Abstract
Since the middle of 1990s the trend of Lake Balaton towards an increasingly trophic status has been reversed, but N2-fixing cyanobacteria are occasionally dominant, endangering water quality in summer. The sources of nitrogen and its uptake by growing phytoplankton were therefore studied. Experiments were carried out on samples collected from the middle of the Eastern (Siófok) and Western (Keszthely) basins between February and October 2001. Ammonium, urea and nitrate uptake and ammonium regeneration were measured in the upper 5-cm layer of sediment using the 15N-technique. Ammonium was determined by an improved microdiffusion assay. N2 fixation rates were measured by the acetylene-reduction method. Ammonium regeneration rates in the sediment were similar in the two basins. They were relatively low in winter (0.13 and 0.16 μg N cm−3 day−1 in the Eastern and Western basin, respectively), increased slowly in the spring (0.38 and 0.45 μg N cm−3 day−1) and peaked in late summer (0.82 and 1.29 μg N cm−3 day−1, respectively). Ammonium uptake was predominant in spring in the Eastern basin and in summer in the Western basin, coincident with the cyanobacterial bloom. The amount of N2 fixed was less than one third of the internal load during summer when external N loading was insignificant. Potentially, the phytoplankton N demand could be supported entirely by the internal N load via ammonium regeneration in the water column and sediment. However, the quantity of N from ammonium regeneration in the upper layer of sediment combined with that from the water column would limit the standing phytoplankton crop in spring in both basins and in late summer in the Western basin, especially when the algal biomass increases suddenly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lake Balaton is the largest shallow lake in Central Europe with a surface area of 594 km2 and a mean depth of 3.5 m. The lake is of international significance as an environmental resource and of great importance to the Hungarian economy as a recreational resort, visited by four million tourists annually; apart from Budapest it is the most important source of tourist income in Hungary. Because of increased nutrient loading during the 1980s the Western part became hypertrophic, the Central and Eastern basins eutrophic (Herodek, 1986). Several measures have been taken to reduce the nutrient load, particularly phosphorus. Reservoirs have been built on the main tributaries feeding Lake Balaton. Phosphorus precipitation has been applied at the sewage treatment plants, and a sewage transfer pipe system diverts wastewater from the catchment area of the lake. Pollution from liquid manure has been stopped and fertiliser use has been reduced. The biologically available phosphorus loads in the Eastern and Western basins have decreased by 60 and 30%, respectively (Somlyódy et al., 1997). Since the most recent bloom in 1994 the phytoplankton biomass has been low, the Eastern basin of the lake has become mesotrophic, while the Western basin remains eutrophic. However, since the algal biomass has decreased, the presence and often the dominance of nitrogen-fixing and potentially toxic cyanobacteria in summer give rise to concern. The most probable reason for their success is that they benefit from extremely warm summers, they are able to take up P very rapidly and they may be able to store it. They also benefit from the low N to P ratio and ability to fix molecular nitrogen as an additional N source if necessary. Due to nitrogen deficiency may contribute to the development of these species (Blomqvist et al., 1994), it seemed worthwhile to study the importance of the different nitrogen sources used by the phytoplankton. Following earlier studies of ammonium regeneration in the water column and sediment (Présing et al., 2001b), the present study was designed to estimate ammonium regeneration in sediment over several seasons, using a newly established measurement method and comparing it with the external nitrogen load in the lake.
Materials and methods
Experiments were carried out between February and October 2001 (Fig. 1) on samples collected from the middle of the Western and Eastern basins of the lake. Details of the methods used for sampling, chlorophyll-a and nutrient analysis of integrated water samples were published previously (Présing et al., 2001b). Ambient nutrient and chlorophyll-a concentrations were determined in three replicates with coefficients of variation less than 10 and 5%, respectively. The mean values of ammonium, urea and nitrate concentrations were compared with paired sample t-test. Ammonium and nitrate concentrations in interstitial water and extractable ammonium in the sediment layers were determined as follows: for each experiment, 10 sediment cores were sliced at 5 cm intervals and the corresponding layers were mixed. From each homogenised sediment layer, 40 g was immediately centrifuged for 10 min at 4,000 rpm in a Hermle Z 320 centrifuge. Extractable ammonium was analysed in another 40 g sample of the same layers. The sediment was mixed with 10 ml of 2 M KCl, incubated for 1 h and centrifuged. The centrifugation supernatants were used for chemical analysis as described above. Ammonium standards were prepared in an equivalent concentration of KCl.
