Abstract
Plant invasiveness was commonly attributed to the invader’s competitive superiority over the native community, but a general pattern supporting this prediction is still lacking. This is particularly enhanced by the fact that competitive dominance and its role in plant invasiveness require the use of scarcely-practiced experimental elements. Here, we used a comprehensive experimental approach to evaluate the competitive superiority of the highly invasive annual Impatiens glandulifera. We used two competition-setting treatments, which independently examine competitive effect versus response of both native and invasive genotypes. As a neighbour species we used Urtica dioica, a vigorous perennial co-occurring with I. glandulifera at both its native and introduced ranges. By examining both components of competitive ability we were able to show that although invasive genotypes exert a weaker competitive pressure compared to their native conspecifics, they are still competitively superior to U. dioica. Our results also suggest that the high competitive ability of I. glandulifera could be attributed it to allelopathic effects on co-occurring native species. We suggest that an appropriate experimental setup, which examines competitive effect and response independently, can provide a more factual evaluation of the competitive ability of invasive plants. Furthermore, the use of a dominant species as a target plant rather than a random species or one with poor competitive ability, renders our results more general, implying that I. glandulifera might exert greater competitive effect on the less robust co-occurring species. We conclude that our approach is highly useful and advocate its application in future tests of invasion success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive plants have often been shown to exhibit vigorous growth and high performance (Thebaud and Simberloff 2001; Bossdorf et al. 2005; Blumenthal and Hufbauer 2007; Schlaepfer et al. 2010; van Kleunen et al. 2010) and invasiveness has consequently been attributed to traits that allow plants to take advantage of high resource levels (Richards et al. 2006; Schlaepfer et al. 2010; Godoy et al. 2011), and confer high competitive abilities (Blossey and Nötzold 1995; Vilà and Weiner 2004; Bossdorf et al. 2005).
Several hypotheses were suggested to account for the potential high competitive ability of invasive plants. For example, high competitive ability was attributed to adaptive changes that are selected for at the introduced range (Blossey and Nötzold 1995; Maron et al. 2004; Bossdorf et al. 2005; Doorduin and Vrieling 2011). Particularly, invasive plants were suggested to experience release from natural enemies, which could select for shifts in resource allocation from herbivore resistance to growth or reproduction and increased competitive ability (evolution of increased competitive ability hypothesis—EICA sensu Blossey and Nötzold 1995). Competitive superiority of invasive plants was also hypothesized to result from their production of allelopathic compounds, which suppress the growth of neighbouring species (Inderjit et al. 2011). These compounds were suggested to be particularly detrimental to the recipient communities, due to their lack of co-evolved defences (novel weapons hypothesis—NWH sensu Callaway and Aschehoug 2000).
These hypotheses were examined in several studies (Vilà et al. 2003; Prati and Bossdorf 2004; Franks et al. 2008; Beaton et al. 2011; Inderjit et al. 2011). However, no general pattern has emerged, particularly regarding the prediction of increased competitive ability in invasive species (Vilà and Weiner 2004; Bossdorf et al. 2005; Dostal 2011).
Testing the role that competitive dominance plays in plant invasiveness is essential for ascertaining the suggested hypotheses. However, this examination requires the use of a few experimental elements that were scarcely practiced. Particularly, for competitive superiority of invasive plants to be supported, their ability to suppress co-occurring species (i.e. competitive effect) should be greater than vice versa (i.e. competitive response). Moreover, the two components of competitive ability could potentially be associated with different traits (Cahill et al. 2005) and with different invasion stages. For example, competitive effect may be important at the beginning of an invasion and enable alien species to outcompete native plants, while competitive response could be important at later stages and allow their stable persistence (Müller-Schärer et al. 2004; Bossdorf et al. 2005). Therefore, distinguishing between these two aspects of competitive ability may provide greater insight into the different mechanisms and stages of invasion (Vilà and Weiner 2004), but to date it has been applied in only a few studies (Suding et al. 2004; Ridenour et al. 2008; He et al. 2009;), and their experimental setup, using a single ‘target-neighbour’ treatment for both competitive effect and response, can not provide an independent evaluation of the two components.
Another important aspect that should be considered when studying the competitive ability of invasive plants is the choice of neighbour species. The competitive ability of invasive plants should be compared using neighbour species with which they co-occur. Ideally, these species should be selected randomly, in which case several species are required for a valid evaluation of their competitive effect. However, if only a single species is to be used, it should preferably be similar to the invasive species in terms of size and life form or phylogenetic relatedness, so as to avoid the random choice of poor competitors, which might bias the results towards competitive superiority of the invasive plant (Vilà and Weiner 2004). Nevertheless, this approach has been scarcely used (Blair and Wolfe 2004; He et al. 2009; Dostal 2011). Furthermore, there has been no study to date which has used a competitor which occurs at both ranges, hampering a direct comparison of competitive ability between ranges.
