Abstract
Effects of herbivory on competition between invasive and native plants have seldom been examined from an above-belowground integrated perspective. We examined the interactions between a monophagous beetle, Agasicles hygrophila, or an oligophagous beetle, Cassida piperata, and a root-knot nematode on the intensity of intra- and interspecific interactions between the invasive Alternanthera philoxeroides and its native congener, Alternanthera sessilis. Plant-plant competition was assessed using the relative neighbour effect (RNE) index. Competitive effects (positive RNE indexes) from conspecifics for A. philoxeroides were detected under herbivory by A. hygrophila alone. The ramet number, stolon length, and/or the biomass of A. philoxeroides were reduced compared to plants without herbivory. The interactions between the two plants without herbivory were facilitative (negative RNE indexes), and the facilitative effect became stronger such that A. philoxeroides produced more biomass under combinative herbivory by C. piperata and the nematode. However, significant competitive effects from conspecifics were detected for A. sessilis under all the AG-BG herbivory treatments, while no apparent competitive or facilitative effects from A. philoxeroides were detected for A. sessilis under all the AG-BG herbivory treatments. These results suggest that intra- or interspecific competition of invasive and native plants can be greatly affected by AG-BG herbivory, and thus interactive effects of AG-BG herbivory and plant competition may influence invasive process of alien plants in the field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasions are widely recognized as a major threat to global biodiversity and community structure (Ehrenfeld 2010; Simberloff et al. 2013); however, why a specific alien plant can successfully invade is still inconclusive. The enemy release hypothesis (ERH) predicts that the proliferation of an invasive plant is due to the fact that it escapes from the regulation by specialized herbivores and other natural enemies in the introduced range (Keane and Crawley 2002). Based on ERH, the evolution of increased competitive ability hypothesis (EICA) assumes that invasive plants may experience evolutionary shifts in resource allocation from defense to growth to increase their competitive ability due to release from coevolved enemies (Blossey and Notzold 1995). Although many studies have provided support for the EICA hypothesis that some invasive plants do have a competitive advantage over their native conspecifics (Beaton et al. 2011; Gruntman et al. 2014; Jakobs et al. 2004), some others have only partially supported this hypothesis (Meyer et al. 2005) or did not support it at all (Parker et al. 2013; Zheng et al. 2015). Thus, it is still unclear whether invasive plants are in general competitively superior to native plants.
In novel environments, invasive plants may confront both heterospecific and conspecific neighbors, and respond to their neighbors either in a positive (facilitation) or a negative (competition) way (Callaway and Walker 1997; Zheng et al. 2015). The pattern and the intensity of both inter- and intraspecific competition might differ greatly between invasive and native species (Huang et al. 2012a; Zheng et al. 2015). Classical competition theory predicts that intraspecific competition should be greater than interspecific competition because individuals within one species require similar resource conditions (Adler et al. 2018); however, some studies have suggested that the intensity of interspecific competition between native and invasive species could exceed the impact of intraspecific competition within species because of limited resources (Sheppard and Burns 2014). Both interspecific and intraspecific competition abilities are significant determinants of invasive success and population dynamics of alien plants in their introduced ranges (Mangla et al. 2011).
Another important factor that may affect the outcomes of competition of invasive and native plants is herbivory, which can potentially alter the magnitude and/or outcomes of competition either by causing greater damage to a dominant competitor or by causing a similar level of damage among species (Huang et al. 2012b; Wang et al. 2017; You et al. 2016). Herbivory can reduce plant fitness as well as the ability of plants to compete with neighbors, and its effects can be increased by competition (Backmann et al. 2019; Center 2005). Invasive plants are considered to be largely released from specialists, but may also be attacked by a diverse array of oligophagous and polyphagous herbivores from both aboveground (AG) and belowground (BG) compartments in their introduced ranges (Joshi and Vrieling 2005). Feeding by those herbivores may influence the outcomes of intraspecific and/or interspecific interactions of an invader with its native neighbors (Ibanez et al. 2013; Kim et al. 2013). Moreover, interactions between AG-BG herbivores may change the relative strengths of plant competition in additive or antagonistic ways through their effects on shared host plants (Bezemer and van Dam 2005; Wardle et al. 2004). Although an increasing number of studies have focusing on invasive plant-mediated interactions between AG-BG herbivores (Bezemer and van Dam 2005; Huang et al. 2012b; Li et al. 2020; Ohgushi et al. 2018; Wardle et al. 2004; Wei et al. 2016), few have tested effects of such interactions on the competition ability of invasive plants while interacting with different heterospecific and conspecific neighbors (Huang et al. 2012a; Jing et al. 2015).