Phytoplankton samples taken from the integrated water samples from the basins were preserved in Lugol’s solution. Algal species were enumerated with an inverted plankton microscope (Utermöhl, 1958). The wet weight of each species was calculated from cell volumes (Németh & Vörös, 1986). At least 25 cells (or filaments) of each species were measured to determine biomass and at least 400 were counted. N2 fixation in both basins was measured by a modified acetylene-reduction technique (Présing et al., 2001a) in the middle of June, July and August. Carbon uptake was estimated using a model developed for the lake (Vörös & V.-Balogh, 1998) based on water temperature and chlorophyll-a concentration. The methods for measuring phytoplankton ammonium, urea and nitrate uptake and the calculations of nitrogen uptake rates were detailed previously (Présing et al., 2001a). The nitrogen contents and 15N/14N ratios of dried seston samples (60°C) were determined by an automated elemental analyser interfaced with an Isotope Ratio Mass Spectrometer (ANCA-MS, Europa Scientific Ltd., UK). Ammonium, urea and nitrate uptake per unit surface area was calculated from the uptake velocities measured at optimal light intensity (110 μmol photon m−2 s−1) at the average depth of the basins. In order to obtain daily uptake, the conversion factors described previously (Présing et al., 2001a) were used, taking account of global irradiation, vertical light attenuation and day–night rhythm.
Ammonium regeneration in the sediment was measured by 15N dilution. The upper 5 cm layers from 10 sediment cores were mixed and supplemented with 15NH4Cl. Filtered lake water (20 ml) was pipetted on top of the mixed, labelled 40 g samples in centrifuge tubes. The samples were incubated in the dark for 1–12 days at the lake temperature. After incubation, the samples were mixed with an equal volume of 2 M KCl for 1 h and then centrifuged. The supernatant was used for microdiffusion and chemical analysis. Ammonium was measured in the supernatant by an improved microdiffusion method after Brooks et al. (1989) and Slawyk & Raimbault (1995) using our modified ammonium trap. Supernatant (10–30 ml) was pipetted into incubation bottles (Duran Schott, 100 ml volume) and 10 mg of MgO and 5 glass beads (4 mm) were added. An aluminium capsule was fixed under the acidified (25 μl of 0.5 N H2SO4) filter trap, separated from the top of the incubation bottle with a specially bent stainless steel wire, to gather the occasional drops and avoid loss of ammonium. The bottles were gently shaken with a vertical shaker for 10 days at 24°C, then the filters were dried for 1–2 days in a helium atmosphere in desiccators containing H3PO4. The dried filters were placed in the same capsule. The regeneration rate was calculated on the basis of the 15N dilution of ammonium at T 0, i.e. the start of the experiment, when the KCl was added. 15N enrichment was analysed using the same isotope ratio mass spectrometer used to measure nitrogen uptake.