Here, we used a comprehensive experimental approach, which applies these methodological aspects, to examine the competitive superiority of the highly invasive annual Impatiens glandulifera (Balsaminaceae). This species is native to the Himalayas and invasive in Europe, North America and New Zealand, where it exhibits fast growth and tends to produce large, monospecific stands (Beerling and Perrins 1993; Tickner et al. 2001). Growing up to 2.5 m, it is considered the tallest annual plant in Europe, implying that competitive ability is likely to be a key factor in its success as an invasive plant (Beerling and Perrins 1993). However, attempts to examine its impact on the recipient communities reveal contrasting and inconclusive results (Beerling and Perrins 1993; Hejda and Pyšek 2006).
To evaluate the competitive ability of I. glandulifera, we used two competition-setting treatments, which independently examined competitive effect versus response. As a neighbour species we used Urtica dioica, which is a tall perennial herb, known to be highly competitive and to often form monospecific stands (Šrůtek 1993; Taylor 2009). U. dioica has been shown to co-occur in high frequencies with I. glandulifera at both its invasive and native range (Beerling and Perrins 1993; Tickner et al. 2001) and to inhibit its growth (Tickner et al. 2001), making it a suitable competitor for I. glandulifera.
Recently, invasive I. glandulifera have been suggested to exert strong allelopathic effects (Scharfy et al. 2011) but no knowledge exists on the effectiveness of allelochemical production in inhibiting the growth of co-occurring species at its invasive range, or on the production of such allelochemicals by native genotypes. Therefore, to further evaluate the competitive mechanisms that promote I. glandulifera invasiveness, we performed an additional experiment where we examined its allelopathic effect of on U. dioica.
In both the competition and allelopathy experiment, we used I. glandulifera from native and invasive populations, which allowed us to examine possible genetic differentiation in their competitive effect and response as well as their allelopathic effects. Differentiation in these traits might indicate post-invasion evolution and elucidate the possible mechanisms that account for I. glandulifera’s invasiveness (Bossdorf et al. 2005; Alpert 2006). For example, greater competitive effect or response of invasive-range plants could provide support for the EICA prediction of evolution of increased competitive ability (Blossey and Nötzold 1995).
We addressed the following predictions: (1) I. glandulifera exhibits greater competitive effect on U. dioica than vice versa (competitive response); (2) I. glandulifera exerts strong allelopathic effects on U. dioica. In addition, we used I. glandulifera from both native-Asian and invasive-European origin to look for potential genetic differentiation and examine the prediction that (3) invasive genotypes exhibit greater growth and competitive effect compared to their native conspecifics. We furthermore addressed the question of whether there are genetic correlations between plant performance with and without competition, or between competitive effect and response to examine the predicted associations between the size of invasive plants and their competitive effect or response.
Methods
Study species
Impatiens glandulifera Royle (1834), is a riparian annual species, occurring mainly along riversides, marshlands, and forest edges (Beerling and Perrins 1993; Pyšek and Prach 1995; Kollmann and Bañuelos 2004; Hejda and Pyšek 2006). It was introduced into Europe in 1839 and has become highly invasive, exhibiting an exponential increase in abundance and distribution (Beerling and Perrins 1993; Pyšek and Prach 1995). I. glandulifera is self-compatible though protandrous and widely visited by pollinators. When ripe, the pods explode at the slightest touch and seeds are dispersed up to 5 m (Beerling and Perrins 1993).
Urtica dioica L. (Urticaceae) is a dioecious perennial herb (Šrůtek 1993; Taylor 2009), which has a wide distribution in Europe, Asia and America, where it inhabits riparian and open areas (Taylor 2009).
Plant sources and seed handling
For the competition experiment, I. glandulifera seeds were collected in autumn 2008. At the invasive range, seeds were collected from two populations in Germany (Baden- Württemberg) and two in the Czech Republic (South Bohemia). Seed collections in the native range were very difficult to realize and we obtained seeds from two populations in India (state of Jammu and Kashmir). Seeds from 20 mother plants (families) per population were grown in summer 2009 at a common garden at Tübingen University. In order to control for maternal effects, which might confound our inference of genetic differentiation among native and invasive genotypes (Moloney et al. 2009), we used F1 genotypes following hand-pollination crosses. Controlled crosses were performed within populations by gently removing the pollen-loaded androecia of a fully expanded flower and coating with it the stigma of a flower in another individual. All pollinated flowers were covered prior to and following pollination with a light fabric organza to avoid access of insect pollinators. 20 additional F1 seeds per population were grown in summer 2010 under control conditions in a common garden at Tübingen University, from which we obtained F2 seeds using the same hand-pollination procedure. This procedure was repeated in 2011 to produce F3 seeds for the allelopathy experiment.
