~70% of Narragansett Bay), then denitrification removed approximately 13–18% of the total N input. However, Nixon et al. (2008) recently revised the annual input to 576 million moles N to Narragansett Bay. The 11% decrease is due to the handling of unmeasured and un-gauged rivers, seeps, or surface flows. If we apply the denitrification rates of Seitzinger et al. (1984) and Nowicki (1994), again restricted to the area of fine-grained sediment, to the revised total N input (576 × 106 mol N year), then denitrification previously removed 15–20% of the total N input.
The net denitrification we measured in 2005 and 2006, when applied to the area of fine grained sediment, could remove about 76 million moles annually, or about 13% of the total annual N input. The situation changed markedly in 2006, when N fixation was found in the summer months. Averaging the summer 2006 net N fixation rates from all Narragansett Bay proper sites and applying them to the area of fine grained sediment, we estimate total N fixation in June, July, and August of about 100 × 106 mol of N. This is almost twice as much as summer N inputs from direct sewage discharge and atmospheric deposition combined (Nixon et al. 2008). Sediment N fixation increased total annual N inputs by almost 16% (Table 7). If we apply the net denitrification rate of 2005/06 (76 × 106 mol year) to the remaining 9 months of the year, denitrification could remove about 57 × 106 mol of N. Thus, over the complete annual cycle, at least in 2006, the sediments added ~40 × 106 mol of N to the bay in contrast to the past, when they were a net sink removing about 80 × 106 mol year. Recent and upcoming N reductions from wastewater treatment plants may further markedly increase the importance of sediment N dynamics in the bay (Nixon et al. 2008).
Conclusion
Despite strong down bay gradients in water column inorganic nutrients, chlorophyll a, and primary production that characterize Narragansett Bay as a whole [e.g. (Oviatt et al. 2002)], we did not observe a down bay gradient in net sediment N2 fluxes. In addition, there was no clear relationship between net N2 fluxes and temperature, SOD, or benthic nutrient fluxes. In some ways, this is surprising as various studies have reported strong connections between these environmental factors and N2 fluxes [e.g., (Piehler and Smyth 2011)]. At the same time, we know that metabolic processes in the sediment can be patchy [coefficients of variation of triplicate benthic inorganic nutrient flux measurements in Narragansett Bay range from 40 to 140%; (Nixon et al. 1980)], and there are probably an equal number of studies reporting no significant relationships between N2 and common environmental parameters. It is possible that our sample size of triplicate cores was not enough to overcome the inherent variability in the system and if we increase the number of cores we may be able to observe spatial and temporal variations. For example, Bartoli et al. (2003) reported that four replicate cores were sufficient for good accuracy for measuring SOD and nitrate fluxes but that over ten replicates were needed for accurate ammonium fluxes. In addition, because the N2/Ar technique provides a net measurement of N2 flux by the entire microbial community within the sediment, it is possible that significant relationships between environmental parameters and either denitrification or N fixation may be masked in the overall net N2 flux.
The natural addition of nitrogen through sediment N fixation in Narragansett Bay leads to an interesting dilemma for scientists and managers alike. Narragansett Bay is in the process of undergoing a substantial reduction in sewage derived nitrogen as the major wastewater treatment plants move toward tertiary treatment from May to October. However, the ecology of at least the central portions of the bay where there are multi decadal records is already responding in surprising ways to climate mediated changes (Nixon et al. 2009). Narragansett Bay is good example of the complex response of ecosystems to human forces both at local (i.e., nutrient loading) and regional/global (i.e., warming water temperatures) scales. These findings highlight that as we move forward with nutrient mitigation in coastal systems we must keep in mind the dynamic nature of ecosystems—as Duarte et al. (2009) have argued, we should not be surprised by their non-linear response to change.