Results
Temperature and phytoplankton succession in Lake Balaton
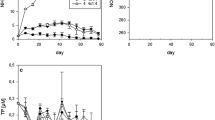
After a relatively mild winter the water temperature of the lake rose to 10°C in March, and a cold April 2001 was followed by a second period of increasing temperature in May (Fig. 1). The water temperature reached 20°C by the middle of June and remained above this level until the beginning of September. In July and August the water temperature reached or exceeded 24–25°C often and for long periods. From September to December the lake cooled rapidly. In the Eastern basin, chlorophyll-a concentrations were low, ranging between 2 and 20 μg l−1. There were two small maxima, one in early spring and the other in late summer–autumn (August–September). In the Western basin, chlorophyll-a concentrations were higher and peaked at around 30 μg l−1 at the end of February and at 75 μg l−1 in August–September. During the period under investigation the phytoplankton biomass varied between 0.5 and 6.1 mg l−1 in the Eastern basin, and Heterocontophyta dominated in spring (Fig. 2a). Of the two classes included in this division, Bacillariophyceae formed the overwhelming majority with a predominance of centric diatoms (Cyclotella spp.), while Chrysophyceae were negligible. In early summer, the phytoplankton community became more diverse with dinoflagellates increasingly noteworthy: (Ceratium hirundinella (O.F.M.) Schrank) and the N2-fixing cyanobacterium (Aphanizomenon flos-aquae (L.) Ralfs). In August and September, N2-fixing cyanobacteria (A. flos-aquae and Cylindrospermopsis raciborskii (Wołosz.) Seenayya and Subba Raju) dominated. In October, filamentous cyanobacteria disappeared; centric diatoms, cryptophytes and picocyanobacteria became the dominant species. Total phytoplankton biomass was much higher in the Western basin of the lake, varying between 1.4 and 21 mg l−1 (Fig. 2b). In April, centric diatoms prevailed, but towards summer they were slowly replaced by cryptophytes (Rhodomonas minuta Skuja), dinoflagellates (C. hirundinella) and N2-fixing blue-greens (Anabaena aphanizomenoides Forti, A. flos-aquae). Phytoplankton biomass was highest (14–21 mg l−1) in August and was dominated by N2-fixing cyanobacteria, A. flos-aquae, Aphanizomenon issatschenkoi (Ussach.) Prosk.-Lavr. and C. raciborskii. From September, the total biomass decreased markedly and cryptophytes along with chlorophytes were the most abundant organisms.
Pelagic inorganic nitrogen and urea concentration, uptake rates and nitrogen fixation
Annual mean concentrations of ammonium (8.01 ± 3.98 μg N l−1) and urea (21.98 ± 10.95 μg N l−1) in pelagic water of both basins were low the entire year and did not follow a seasonal pattern. Nitrate concentrations changed during the year in both basins with higher values from late autumn to spring (55.6 ± 21.7 μg N l−1) and significantly (P < 0.001) lower ones during summer (5.7 ± 2.53 μg N l−1). Nitrate and ammonium concentrations were similarly low and did not differ significantly (P > 0.05) in summer. However annual mean concentrations of urea were also relatively low, significantly (P < 0.001) exceeded those of ammonium and summer values of nitrate. Nitrate concentrations from autumn to spring were far above (P < 0.001) the highest ambient nitrogen concentrations of other N-forms during the whole year.
The results of the nitrogen uptake experiments are shown in Table 1. In the Eastern basin the uptake rates were mostly below 1 μg N l−1 h−1 with peaks of 3.24 μg N l−1 h−1 for ammonium in August. Because of the higher algal biomass, uptake rates were higher in the Western than the Eastern basin, especially for ammonium at the time of the cyanobacterial bloom in August (6.18 μg N l−1 h−1). For the most part, ammonium assimilation was the most pronounced, followed by nitrate and/or urea uptake. However, the nitrate uptake rate exceeded that of ammonium in the Western basin in February and in the Eastern basin in June. In the Eastern basin with only one exception, and in the Western basin always, the V max was highest for ammonium. The greatest values were determined in May and August in both basins. On the basis of the calculated daily uptake per unit surface area, ammonium was taken up in highest amount in both basins between March and October. Its contribution to the daily nitrogen supply of the phytoplankton usually exceeded 50% (up to 66% in May). The contribution of urea to the daily nitrogen uptake was between 18 and 53% and was the largest portion of daily nitrogen demand in June. While ammonium was the preferred nitrogen source of phytoplankton, urea sometimes contributed more than ammonium to daily total nitrogen uptake during 2001 because the ambient urea concentrations were higher. Nitrate uptake was not dominant in the nitrogen supply to the phytoplankton. In the Eastern basin, its highest value (24 mg N m−2 day−1 in June) represented 31% of total daily uptake. At other times it contributed only 4–15%. N2 fixation was negligible in June and July (only 2–5%) and reached only 5% even when 70% of the phytoplankton biomass comprised N2-fixing cyanobacteria. The amount of fixed nitrogen was only a few percent of the total nitrogen demand of the phytoplankton. In the Western basin the ratios of daily ammonium, urea and nitrate uptake per unit surface area to the total nitrogen utilisation by phytoplankton were similar to those in the Eastern basin. Nitrogen fixation played a more important role in the Western than the Eastern basin. Considering the measured N fixation rate (50.68 mg N m−2 day−1) and the surface area of the Western basin (38 km2), the amount of nitrogen fixed during August may be about 60 ton.