Seeds for both experiments were kept in paper bags according to their family. Prior to the each experiment, the seeds were placed in Petri dishes with moist filter paper and were cold stratified at 4 °C for four weeks to initiate germination (Baskin and Baskin 1998).
Seeds of U. dioica were collected in autumn 2009 and 2011 for the competition and allelopathy experiments, respectively, at 10 randomly chosen locations around Tübingen, which are also inhabited by I. glandulifera.
Competition experiment
At the end of March 2010, seeds from six families per population of both I. glandulifera and U. dioica were sown in 1.5 L pots filled with potting soil (Topferde, Einheitserde, Gebr. Patzer GmbH & Co. KG, Kreutztal, Germany) and placed in a heated greenhouse. 20 seeds from each family were sown per pot.
At the end of April 2010, seedlings were transplanted into 20 L pots filled with potting soil and randomly assigned to one of the following four treatments (Fig. 1): I. glandulifera control with a single plant per pot; U. dioica control with a single plant per pot; competitive response, with I. glandulifera as a single target plant surrounded by four U. dioica neighbours; competitive effect, with U. dioica as a single target plant surrounded by three I. glandulifera neighbours. The two competition treatments had different numbers of neighbour plants in order to compensate for the weaker competitive ability of U. dioica compared to I. glandulifera (Tickner et al. 2001). The experimental design resulted in a total of 144 pots [4 competition treatments × 6 I. glandulifera origins (2 native + 4 invasive) × 6 families].
Following transplant, the pots were placed in a common garden at Tübingen University, where they were arranged in blocks according to families. The pots were placed at a minimum distance of 90 cm to avoid light competition effects (Fig. 1) and stabilized with planting sticks to avoid their overturn by wind. 10 g of slow-release fertilizer (Osmocote Classic 14 % N, 14 % P2O5, 14 % K2O; Scotts, Geldermalsen, The Netherlands) and an insecticide treatment (Neudosan, H. Nitsch und Sohn GmbH und Co. KG, Kreutztal, Germany) were applied to each pot. The latter was applied to control for confounding differences in herbivore resistance between plants. Plants were irrigated to field capacity two to four times a week, according to weather conditions.
During the experiment, I. glandulifera and U. dioica were recorded for their onset of reproduction. As maturation did not occur simultaneously for I. glandulifera plants from the different origins, harvest of target plants in the competitive response treatment took place when plants exhibited signs of senescence and ceased to produce new flowers (end of August and late September, for invasive and native plants, respectably). U. dioica target plants in the competitive effect treatment were all harvested at the end of August, to ensure the same effect from both native and invasive plants.
Growth parameters were estimated following harvest, including height and above-ground vegetative biomass of both species, the latter of which was measured after pre-drying the plants for at least 10 days in the greenhouse and oven-drying them in 90 °C for 4.5 and 2 days, for I. glandulifera and U. dioica, respectively. For I. glandulifera, reproductive output was estimated by collecting seeds every 1–2 weeks during the reproductive period (June–September) from six ripe pods at various locations on the plant. Seeds were counted and weighed on the day of collection. Pod number was not estimated as ripe pods explode and fall upon the slightest vibration. For U. dioica, reproductive output was estimated by counting the number of inflorescences on both gynoecious and androecious plants, which did not exhibit significant differences in either dry mass (ANOVA control: F1,46 = 0.63, P = 0.43; competition: F1,46 = 0.86, P = 0.36) or number of inflorescences (ANOVA control: F1,46 < 0.001, P = 0.99; competition: F1,46 = 0.02; P = 0.90).
Competitive effect and response were estimated for all response variables using the Relative Interaction Index (Armas et al. 2004), namely the relative change in the performance of a target individual in the competitive effect or response treatments (Pcompetition) compared to that of its sibling under competition-free conditions (Pcontrol):
Values of this index range from −1 to 1. Negative values indicate suppressive effect by neighbours and higher (less negative) values indicate greater tolerance of competition.