References
Bartoli M, Nizzoli D, Viaroli P (2003) Microphytobenthos activity and fluxes at the sediment-water interface: interactions and spatial variability. Aquat Ecol 37:341–349
Boyd PW, Strzepek R, Fu FX, Hutchins DA (2010) Environmental control of open-ocean phytoplankton groups: now and in the future. Limnol Oceanogr 55:1353–1376
Boynton WR, Kemp WM (1985) Nutrient regeneration and oxygen-consumption by sediments along an estuarine salinity gradient. Mar Ecol-Prog Ser 23:45–55
Burdige DJ, Zheng SL (1998) The biogeochemical cycling of dissolved organic nitrogen in estuarine sediments. Limnol Oceanogr 43:1796–1813
Cabrita MT, Brotas V (2000) Seasonal variation in denitrification and dissolved nitrogen fluxes in intertidal sediments of the Tagus estuary, Portugal. Mar Ecol-Prog Ser 202:51–65
Caffrey JM, Cloern JE, Grenz C (1998) Changes in production and respiration during a spring phytoplankton bloom in San Francisco Bay, California, USA: implications for net ecosystem metabolism. Mar Ecol-Prog Ser 172:1–12
Capone DG (2001) Marine nitrogen fixation: what’s the fuss? Curr Opin Microbiol 4:341–348
Carrit DE, Carpenter JH (1966) Recommendation procedure for Winkler analyses of sea water for dissolved oxygen. J Mar Res 24:313–318
Chapin DM, Bliss LC, Bledsoe LJ (1991) Environmental-regulation of nitrogen-fixation in a high arctic lowland ecosystem. Can J Bot-Revue Canadienne De Botanique 69:2744–2755
Colt J (1984) Computation of dissolved gas concentrations in water as functions of temperature, salinity, and pressure
Cornwell JC, Kemp WM, Kana TM (1999) Denitrification in coastal ecosystems: methods, environmental controls, and ecosystem level controls: a review. Aquat Ecol 33:41–54
Cowan JLW, Boynton WR (1996) Sediment-water oxygen and nutrient exchanges along the longitudinal axis of Chesapeake Bay: seasonal patterns, controlling factors and ecological significance. Estuaries 19:562–580
Coyer JA, CabelloPasini A, Swift H, Alberte RS (1996) N-2 fixation in marine heterotrophic bacteria: dynamics of environmental and molecular regulation. Proc Natl Acad Sci USA 93:3575–3580
Davis CS, McGillicuddy DJ (2006) Transatlantic abundance of the N-2-fixing colonial cyanobacterium Trichodesmium. Science 312:1517–1520
DiMilla PA, Nixon SW, Oczkowski AJ, Altabet MA, McKinney RA (2011) Some challenges of an “upside down” nitrogen budget: science and management in Greenwich Bay, RI (USA). Mar Pollut Bull 62:672–680
Duarte CM, Conley DJ, Carstensen J, Sánchez-Camacho M (2009) Return to Neverland: shifting baselines affect eutrophication restoration targets. Estuaries and Coasts 32:29–36
Elderfield H, Luedtke N, Mccaffrey RJ, Bender M (1981) Benthic flux studies in Narragansett Bay. Am J Sci 281:768–787
Eyre BD, Rysgaard S, Dalsgaard T, Christensen PB (2002) Comparison of isotope pairing and N-2: Ar methods for measuring sediment-denitrification-assumptions, modifications, and implications. Estuaries 25:1077–1087
Eyre BD, Rysgaard S, Dalsgaard T, Christensen PB (2004) Reply to comment on “Comparison of isotope pairing and N-2: Ar methods for measuring sediment denitrification”. Estuaries 27:177–178
Ferguson AJP, Eyre BD (2010) Carbon and nitrogen cycling in a shallow productive sub-tropical coastal embayment (Western Moreton Bay, Australia): the importance of pelagic-benthic coupling. Ecosystems 13:1127–1144
Fulweiler RW, Nixon SW (2009) Responses of benthic-pelagic coupling to climate change in a temperate estuary. Hydrobiologia 629:147–156
Fulweiler RW, Nixon SW, Buckley BA, Granger SL (2007) Reversal of the net dinitrogen gas flux in coastal marine sediments. Nature 448:180–182
Fulweiler RW, Nixon SW, Buckley BA, Granger SL (2008) Net sediment N-2 Fluxes in a coastal marine system-experimental manipulations and a conceptual model. Ecosystems 11:1168–1180