External nitrogen load and ammonium generation in the sediment
It was assumed that the external load of the Western basin was determined by the single significant inflow, the River Zala, which transports up to 90% of the total nutrient load of the basin. The load was calculated from daily water discharge and nutrient concentrations and was summarised monthly (Table 2). It showed a clear annual trend, with higher quantities in autumn and winter and lowest in the summer months. The total DIN charged by Zala to the lake was only 0.22 ton in August.
At the end of February, when the lake temperature was 5°C, the daily ammonium productions per cm3 wet sediment were 0.13 and 0.16 μg in the Eastern and Western basins, respectively. This means that ammonium regeneration in the upper 5-cm layer of 1 m2 sediment can produce 6.6 and 8 mg nitrogen daily in the Eastern and Western basins, respectively (Fig. 3). By the time of the experiments in spring, the water temperature had increased to 10°C in the Eastern basin and to 15°C in the shallower Western basin. The daily ammonium production increased to 0.4 μg N cm−3 wet sediment, which meant around 20 mg m−2 of ammonium nitrogen production in both basins. At the end of June the water temperature in both basins was 20°C. Ammonium regeneration per cm3 wet weight was 1.15 and 0.82 μg, and 57.3 and 41.2 mg m−2, in the Eastern and Western basins, respectively. At the time of the experiments in August the water in the Eastern basin was at 23°C while that in the shallower Western basin was at 26°C. Ammonium regeneration values in the Eastern basin were close to those measured in June. In the Western basin the ammonium production had almost doubled and reached its yearly maximum of 1.3 μg cm−3 and 64.2 mg m−2. Scaling this over the 38 km2 surface area of the Western basin, the amount of nitrogen originating from the sediment via ammonium regeneration may reach 75 ton during August. By the end of October, the water temperature had dropped to around 10°C. In the Eastern part of the lake the ammonium regeneration fell to half of that measured in the summer. In the Western basin the decrease was slower and the values remained around 1 μg cm−3 and 53.6 mg m−2.
Discussion
Kinetic parameters (K s and V max) determined in our experiments are conventionally used to evaluate the possible nutrient limitation or substrate affinity of phytoplankton. Maximal uptake velocity (V max) rather than the half saturation constant (K s) was used to characterise nitrogen uptake; determination of K s is less accurate and the value generally increases with increasing V max (Takamura et al., 1987), which is a more independent indicator and usually increases with increasing nitrogen limitation (Lomas et al., 1996). The initial slope of the uptake rate versus nutrient concentration curve (α) is also thought to be a better indicator of nutrient competition than K s alone (Cochlan & Harrison, 1991). Based on these kinetic parameters the order of preference of phytoplankton for nitrogen forms in both basins of Lake Balaton was ammonium > urea ≫ nitrate uptake. The highest affinity of algae for nitrogen especially for ammonium occurred in spring and coinciding with the cyanobacterial blooms in summer in both basins. Generally, the contribution of urea to the total N utilised by the phytoplankton is intermediate between those of ammonium and nitrate, somewhat closer to the former (Takamura et al., 1987; Mitamura et al., 1995). The contribution of urea can be as much as 70–80% of the daily nitrogen demand (Glibert et al., 1991). Measurements during 1998 showed that the contribution of urea to phytoplankton nitrogen supply in Lake Balaton reached 50–70% of ammonium uptake (Présing et al., 2001a). The contributions of reduced nitrogen forms (ammonium and urea) were far more important than that of nitrate, which was some 10% of the daily nitrogen supply to the algae. However, the contribution of nitrogen fixation was also around 10% but this can be a critically important additional nitrogen source for cyanobacteria, conferring a competitive advantage.