Alternanthera philoxeroides is a perennial clonal herb that is native to South America and is aggressively invasive in temperate, tropical and subtropical areas of both aquatic and terrestrial environments of China (Pan et al. 2007) and elsewhere. In China, A. philoxeroides commonly reproduces via regeneration from clonal fragments. Alternanthera sessilis is the only native congener of A. philoxeroides and is often sympatric with A. philoxeroides in China (He et al. 2014; Lu and Ding 2010; Wei et al. 2016). However, A. philoxeroides always outperforms A. sessilis in the field. Previous studies have suggested that A. philoxeroides is more phenotypically plastic than A. sessilis across a wide range of growth, morphological, physiological and fitness-related traits (Geng et al. 2006; Zhang et al. 2021), and these advantages are believed to enhance the competitive capacity of A. philoxeroides and promote its invasion success in heterogeneous and adverse environments (Geng et al. 2006; Wang et al. 2018, 2021). In China, A. philoxeroides is damaged by a series of AG and BG herbivores, including the monophagous leaf beetle A. hygrophila (Coleoptera: Chrysomelidae) introduced from the native range, the indigenous oligophagous tortoise beetle C. piperata (Coleoptera: Chrysomelidae), and several generalists such as a root-knot nematode Meloidogyne incognita (He et al. 2014; Wei et al. 2016). Alternanthera philoxeroides exhibits higher tolerance to simulated and actual herbivory than A. sessilis in many environments (Lu and Ding 2012; Sun et al. 2010), and the invader can allocate resource flexibly from leaves to roots to tolerate AG herbivory or joint AG-BG herbivory (Sun et al. 2010; Wei et al. 2016). Invasive plants may have evolved in response to different monophagous, oligophagous, and generalist herbivores (Agrawal and Fishbein 2006), and thus, it is necessary to assess the impacts of individual herbivores and species combinations to provide new insights into the evolutionary changes of invasive plants. However, it is still unclear how the performance of A. philoxeroides and its functional counterpart A. sessilis would be affected by biotic interactions between multiple AG and BG herbivores under intra- and interspecific competition environments.
In this study, we conducted a common garden experiment by planting A. philoxeroides and its indigenous congener A. sessilis, either mixed with each other or planted individually, while subjecting both plants to AG herbivory by the leaf beetle A. hygrophila or the tortoise beetle C. piperata, and BG herbivory by the root-knot nematode M. incognita. We sought to compare how the growth and inter- and intraspecific competitive ability of A. philoxeroides and A. sessilis were differently affected by the interactions of AG and BG herbivores. Specifically, we asked the following questions: (1) How does AG-BG herbivory affect the outcomes of intra- and interspecific competition of the two plants? (2) How does joint herbivory by A. hygrophila (or C. piperata) and M. incognita differ in its effects on competitive outcome? (3) How do invasive and native plants vary in their responses to interactive effects between competition and damage by different types of herbivores? We predicted that: (1) both competition and AG-BG herbivory interactions would influence plant performance, but would be highly dependent on competition type and herbivore species; (2) AG-BG herbivore interactions would alter the outcomes of intra- and/or interspecific competition through differential impact on neighbour plants.
Material and methods
Rearing of plants and herbivores
Plants of each species (A. philoxeroides and A. sessilis) were collected from agricultural land in Nanning (108°24′E, 22°84′N), Guangxi Province, China, and propagated vegetatively at ambient temperature and light in a greenhouse at Guangxi University. Day temperatures in the greenhouse were about 28–33°C, night temperatures were about 24–28°C, relative humidity was about 75%. Stem fragments (2 cm) with a stem node centered around the node were cut from shoots of A. philoxeroides and A. sessilis and then planted in the seedling trays (54 × 28 cm, 50 holes) containing mixtures of loam and peat soil (1:1). Plants were grown under a natural photoperiod and watered regularly to maintain soil moisture. Three weeks later, the fragments of A. philoxeroides and A. sessilis that had similar growth states were used for the experiments.
Adults of the leaf beetle and the tortoise beetle were collected from alligator weed at a field adjacent to Guangxi University, Nanning, China, and each species was reared separately in containers (10 cm in diameter, 18 cm in depth, covered with gauze) with fresh ramets of A. philoxeroides as food. After the adult beetles (of each species) produced eggs, the leaves with eggs were transferred to new Petri dishes. Newly hatched larvae were reared with fresh ramets of A. philoxeroides until they pupated. Newly emerged adults were raised individually in Petri dishes to prevent mating and were used in the experiment. The root-knot nematode M. incognita was also collected from the roots of A. philoxeroides and A. sessilis at a farmland adjacent to Guangxi University, Nanning, China.
The common-garden experiment
We designed a 24-factorial common-garden experiment consisting of 2 plant origins (invasive, and native plants) × 2 competition types (intraspecific, and interspecific competition) × 2 BG herbivory treatments (with nematodes, and without nematodes) × 3 AG herbivory treatments (A. hygrophila, C. piperata, and without herbivory) (Fig. 1). Each treatment was replicated for 6 times (144 replicated combinations).
Experimental design. The experiment employed a four-way factorial design including two plant origins (invasive Alternanthera philoxeroides and native Alternanthera sessilis) × two competition types (intraspecific competition, or interspecific competition) × two belowground (BG) herbivory treatments (no nematodes, or nematodes) × three aboveground (AG) herbivory treatments (no herbivory [= “control”], herbivory by the leaf beetle Agasicles hygrophila [= “leaf beetle”], or herbivory by the tortoise beetle C. piperata [= “tortoise beetle”])
For the interspecific competition treatment, one fragment of A. philoxeroides was cultivated in a pot (25 cm diameter × 16 cm height) along with one fragment of A. sessilis; but for the intraspecific competition treatment, two fragments of A. philoxeroides or A. sessilis were planted in the same pot. In addition, one fragment of A. philoxeroides or A. sessilis were planted in monoculture, which were set as the control for calculating relative neighbour effect (RNE) in the mixed culture experiment. Each pot was covered with a 0.15 mm nylon mesh to exclude herbivores other than the test species.