Fulweiler RW, Nixon SW, Buckley BA (2010) Spatial and temporal variability of benthic oxygen demand and nutrient regeneration in an anthropogenically impacted New England Estuary. Estuar Coasts 33:1377–1390
Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ (2003) The nitrogen cascade. Bioscience 53:341–356
Gardner WS, McCarthy MJ, An SM, Sobolev D, Sell KS, Brock D (2006) Nitrogen fixation and dissimilatory nitrate reduction to ammonium (DNRA) support nitrogen dynamics in Texas estuaries. Limnol Oceanogr 51:558–568
Granger S, Brush M, Buckley B, Traber M, Richardson M, Nixon SW (2000) An assessment of eutrophication in Greenwich Bay. Paper No. 1. In: M. Schwartz (ed) Restoring Water Quality in Greenwich Bay: a whitepaper series. Rhode Island Sea Grant, Narragansett, RI, p 20. http://seagrant.gso.uri.edu/G_Bay/whitepapers/GBeutrophication.pdf
Grasshoff K (1976) Methods of seawater analysis, 2nd edn. Weinheim, Chemie
Groffman PM, Altabet MA, Bohlke JK, Butterbach-Bahl K, David MB, Firestone MK, Giblin AE, Kana TM, Nielsen LP, Voytek MA (2006) Methods for measuring denitrification: diverse approaches to a difficult problem. Ecol Appl 16:2091–2122
Hale S (1975) The role of benthic communities in the nitrogen and phosphorus cycles of an estuary. In: Howell F, Gentry J, Smith M (eds) Mineral cycling in southeastern ecosystems. Nat Tech Inf Ser, US DOC, Springfield, pp 291–308
Howarth RW, Marino R, Cole JJ (1988a) Nitrogen-fixation in fresh-water, estuarine, and marine ecosystems 2. Biogeochemical controls. Limnol Oceanogr 33:688–701
Howarth RW, Marino R, Lane J, Cole JJ (1988b) Nitrogen-fixation in fresh-water, estuarine, and marine ecosystems 1. Rates and importance. Limnol Oceanogr 33:669–687
Howarth R, Chan F, Conley DJ, Garnier J, Doney SC, Marino R, Billen G (2011) Coupled biogeochemical cycles: eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front Ecol Environ 9:18–26
Jenkins MC, Kemp WM (1984) The coupling of nitrification and denitrification in 2 estuarine sediments. Limnol Oceanogr 29:609–619
Jorgensen KS (1989) Annual Pattern of denitrification and nitrate ammonification in estuarine sediment. Appl Environ Microbiol 55:1841–1847
Kana TM, Darkangelo C, Hunt MD, Oldham JB, Bennett GE, Cornwell JC (1994) Membrane inlet mass-spectrometer for rapid high-precision determination of N-2, O-2, and ar in environmental water samples. Anal Chem 66:4166–4170
Kana TM, Sullivan MB, Cornwell JC, Groszkowski KM (1998) Denitrification in estuarine sediments determined by membrane inlet mass spectrometry. Limnol Oceanogr 43:334–339
Kemp WM, Boynton WR, Adolf JE, Boesch DF, Boicourt WC, Brush G, Cornwell JC, Fisher TR, Glibert PM, Hagy JD, Harding LW, Houde ED, Kimmel DG, Miller WD, Newell RIE, Roman MR, Smith EM, Stevenson JC (2005) Eutrophication of Chesapeake Bay: historical trends and ecological interactions. Mar Ecol-Prog Ser 303:1–29
Kremer JN, Nixon SW (1978) A coastal marine ecosystem: simulation and analysis. Springer, New York
Laursen AE, Seitzinger SP (2002) The role of denitrification in nitrogen removal and carbon mineralization in Mid-Atlantic Bight sediments. Cont Shelf Res 22:1397–1416
Li L, Yao X, Sun CH, Du AJ, Cheng LN, Zhu ZH, Yu CZ, Zou J, Smith SC, Wang P, Cheng HM, Frost RL, Lu GQM (2009) Lithium-catalyzed dehydrogenation of ammonia borane within mesoporous carbon framework for chemical hydrogen storage. Adv Funct Mater 19:265–271
Marcarelli AM, Wurtsbaugh WA (2006) Temperature and nutrient supply interact to control nitrogen fixation in oligotrophic streams: an experimental examination. Limnol Oceanogr 51:2278–2289
Morlock S, Taylor D, Giblin A, Hopkinson C, Tucker J (1997) Effect of salinity on the fate of inorganic nitrogen in sediments of the Parker river estuary, Massachusetts. Biol Bull 193:290–292
Murray DW, Prell WL, Rincon CE, Saarman E (2007) Physical properties and chemical characteristics of surface sediment grab samples from Narragansett Bay and the Providence and Seekonk Rivers, a summary of the Brown University Narragansett Bay Sediment Project (BUNBSP). http://www.nbep.org/publications/nbep07-127OPTIMIZED.pdf