The measured ammonium regeneration rates are close to the very few values in the literature (Bowden, 1984). One reason why there are so few ammonium regeneration results based on direct measurements is the lack of appropriate method. Our modified microdiffusion method has proved suitable for both extraction and concentration of ammonium. It does not need specialised laboratory equipment and gives 80–100% recoveries.
We assumed that the nitrogen assimilated by phytoplankton can be calculated from its primary production and Redfield’s classical C to N (weight:weight) ratio of 5.6. By this calculation we estimated the total phytoplankton N demand (open columns in Fig. 3) independently of our different N uptake measurements. In the water column, approximately 50% of primary phytoplankton production can be utilised by bacteria (Vörös et al., 1996). On average, a similar proportion of the phytoplankton nitrogen demand was provided by ammonium regeneration in the water column (Présing et al., 2001b). The balance of the nitrogen demand may be supplied by ammonium regeneration in the sediment together with external sources, such as inflowing water and fixation of atmospheric nitrogen. From autumn to spring, nitrogen demand can be met by the relatively high inflowing nitrogen load (Table 2). In summer, especially in the Western part of the lake, phytoplankton nitrogen demand is met only by adding the input from nitrogen fixation to the ammonium supplied by elevated regeneration. It should be noted that these calculations do not consider N loss via denitrification. A portion of the regenerated ammonium will be oxidised to nitrate. This, together with exogenous nitrate, will be subject to loss via denitrification, which will result in even less available combined nitrogen to support the standing crop.
Potentially, more of the phytoplankton nitrogen demand could be met by inflowing external load and by internal sources via ammonium regeneration, which occurs in the water column and the upper layer of sediment. These sources could barely supply enough combined nitrogen to maintain the standing phytoplankton crop in late summer, especially in the Western basin, when the algal biomass increases rapidly, and at the same time compensate for nitrogen loss by denitrifcation. External nitrogen load is insignificant in summer, when the atmospheric N2 can supplement available nitrogen, but only for cyanobacteria capable of nitrogen fixation. The impact of nuisance algae may possibly be minimised by managing the nutrient loading: decreasing the external P while not reducing the external N load.
References
Blomqvist, P., A. Peterson & P. Hyenstrand, 1994. Ammonium nitrogen: A key regulatory factor causing dominance of non-nitrogen-fixing cyanobacteria in aquatic systems. Archiv für Hydrobiologie 132: 141–164.
Bowden, W. B., 1984. A nitrogen-15 isotope dilution study of ammonium production and consumption in a marsh sediment. Limnology and Oceanography 29: 1004–1015.
Brooks, P. D., J. M. Stark, B. B. McInteer & T. Preston, 1989. Diffusion method to prepare soil extracts for automated nitrogen-15 analysis. Soil Science Society of America Journal 53: 1707–1711.
Cochlan, W. P. & P. J. Harrison, 1991. Kinetics of nitrogen (nitrate, ammonium and urea) uptake by the picoflagellate Micromonas pusilla (Prasinophyceae). Journal of Experimental Marine Biology and Ecology 153: 129–141.
Glibert, P. M., C. Garside, J. A. Fuhrman & M. R. Roman, 1991. Time-dependent coupling of inorganic and organic nitrogen uptake and regeneration in the plume of the Chesapeake Bay estuary and its regulation by large heterotrophs. Limnology and Oceanography 36: 895–909.