After one month of growth, each plant was randomly assigned to one of two groups of nematode treatment before nematode inoculation (0 or 10,000 M. incognita eggs per plant). The nematode suspension was prepared according to Liu (2000). In brief, the roots of tomatoes infested by M. incognita were rinsed with water and cut into about 1 cm length. These segments were blended in a juicer, and then the suspension was successively passed through sterilized 200 mesh and 500 mesh suspension screens, being washed with sterilized water. Finally, the filter on the 500 mesh was washed into a centrifuge tube, centrifuged, the concentration of the suspension was adjusted to 1000 eggs·mL-1 with distilled water. To inoculate plants with nematodes, five holes (3 mm deep) were created with a hole puncher near the plant’s roots, and 2 mL of nematode suspension were injected into each hole. The injection site was then covered with soil. For the control plants with no nematodes, similar holes were created and injected with 2 mL of sterilized water.
One month after nematode inoculation, one adult of the leaf beetle or the tortoise beetle was released individually on each plant under the corresponding AG herbivory treatment. After 10 days of feeding, all the beetles were removed. One week later, the stem diameter, the ramet number, the stolon length, and the root length of each plant were measured, respectively. Upon harvest, all the plants were cut, AG and BG plant parts were dried separately at 65°C until constant weight and weighed. The total biomass of each plant was then calculated.
Statistical analyses
Both interspecific and intraspecific interactions between A. philoxeroides or A. sessilis were quantified using the RNE index (Markham and Chanway 1996):
where P-N is the total biomass of the species planted in monoculture and P+N is the total biomass of the same species in the presence of a neighbour species. The term x depends on which value of P is greater. If P-N>P+N, then x = P-N; however, if P-N < P+N, then x = P+N. RNE index values fall into a symmetrical range from +1 to −1 with negative values indicating facilitation and positive values indicating competition. An RNE value of 0 indicates that no interaction is occurring.
A series of two-way ANOVAs were conducted to test the effects of AG herbivory treatment (leaf beetle, tortoise beetle, or control), BG herbivory treatment (no nematodes, or nematodes), and their interactions on the growth traits (stem diameter, ramet number, stolon length, root length, and biomass allocation) and on the resultant RNE indexes of A. philoxeroides and A. sessilis under intra- or interspecific competition conditions, separately. AG herbivory treatment and BG herbivory treatment were taken as fixed factors. Tukey’s HSD tests were applied to test for pairwise differences among treatments. Significant level was set at P < 0.05. All data were statistically analyzed by the statistical software package SPSS 18.0 (SPSS Inc., Chicago, USA), and all figures were plotted by SigmaPlot 12.5.
Results
Growth of the two test plants
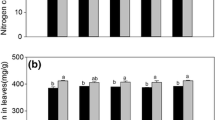
When the two plants were grown in monoculture (intraspecific competition), the stem diameter, ramet number, stolon length, AG biomass, and total biomass of A. philoxeroides and the BG biomass of A. sessilis were all significantly affected by the AG herbivory treatment (Table 1). The above mentioned growth parameters of A. philoxeroides were only significantly reduced by the leaf beetle A. hygrophila, except for the stem diameter of A. philoxeroides and the BG biomass of A. sessilis, which were also significantly reduced by the tortoise beetle C. piperata, compared to the plants with no AG herbivory (Figs. 2, 3). The ramet number and BG biomass of A. philoxeroides and the ramet number of A. sessilis were significantly affected by the BG herbivory treatment (Table 1): nematode-infested A. philoxeroides plants had more ramets and BG biomass, but nematode-infested A. sessilis plants conversely had fewer ramets, compared to conspecific plants with no nematodes (Figs. 2, 3). The root length of A. philoxeroides and the AG biomass and total biomass of A. sessilis were significantly affected by interactions between the AG herbivory treatment and the BG herbivory treatment (Table 1). The roots of A. philoxeroides were significantly longer under herbivory by the nematode alone compared to other AG-BG herbivory treatments (Fig. 2D). However, both the AG biomass and total biomass of A. sessilis were significantly reduced by all the AG-BG herbivory treatments compared to the control plants with no herbivory (Fig. 3).
Effects of aboveground (AG) and belowground (BG) herbivory treatments on the stem diameter (A), ramet number (B), stolon length (C), and root length (D) of Alternanthera philoxeroides and Alternanthera sessilis under intra- or interspecific competition. No nema = no nematodes; Nema = herbivory by nematodes. Different capital letters and lowercase letters indicate significant difference of the growth traits of A. philoxeroides and A. sessilis, respectively, among AG- and BG herbivory treatments at P < 0.05 level
Effects of aboveground (AG) and belowground (BG) herbivory treatment on the biomass of Alternanthera philoxeroides and Alternanthera sessilis under intra- or interspecific competition. No nema = no nematodes; Nema = herbivory by nematodes. Different capital letters and lowercase letters indicate significant difference of the AG biomass and BG biomass of A. philoxeroides and A. sessilis, respectively, under AG- and BG herbivory treatments at P < 0.05 level
When the two plant species were grown together, only the stem diameter and ramet number of A. philoxeroides were significantly affected by interactions between the AG herbivory treatment and the BG herbivory treatment (Table 2). Agasicles hygrophila herbivory alone significantly reduced the stem diameter, but the combinative herbivory by A. hygrophila and the nematode conversely increased the ramet number by 180% (Figs. 2B, C). The AG biomass and total biomass of A. philoxeroides were significantly affected by the AG herbivory treatment (Table 2). Both of these parameters were significantly reduced by A. hygrophila, compared to other AG herbivory treatment (Fig. 3). In contrast, none of the growth traits of A. sessilis were significantly affected by AG-BG herbivory treatments, except for the stolon length (Table 2), which was significantly reduced by C. piperata herbivory alone or the combinative herbivory by A. hygrophila and the nematode, compared to the control plants with no herbivory (Fig. 2C).