Nixon SW (1995) Coastal marine eutrophication: a definition, social causes, and future concerns. Ophelia 41:199–219
Nixon SW, Fulweiler RW (2009) Nutrient pollution, eutrophication, and the degradation of coastal marine ecosystems. In: Duarte CM (ed) Global loss of coastal habitats: rates, causes and consequences. Fundacion BBVA, Bilbao
Nixon SW, Oviatta CA, Hale S (1976) Nitrogen regeneration and the metabolism of coastal bottom communities. In: Anderson JM, Macfayden A (eds) The role of terrestrial and aquatic organisms in decomposition processes. Blackwell Scientific Publications, Oxford
Nixon SW, Kelly J, Furnas B, Oviatt C, Hale S (1980) Phosphorus regeneration and the metabolism of coastal marine bottom communities. In: Tenore KR, Coull BC (eds) Marine benthic dynamics. Univ. of South Carolina Press, Columbia, pp 219–242
Nixon S, Nowicki B, Buckley B (1990a) Report the the Narragansett Bay project on the measurement of sediment oxygen demand in the Providence River, Narragansett
Nixon S, Nowicki B, Buckley B (1990b) Report to the Narragansett Bay Project on the measurement of nutrient flux data obtained from incubations of the Providence River benthos, Rhode Island Sea Grant, Narragansett
Nixon SW, Granger SL, Nowicki BL (1995) An assessment of the annual mass balance of carbon, nitrogen, and phosphorus in Narragansett Bay. Biogeochemistry 31:15–61
Nixon SX, Granger S, Buckley BA, Lamont M, Rowell B (2004) A one hundred and seventeen year coastal water temperature record from Woods Hole, Massachusetts. Estuaries 27:397–404
Nixon SW, Granger SL, Buckley BA, Harris L, Oczkowski A, Cole L, Fulweiler RW (2005) Anthropogenic nutrients to Narragansett Bay: a twenty-five year perspective: a report to the Narragansett Bay Commission and Rhode Island Sea Grant
Nixon S, Buckley B, Granger S, Harris L, Oczkowski A, Fulweiler R, Cole L (2008) Nitrogen and phosphorus inputs to Narragansett Bay: past, present, and future. In: Desbonnet A, Costa-Pierce B (eds) Science for ecosystem-based management, Narragansett Bay in the 21st Century. Springer, New York, pp 101–175
Nixon SW, Fulweiler RW, Buckley BA, Granger SL, Nowicki BL, Henry KM (2009) The impact of changing climate on phenology, productivity, and benthic-pelagic coupling in Narragansett Bay. Estuar Coast Shelf Sci 82:1–18
Nowicki BL (1994) The effect of temperature, oxygen, salinity, and nutrient enrichment on estuarine denitrification rates measured with a modified nitrogen gas flux technique. Estuar Coast Shelf Sci 38:137–156
Nowicki BL, Oviatt CA (1990) Are estuaries traps for anthropogenic nutrients: evidence from estuarine mesocosms. Marine Ecology-Progress Series 66:131–146
Oakley BA, Boothyroyd JC (2006) Benthic geologic habitats of Greenwich Bay, R.I. Geological Society of America, p 11
Oviatt CA (2004) The changing ecology of temperate coastal waters during a warming trend. Estuaries 27:895–904
Oviatt C, Keller A, Reed L (2002) Annual primary production in Narragansett Bay with no bay-wide winter-spring phytoplankton bloom. Estuar Coast Shelf Sci 54:1013–1026
Paerl HW, Carlton RG (1988) Control of nitrogen-fixation by oxygen depletion in surface-associated microzones. Nature 332:260–262
Paerl HW, Valdes LM, Pinckney JL, Piehler MF, Dyble J, Moisander PH (2003) Phytoplankton photopigments as indicators of estuarine and coastal eutrophication. Bioscience 53:953–964
Piehler MF, Smyth AR (2011) Habitat-specific distinctions in estuarine denitrification affect both ecosystem function and services. Ecosphere 2:1–16
Pilson MEQ (1985) On the residence time of water in Narragansett Bay. Estuaries 8:2–14
Pinckney JL, Richardson TL, Millie DF, Paerl HW (2001) Application of photopigment biomarkers for quantifying microalgal community composition and in situ growth rates. Org Geochem 32:585–595