Herodek, S., 1986. Phytoplankton changes during eutrophication and P and N metabolism. In Somlyódy, L. & G. van Straten (eds), Modeling and Managing Shallow Lake Eutrophication. Springer Verlag, Berlin, 183–204.
Lomas, M. W., P. M. Glibert, G. M. Berg & M. Burford, 1996. Characterization of nitrogen uptake by natural populations of Aureococcus anophagefferens (Chrysophyceae) as a function of incubation duration, substrate concentration, light and temperature. Journal of Phycology: 32: 907–916.
Mitamura, O., Y. Saijo, K. Hino & F. A. R. Barbosa, 1995. The significance of regenerated nitrogen for phytoplankton productivity in the Rio Doce Valley Lakes, Brazil. Archiv für Hydrobiologie 134: 179–194.
Németh, J. & L. Vörös, 1986. Koncepció és módszertan felszíni vizek algológiai monitoringjához. OKTH, Budapest, 135 pp. (in Hungarian).
Présing, M., S. Herodek, T. Preston & L. Vörös, 2001a. Nitrogen uptake and the importance of internal nitrogen loading in Lake Balaton. Freshwater Biology 46: 125–139.
Présing, M., T. Preston, A. Kovács & P. Sprőber, 2001b. Internal and external nitrogen supply of phytoplankton in Lake Balaton (Hungary). Biwako 2001, 9th International Conference on the Conservation and Management of Lakes, Kyoto, Japan. Partnerships for Sustainable Life in Lake Environments, Conference Proceedings, Session 5: 272–275.
Slawyk, G. & P. Raimbault, 1995. Simple procedure for simultaneous recovery of dissolved inorganic and organic nitrogen in 15N-tracer experiments and improving the isotopic mass balance. Marine Ecology Progress Series 124: 289–299.
Somlyódy, L., S. Herodek, Cs. Aradi, A. Clement, Gy. Dévai, V. Istvánovics & Gy. Varga, 1997. Revision of the Lower Kis-Balaton Reservoir. Synthesis Report, Technical University, Budapest.
Takamura, N., T. Iwakuma & M. Yasuno, 1987. Uptake of 13C and 15N (ammonium, nitrate and urea) by Microcystis in Lake Kasumigaura. Journal of Plankton Research 9: 151–165.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitteilungen Internationale Vereinigung für Theoretische und Angewandte Limnologie 9: 1–38.
Vörös, L. & K. V.-Balogh, 1998. A Balaton Keszthelyi-medencéjének szénforgalma. Hidrológiai Közlöny 78: 385–386 (in Hungarian).
Vörös, L., K. V.-Balogh & S. Herodek, 1996. Microbial food web in a large shallow lake (Lake Balaton, Hungary). Hydrobiologia 339: 57–65.
Acknowledgements
This work was supported by the Office of the Prime Minister of Hungary (MeH). Support from NATO in the form of travelling expenses to Tom Preston is gratefully acknowledged. We are very grateful to Gerd Slawyk for consultation about the microdiffusion method. We wish to thank the Western-Transdanubian Environmental and Water Authority for nutrient loading data. We are extremely grateful for Terézia Horváth, Erika Kozma and Géza Dobos for their assistance with the sampling and laboratory experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: T. Nõges, R. Eckmann, K. Kangur, P. Nõges, A. Reinart, G. Roll, H. Simola & M. Viljanen
European Large Lakes—Ecosystem changes and their ecological and socioeconomic impacts
Rights and permissions
About this article
Cite this article
Présing, M., Preston, T., Takátsy, A. et al. Phytoplankton nitrogen demand and the significance of internal and external nitrogen sources in a large shallow lake (Lake Balaton, Hungary). Hydrobiologia 599, 87–95 (2008). https://doi.org/10.1007/s10750-007-9191-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-007-9191-1