The intra- and interspecific interactions between the two plants
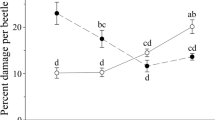
For plants under intraspecific competition conditions, the RNE index values of A. philoxeroide were only significantly affected by the AG herbivory treatment, while the RNE index values of A. sessilis were significantly affected by interactions between the AG herbivory treatment and the BG herbivory treatment (Table 1). Specifically, neighboring conspecifics had weakly competitive effects (positive RNE index values) on A. philoxeroide but significant facilitative effects (negative RNE index values) on A. sessilis under intraspecific competition conditions with no herbivory (Fig. 4). Significant competitive effects from neighboring conspecifics were detected for A. philoxeroides under herbivory by A. hygrophila alone (Fig. 4). However, strong shifts from facilitative processes to significant competitive effects from conspecific neighbors were found for A. sessilis under attack by all the AG and/or BG herbivory treatments (Fig. 4).
Effects of aboveground (AG) and belowground (BG) herbivory treatment on the relative neighbour effect (RNE) values between Alternanthera philoxeroides and Alternanthera sessilis under intra- or interspecific competition. No nema = no nematodes; Nema = herbivory by nematodes. Asterisks indicate a significant positive or negative RNE index values (i.e., greater than one minimum significant difference from zero: Tukey HSD test, P < 0.05)
In contrast, for plants under interspecific competition conditions, the RNE index values of A. philoxeroide were significantly affected by interactions between the AG herbivory treatment and the BG herbivory treatment, while the RNE index values of A. sessilis were only significantly affected by the AG herbivory treatment (Table 2). Specifically, interspecific relationships between A. philoxeroide and A. sessilis were facilitative when there was no herbivory, and the facilitative effect of A. sessilis on A. philoxeroides became stronger under combinative herbivory by C. piperata and the nematode (Fig. 4). However, no apparent competitive or facilitative effects from A. philoxeroides were detected for A. sessilis under all the AG-BG herbivory treatments (Fig. 4).
Discussion
An important factor affecting invasion success of plants is the species’ exact biology, including clonal traits (Cornelissen et al. 2014; Pan and Price 2002). It is well known that many important plant invaders, such as A. philoxeroides, grow and spread primarily or exclusively by vegetative growth and clonal propagation in their introduced regions (Liu et al. 2006; Wang et al. 2017). Stronger clonal potential of invasive clonal plants compared to their co-occurring, non-invasive clonal native plants may facilitate the establishment of invasive species in recipient habitats (Wang et al. 2017; You et al. 2016). Alternanthera philoxeroides generally had longer stolons and roots. Longer stolon length indicates a stronger capacity for dispersal and occupation in stoloniferous clonal plants (Oborny and Kun 2002). Wang et al. (2016) found that the cost of stolon elongation for A. philoxeroides was lower than that for A. sessilis, which may let A. philoxeroides more rapidly colonize novel environments. We also found that A. philoxeroides had higher BG biomass in comparison to A. sessilis, regardless of competition type. Higher total biomass reflects the better performance for A. philoxeroides, and its longer roots and higher root biomass may enable A. philoxeroides to use soil water and nutrients more efficiently and produce more storage and propagative organs compared to A. sessilis (Poorter and Nagel 2000; Wang et al. 2016). Some other studies have also demonstrated that many successful plant invaders likely benefit more from biomass allocation to BG parts compared to co-occurring native plants (Drenovsky et al. 2012; Ren et al. 2019).
Another important invasion mechanism of successful invasive plants is phenotypic plasticity (Davidson et al. 2011), which has been considered as a critical factor contributing to the invasiveness of A. philoxeroides, which occupies variable environments (Geng et al. 2006; Zhang et al. 2021). In this study, we found that A. philoxeroides produced a greater number of ramets, longer roots, and more biomass under AG and/or BG herbivory. The type of herbivory (A. hygrophila, C. piperata, or joint herbivory with the nematode) was found to affect the level of phenotypic plasticity. However, its native congener A. sessilis was greatly suppressed by herbivory under most conditions. Ramet number is a functional trait that is positively correlated with reproductive allocation because having more ramets can help plants occupy more space, leading to higher reproductive output. Increases in ramet number may lead clonal plants like A. philoxeroides to produce more reproductive parts to cope with biomass loss caused by AG-BG herbivores. More AG and/or BG biomass after herbivory indicates A. philoxeroides has both a higher compensatory growth ability and higher tolerance to herbivory (He et al. 2014; Lu and Ding 2012; Lu et al. 2014). Adaptive phenotypic plasticity may promote the optimal trait expression of invasive species and potentially facilitate successful invasion in a broad range of environments such as those that have multiple herbivores.