Postgate JR (1982) Biological nitrogen-fixation: fundamentals. Philos Trans R Soc London Ser B-Biol Sci 296:375–385
Postgate JR, Elmerich C, Sprent J, Kennedy C, Johnston AWB, Nutman PS, Stouthamer AH, Eady RR, Witty J, Newton WE, Pickett C, Thorneley RNF, Leigh JG, Hill S, Becking J (1987) A century of nitrogen-fixation research: present status and future-prospects; general discussion. Philos Trans R Soc London Ser B-Biol Sci 317:291–294
Propp MV, Tarasoff VG, Cherbadi I, Lootzkik NK (1980) Benthic pelagic oxygen and nutrient exchange in a coastal region of the Sea of Japan. In: Tenore KR, Coull BC (eds) Marine benthic dynamics. Univ. of South Carolina Press, Columbia
Risgaard-Petersen N (2003) Coupled nitrification-denitrification in autotrophic and heterotrophic estuarine sediments: on the influence of benthic microalgae. Limnol Oceanogr 48:93–105
Ryther JH, Dunstan WM (1971) Nitrogen, phosphorus, and eutrophication in the coastal marine environment. Science 171:1008–1013
Scott JT, McCarthy MJ (2010) Nitrogen fixation may not balance the nitrogen pool in lakes over timescales relevant to eutrophication management. Limnol Oceanogr 55:1265–1270
Seitzinger SP (1987) Nitrogen biogeochemistry in an unpolluted estuary: the importance of benthic denitrification. Mar Ecol-Prog Ser 41:177–186
Seitzinger SP (1988) Denitrification in fresh-water and coastal marine ecosystems: ecological and geochemical significance. Limnol Oceanogr 33:702–724
Seitzinger SP, Giblin AE (1996) Estimating denitrification in North Atlantic continental shelf sediments. Biogeochemistry 35:235–260
Seitzinger SP, Nixon SW (1985) Eutrophication and the rate of denitrification and N2O production in coastal marine-sediments. Limnol Oceanogr 30:1332–1339
Seitzinger S, Nixon S, Pilson MEQ, Burke S (1980) Denitrification and N2O production in near-shore marine-sediments. Geochim Cosmochim Acta 44:1853–1860
Seitzinger SP, Nixon SW, Pilson MEQ (1984) Denitrification and nitrous-oxide production in a coastal marine ecosystem. Limnol Oceanogr 29(1):73–83
Sisler FD, Zobell CE (1951) Nitrogen fixation by sulfate reducing bacteria indicated by Nitrogen/Argon ratios. Science 113:511
Sullivan BK, Van Keuren D, Clancy M (2001) Timing and size of blooms of the ctenophore Mnemiopsis leidyi in relation to temperature in Narragansett Bay, RI. Hydrobiologia 451:113–120
Sundback K, Enoksson V, Graneli W, Pettersson K (1991) Influence of sublittoral microphytobenthos on the oxygen and nutrient flux between sediment and water: a laboratory continuous-flow study. Mar Ecol-Prog Ser 74:263–279
Tibbles BJ, Lucas MI, Coyne VE, Newton ST (1994) Nitrogenase activity in marine-sediments from a temperate salt-marsh lagoon: modulation by complex polysaccharides, ammonium and oxygen. J Exp Mar Biol Ecol 184:1–20
Weston N, Carini S, Giblin A, Banta G, Hopkinson C, Tucker J (1996) Estimating denitrification in sediments of the Parker River estuary, Massachusetts. Biol Bull 191:334–335
Yoch DC, Whiting GJ (1986) Evidence for Nh4 + switch-off regulation of nitrogenase activity by bacteria in salt-marsh sediments and roots of the grass spartina-alterniflora. Appl Environ Microbiol 51:143–149
Acknowledgments
The authors would like to thank Luke Cole for the map. In addition, we thank the editor who handled this manuscript for their helpful guidance as well as two anonymous reviewers for their thoughtful and insightful comments that much improved this manuscript. For field assistance, we thank S. Granger, P. DiMilla, K. Hanks, K. Henry, M. Horn, J. Krumholz, C. Mueller, A. Oczkowski, A. Pimenta, M. Traber, and N. Hovey.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fulweiler, R.W., Nixon, S.W. Net sediment N2 fluxes in a southern New England estuary: variations in space and time. Biogeochemistry 111, 111–124 (2012). https://doi.org/10.1007/s10533-011-9660-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-011-9660-5