The invasive success of alien plants may reflect an evolutionary response to changes in natural enemy pressure in their invaded ranges (Müller-Schärer et al. 2004). Joshi and Vrieling (2005) proposed that invasive species will become better protected against polyphagous enemies but concurrently lose their defenses against monophagous or oligophagous enemies that are absent in the invaded range. Thus, coevolved specialists will have a greater impact on invasive plants, while indigenous generalist herbivores will have a greater impact on native plants in the introduced range (Joshi and Vrieling 2005; Müller-Schärer et al. 2004). Consistent with the above predictions, we also found that the specialist A. hygrophila reduced the growth of A. philoxeroides more seriously than did the native C. piperata, indicating that under the same population density, the co-evolved specialist beetle performed better on A. philoxeroides than those oligophagous herbivores from introduced ranges. Similarly, A. hygrophila could suppress the leaf biomass of A. philoxeroides more strongly than could two generalist insects (Atractomorpha sinensis and Hymenia recurvalis) (Yu and Fan 2018), while herbivory by C. piperata could not suppress the growth and expansion of A. philoxeroides (Wei et al. 2016). These findings support the hypothesis that release from co-evolved specialists (from the native range) may contribute to better performance of invasive plants in introduced regions, as proposed by the ERH (Keane and Crawley 2002).
Little is known about the effects of herbivory on the competitive ability of exotic plants (Huang et al. 2012a; Mangla et al. 2011). Stoloniferous clonal plants usually grow in diameter rather than height, and therefore intraspecific competition within clonal plants is likely to be very strong given that many invasive clonal plants form thick monospecific stands in the invade range (Wan et al. 2019). Yu et al. (2019) suggested that intraspecific competition can limit the growth and establishment of A. philoxeroides by suppressing its root sprouting and ramet growth. When resources are limited due to intraspecific competition, population growth of clonal plants may be easily affected by environmental disturbances such as herbivory (Huang et al. 2012a; Kim et al. 2013), which can influence the fitness and the ability of plants to compete with neighbors (Center 2005). In this study, we found AG-BG herbivory exerted different effects on intraspecific competition of A. philoxeroides and A. sessilis. Notably, negative effects from neighboring plants on A. philoxeroides were detected under herbivory by A. hygrophila. Thus, A. hygrophila could potentially prevent A. philoxeroides from aggressively expanding under competitive conditions. If so, native species such as A. sessilis would benefit from the feeding of A. hygrophila on the dominant A. philoxeroides. Similarly, following successful control of invasive leafy spurge (Euphorbia esula L.) by flea beetles (Aphthona spp.), several sod-forming grasses become dominant in northern Great Plains grasslands (Larson and Larson 2010). Moreover, intraspecific competition has the potential to generate feedbacks between plants and herbivores as intraspecific competition would result in a decrease in plant size and plant nutritional quality (Louda et al. 1990).
Interspecific competition and herbivory can both lead to reductions in plant growth, biomass, and reproduction (Adler et al. 2018; Ibanez et al. 2013; Jing et al. 2015). Herbivore damage may change the outcome of the interspecific competition based on the herbivores’ feeding preferences (Hambäck and Beckerman 2003; Ibanez et al. 2013; Jing et al. 2015). In this study, we found A. philoxeroides and A. sessilis differed in overall performance and their responses to interspecific competition and either AG and/or BG herbivory. The interspecific interactions between A. philoxeroides and A. sessilis was facilitative when there was no herbivory, suggesting that both A. philoxeroides and A. sessilis benefit from their interspecific competition. Many studies have also demonstrated that herbivory can affect the intensity of interspecific competition in plants (Center 2005; Huang et al. 2012a; Wei et al. 2016). In this study, we found that herbivory by A. hygrophila or A. hygrophila + nematodes tended to increase the intensity of interspecific competition between A. philoxeroides and A. sessilis. These findings suggest that we may be able to manage a specific invader by increasing interspecific competition intensity combined with manipulating of herbivory. Contrary to classical competition theory (Adler et al. 2018), interspecific competition should be more intense between native and alien plants occupying similar ecological habits or niches (Sheppard and Burns 2014). Indeed, A. philoxeroides always forms denser populations and grows larger when it occurs in sympatry with A. sessilis in the field. The high densities of invasive plants may monopolize resources and inhibit the growth of other plants (Silveira and Thiébaut 2020), and differences in aggressiveness and body size may lead to asymmetric competition between the two plants.
In contrast to competitive interactions among individuals, intra- or interspecific facilitation may enhance plant tolerances to herbivores through compensatory growth (Arsenault and Owen-Smith 2002; Rand 2004). In this study, the strong facilitative effect of A. sessilis on A. philoxeroides was detected under combinative herbivory by C. piperata and the nematode. However, the negative effects of the root-knot nematode and C. piperata appeared to be additive on some growth traits of A. philoxeroides grown in monoculture. This suggest that those indigenous natural enemies or their interactions, in some cases, may also be able to influence the growth of invasive plants like A. philoxeroides. This outcome, however, is highly dependent on the identity of neighboring plants. Similar to our results, Lu and Ding (2010) found that intraspecific interactions can increase the compensatory ability of A. philoxeroides to AG herbivory. Thus, combinative herbivory by indigenous AG-BG herbivores in the invaded range (which often occurs in the field), may potentially influence the further spread of some invasive plants like A. philoxeroides. Backmann et al. (2019) also found that timely elimination of herbivores can reduce competition from neighboring plants, and that herbivores may be a part of a plant’s strategy for reducing competition and increasing plant fitness. However, similar facilitative effects from A. philoxeroides were not detected for A. sessilis, suggesting that the competitive outcomes between invasive alien plants and their co-occurring native plants are highly dependent on AG and BG herbivores (He et al. 2014; Jing et al. 2015).
Conclusion
The results of our study indicate that the intra- or interspecific interactions of the invasive A. philoxeroides and its native congener A. sessilis were affected differently by AG-BG herbivore interactions, and significant competitive effects from conspecific plants were detected for A. philoxeroides under herbivory by the monophagous A. hygrophila alone. However, A. sessilis exerted strong facilitative effects on A. philoxeroides under combinative herbivory by the oligophagous C. piperata and the nematode. Our results suggest that intra- and interspecific competition of native and invasive plants can be greatly affected by AG-BG herbivory, and thus the interactive effects between AG-BG herbivory and plant competition will have the potential to greatly influence the establishment and expansion of invasive plants in the field.
Data availability
Data are available in the electronic appendices and are also available from the corresponding author on reasonable request.
References
Adler PB, Smull D, Beard KH et al (2018) Competition and coexistence in plant communities: intraspecific competition is stronger than interspecific competition. Ecol Lett 21:1319–1329. https://doi.org/10.1111/ele.13098
Agrawal AA, Fishbein M (2006) Plant defense syndrom. Ecology 87:132–149. https://doi.org/10.1890/0012-9658(2006)87[132:PDS]2.0.CO;2
Arsenault R, Owen-Smith N (2002) Facilitation versus competition in grazing herbivore assemblages. Oikos 97:313–318. https://doi.org/10.1034/j.1600-0706.2002.970301.x
Backmann P, Grimm V, Jetschke G et al (2019) Delayed chemical defense: timely expulsion of herbivores can reduce competition with neighboring plants. Am Nat 193:125–139. https://doi.org/10.1086/700577
Beaton LL, Van Zandt PA, Esselman EJ et al (2011) Comparison of the herbivore defense and competitive ability of ancestral and modern genotypes of an invasive plant, Lespedeza cuneata. Oikos 120:1413–1419. https://doi.org/10.1111/j.1600-0706.2011.18893.x
Bezemer TM, van Dam NM (2005) Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol Evol 20:617–624. https://doi.org/10.1016/j.tree.2005.08.006
Blossey B, Notzold R (1995) Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. J Ecol 83:887–889. https://doi.org/10.2307/2261425
Callaway RM, Walker LR (1997) Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology 78:1958–1965. https://doi.org/10.1890/0012-9658(1997)078[1958:CAFASA]2.0.CO;2
Center TD (2005) Herbivory alters competitive interactions between two invasive aquatic plants. Biol Control 33:173–185. https://doi.org/10.1016/j.biocontrol.2005.02.005
Cornelissen JH, Song YB, Yu FH et al (2014) Plant traits and ecosystem effects of clonality: a new research agenda. Ann Bot 114:369–376. https://doi.org/10.1093/aob/mcu113
Davidson AM, Jennions M, Nicotra AB (2011) Do invasive species show higher phenotypic plasticity than native species and if so, is it adaptive? A meta-analysis. Ecol Lett 14:419–431. https://doi.org/10.1111/j.1461-0248.2011.01596.x
Drenovsky RE, Grewell BJ, D’Antonio CM et al (2012) A functional trait perspective on plant invasion. Ann Bot 110:141–153. https://doi.org/10.1093/aob/mcs100
Ehrenfeld JG (2010) Ecosystem consequences of biological invasions. Annu Rev Ecol Evol S 41:59–80. https://doi.org/10.1146/annurev-ecolsys-102209-144650
Geng YP, Pan XY, Xu CY et al (2006) Phenotypic plasticity of invasive Alternanthera philoxeroides in relation to different water availability, compared to its native congener. Acta Oecol 30:380–385. https://doi.org/10.1016/j.actao.2006.07.002
Gruntman M, Pehl AK, Joshi S et al (2014) Competitive dominance of the invasive plant Impatiens glandulifera: using competitive effect and response with a vigorous neighbour. Biol Invasions 16:141–151. https://doi.org/10.1007/s10530-013-0509-9
Hambäck PA, Beckerman AP (2003) Herbivory and plant resource competition: a review of two interacting interactions. Oikos 101:26–37. https://doi.org/10.1034/j.1600-0706.2003.12568.x
He MY, Ding JQ, Lu XM (2014) Increased compensatory ability of an invasive plant to above- and below-ground enemies in monocultures. Plant Ecol 215:253–260. https://doi.org/10.1007/s11258-013-0294-7
Huang W, Carrillo J, Ding JQ et al (2012a) Interactive effects of herbivory and competition intensity determine invasive plant performance. Oecologia 170:373–382. https://doi.org/10.1007/s00442-012-2328-6
Huang W, Carrillo J, Ding JQ et al (2012b) Invader partitions ecological and evolutionary responses to above- and belowground herbivory. Ecology 93:2343–2352. https://doi.org/10.1890/11-1964.1
Ibanez S, Bison M, Lavorel S et al (2013) Herbivore species identity mediates interspecific competition between plants. Community Ecol 14:41–47. https://doi.org/10.1556/ComEc.14.2013.1.5
Jakobs G, Weber E, Edwards PJ (2004) Introduced plants of the invasive Solidago gigantea (Asteraceae) are larger and grow denser than conspecifics in the native range. Divers Distrib 10:11–19. https://doi.org/10.1111/j.1472-4642.2004.00052.x
Jing J, Raaijmakers C, Kostenko O et al (2015) Interactive effects of above- and belowground herbivory and plant competition on plant growth and defence. Basic Appl Ecol 16:500–509. https://doi.org/10.1016/j.baae.2015.04.009
Joshi J, Vrieling K (2005) The enemy release and EICA hypothesis revisited: incorporating the fundamental difference between specialist and generalist herbivores. Ecol Lett 8:704–714. https://doi.org/10.1111/j.1461-0248.2005.00769.x
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170. https://doi.org/10.1016/S0169-5347(02)02499-0
Kim TN, Underwood N, Inouye BD (2013) Insect herbivores change the outcome of plant competition through both inter- and intraspecific processes. Ecology 94:1753–1763. https://doi.org/10.1890/12-1261.1
Larson DL, Larson JL (2010) Control of one invasive plant species allows exotic grasses to become dominant in northern Great Plains grasslands. Biol Conserv 143:1901–1910. https://doi.org/10.1016/j.biocon.2010.04.045
Li XQ, Gao X, Siemann E et al (2020) Effects of above- and belowground herbivory of specialists and generalists on the growth and defensive chemicals of introduced and native Chinese tallow seedlings. Plant Soil 455:65–78. https://doi.org/10.1007/s11104-020-04666-2
Liu J, Dong M, Miao SL et al (2006) Invasive alien plants in China: role of clonality and geographical origin. Biol Invasions 8:1461–1470. https://doi.org/10.1007/s10530-005-5838-x
Liu WZ (2000) Plant pathogen nematology. China Agricultural Press, Beijing, China
Louda SM, Keeler KH, Holt RD (1990) Herbivore influences on plant performance and competitive interactions. In: Grace JB, Tilman D (eds) Perspectives on plant competition. Academic Press, New York, USA, pp 413–444
Lu XM, Ding JQ (2010) Flooding compromises compensatory capacity of an invasive plant: implications for biological control. Biol Invasions 12:179–189. https://doi.org/10.1007/s10530-009-9441-4
Lu XM, Ding JQ (2012) History of exposure to herbivores increases the compensatory ability of an invasive plant. Biol Invasions 14:649–658. https://doi.org/10.1007/s10530-011-0106-8
Lu XM, Shao X, Ding JQ (2014) No impact of a native beetle on exotic plant performance and competitive ability due to plant compensation. Plant Ecol 215:275–284. https://doi.org/10.1007/s11258-014-0296-0
Müller-Schärer H, Schaffner U, Steinger T (2004) Evolution in invasive plants: implications for biological control. Trends Ecol Evol 19:417–422. https://doi.org/10.1016/j.tree.2004.05.010
Mangla S, Sheley RL, James JJ et al (2011) Intra and interspecific competition among invasive and native species during early stages of plant growth. Plant Ecol 212:531–542. https://doi.org/10.1007/s11258-011-9909-z
Markham JH, Chanway CP (1996) Measuring plant neighbour effects. Funct Ecol 10:548–549
Meyer G, Clare R, Weber E (2005) An experimental test of the evolution of increased competitive ability hypothesis in goldenrod, Solidago gigantea. Oecologia 144:299–307. https://doi.org/10.1007/s00442-005-0046-z
Oborny B, Kun Á (2002) Fragmentation of clones: how does it influence dispersal and competitive ability? In: Stuefer JF, Erschbamer B, Huber H, Suzuki J (eds) Ecology and evolutionary biology of clonal plants. Springer, Dordrecht, pp 97–124
Ohgushi T, Wurst S, Johnson SN (2018) Current knowledge and future challenges of aboveground and belowground community ecology. Aboveground belowground community ecology. Springer, NewYork, pp 345–361
Pan JJ, Price JS (2002) Fitness and evolution in clonal plants: the impact of clonal growth. In: Stuefer JF, Erschbamer B, Huber H, Suzuki J (eds) Ecology and evolutionary biology of clonal plants. Springer, Dordrecht., pp 361–378
Pan XY, Geng YP, Alejandro S et al (2007) Invasive Alternanthera philoxeroides: biology, ecology and management. J Syst Evol 45:884–900. https://doi.org/10.1360/aps06134
Parker JD, Torchin ME, Hufbauer RA et al (2013) Do invasive species perform better in their new ranges? Ecology 94:985–994. https://doi.org/10.1890/12-1810.1
Poorter H, Nagel O (2000) The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Funct Plant Biol 27:1191–1191. https://doi.org/10.1071/PP99173_CO
Rand TA (2004) Competition, facilitation, and compensation for insect herbivory in an annual salt marsh forb. Ecology 85:2046–2052. https://doi.org/10.1890/03-3087
Ren GQ, Li Q, Li Y et al (2019) The enhancement of root biomass increases the competitiveness of an invasive plant against a co-occurring native plant under elevated nitrogen deposition. Flora 261:151486. https://doi.org/10.1016/j.flora.2019.151486
Sheppard CS, Burns BR (2014) Effects of interspecific alien versus intraspecific native competition on growth of native woody plants. Plant Ecol 215:1527–1538. https://doi.org/10.1007/s11258-014-0411-2
Silveira MJ, Thiébaut G (2020) Effect of density and neighbours on interactions between invasive plants of similar growth form. Aquat Ecol 54:463–474. https://doi.org/10.1007/s10452-020-09753-1
Simberloff D, Martin JL, Genovesi P et al (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66. https://doi.org/10.1016/j.tree.2012.07.013
Sun Y, Ding JQ, Frye MJ (2010) Effects of resource availability on tolerance of herbivory in the invasive Alternanthera philoxeroides and the native Alternanthera sessilis. Weed Res 50:527–536. https://doi.org/10.1111/j.1365-3180.2010.00822.x
Wan JZ, Wang CJ, Yu FH (2019) Large-scale environmental niche variation between clonal and non-clonal plant species: Roles of clonal growth organs and ecoregions. Sci Total Environ 652:1071–1076. https://doi.org/10.1016/j.scitotenv.2018.10.280
Wang T, Hu JT, Miao LL et al (2016) The invasive stoloniferous clonal plant Alternanthera philoxeroides outperforms its co-occurring non-invasive functional counterparts in heterogeneous soil environments-invasion implications. Sci Rep 6:38036. https://doi.org/10.1038/srep38036
Wang T, Hu JT, Wang RQ et al (2018) Tolerance and resistance facilitate the invasion success of Alternanthera philoxeroides in disturbed habitats: A reconsideration of the disturbance hypothesis in the light of phenotypic variation. Environ Exp Bot 153:135–142. https://doi.org/10.1016/j.envexpbot.2018.05.011
Wang Y, Xiong YT, Wang Y et al (2021) Long period exposure to serious cadmium pollution benefits an invasive plant (Alternanthera philoxeroides) competing with its native congener (Alternanthera sessilis). Sci Total Environ 786:147456. https://doi.org/10.1016/j.scitotenv.2021.147456
Wang YJ, Müller-Schärer H, van Kleunen M et al (2017) Invasive alien plants benefit more from clonal integration in heterogeneous environments than natives. New Phytol 216:1072–1078. https://doi.org/10.1111/nph.14820
Wardle DA, Bardgett RD, Klironomos JN et al (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633. https://doi.org/10.1126/science.1094875
Wei H, He MY, Lu XM et al (2016) Differences in interactions of aboveground and belowground herbivores on the invasive plant Alternanthera philoxeroides and native host A. sessilis. Biol Invasions 18:3437–3447. https://doi.org/10.1007/s10530-016-1234-y
You WH, Han CM, Fang LX et al (2016) Propagule pressure, habitat conditions and clonal integration influence the establishment and growth of an invasive clonal plant Alternanthera Philoxeroides. Front Plant Sci 7:568. https://doi.org/10.3389/fpls.2016.00568
Yu HH, Fan SF (2018) Differences in physiological traits and resistances of Alternanthera philoxeroides after herbivory by generalists and specialists. Aquat Ecol 52:323–332. https://doi.org/10.1007/s10452-018-9666-3
Yu HW, Shen N, Guan X et al (2019) Influence of soil nutrient heterogeneity and competition on sprouting and ramets growth of Alternanthera philoxeroides. CLEAN-Soil, Air, Water 47:1800182. https://doi.org/10.1002/clen.201800182
Zhang JL, Huang W, Ding JQ (2021) Phenotypic plasticity in resource allocation to sexual trait of alligatorweed in wetland and terrestrial habitats. Sci Total Environ 757:143819. https://doi.org/10.1016/j.scitotenv.2020.143819
Zheng YL, Feng YL, Valiente-Banuet A et al (2015) Are invasive plants more competitive than native conspecifics? Patterns vary with competitors. Sci Rep 5:15622. https://doi.org/10.1038/srep15622
Acknowledgements
We would like to thank Yao Xiang, Zhongcai Ma, and Shunliang Feng for insect collection and experimental assistance. We especially thank two anonymous reviewers for their valuable comments on earlier versions of this paper. We would also like to thank Van Driesche Scientific Editing for editing of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (31800423), the Natural Science Foundation of Guangxi Province (2018GXNSFBA281172), and the Science Development Foundation of Guangxi Academy of Agricultural Sciences (2021YT119).
Author information
Authors and Affiliations
Contributions
SS conceptualization, data manipulation, formal analysis, methodology, writing-original draft. WG data manipulation, software, investigation, writing-review and editing. XL project administration, funding acquisition, writing-review and editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shen, S., Guo, W. & Li, X. Above- and belowground herbivory alters the outcome of intra- and interspecific competition between invasive and native Alternanthera species. Biol Invasions 24, 801–813 (2022). https://doi.org/10.1007/s10530-021-02694-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-021-02694-2