Allelopathy experiment
In April 2012, U. dioica seeds were sown in Petri dishes with moist filter paper and placed for 4–5 days in a heated greenhouse. Following germination, the seedlings were planted in 1 L pots either alone, with a single invasive-range I. glandulifera seedling or a native-range seedling. The pots were filled with potting soil, and for half of the pots the soil was thoroughly mixed with 20 mL of activated carbon (particle size 0.15 mm; neoLab Migge Laborbedarf-Vertriebs GmbH). Activated carbon is commonly used for investigating the allopathic effects of root exudates due to its high capacity to absorb organic compounds, which could thus alleviate the negative effects of allelochemicals and differentiate between their effect and that of resource depletion by neighbours (Callaway and Aschehoug 2000; Inderjit and Callaway 2003). The pots were arranged in blocks according to families within I. glandulifera origin. The experimental design resulted in a total of 108 pots [3 competition treatments (control + 2 I. glandulifera origins) × 2 activated-carbon treatments × 18 blocks].
All pots were supplemented with 2 g of a slow release fertilizer to reduce possible confounding effects of activated carbon on nutrient availability (Lau et al. 2008) and treated with a systemic insecticide. The plants were harvested in July 2012 and response parameters of U. dioica were estimated, including height, total length of inflorescences and above-ground vegetative biomass, after oven-drying the plants in 90 °C for 2 days.
As for the competitive effect and response, allelopathic effects of I. glandulifera were estimated using a Relative Allelopathy Index, similar to the RII (Armas et al. 2004), which is the relative change in the performance of each U. dioica genotype without activated carbon (Pno carbon) compared to that of their siblings grown in soil with activated carbon (Pcarbon):
Values of this index also range from −1 to 1. Negative values indicate allelopathic effect by neighbours, but might also suggest facilitative effects of activated carbon (Inderjit and Callaway 2003). This can be resolved by examining RAI values for control plants growing without I. glandulifera, for which zero values would indicate that the addition of activated carbon has no confounding effects on the performance of U. dioica.
Data analyses
Due to the unbalanced number of populations per region, differences between competitive effect and response were examined using linear mixed-effects models based on restricted maximum likelihood for all response variables, with competition setting (effect and response) and plant origin as fixed factors and country and population as random factors nested within origin and country within origin, respectively. Separate analyses for competitive effect and response, with plant origin as a fixed factor and country and population as random factors, where then used to determine significant differences among origins within a competitive setting.
Additionally, differences in plant performance among invasive and native plants growing under competition-free conditions were examined using linear mixed-effects models for all response variables, with origin as a fixed factor and country and population as random factors nested within origin and country within origin, respectively. Differences in onset of flowering were examined using Pearson’s χ2 test.
Genetic correlations between siblings’ performance under competition-free conditions and competitive effect or response, and between siblings’ competitive effect and response were examined using Pearson correlation for all response variables. Separate analyses were performed for invasive and native plants.
Differences in allelopathic effect between U. dioica plants growing alone, with native or with invasive I. glandulifera were examined using univariate ANOVAs for height and above-ground biomass, with competition as a fixed factor and block as a random factor. Additionally, one-sample t-tests were used to examine whether RAI values are significantly different from zero (i.e. no effect of activated carbon) (Sokal and Rohlf 1995). Because many U. dioica plants had no inflorescences, RAI values could not have been calculated for total inflorescence length and the response of U. dioica to activated carbon was thus analysed for the original values. As these values did not meet the assumption of normality, the effect of activated carbon was examined separately for plants growing alone, with native or with invasive I. glandulifera using non-parametric Kruskal–Wallis tests. All statistical analyses were performed using PASW 18 (SPSS Inc., Chicago, IL, USA).
Results
Competitive effect and response
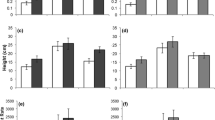
Regardless of their origin, I. glandulifera plants exhibited a 285 % greater competitive effect on U. dioica than vice versa (i.e., competitive response) in above-ground biomass and 28 % greater competitive effect in height (Table 1; Fig. 2a, b), despite the fact that U. dioica was subjected to competition with only three individuals, compared to four for I. glandulifera.
Competitive effect and response in growth and reproductive parameters of Impatiens glandulifera from native (Nat) and invasive (Inv) origin grown under competition with Urtica dioica. Values are Relative Interaction Index (mean ± SE) [RII = (competition − control)/(competition + control)]. Reproductive output represents total inflorescence length (cm) and total seed biomass (g) for U. dioica and I. glandulifera, respectively. Significant differences between native and invasive genotypes are indicated above bars (*P < 0.05; Separate linear mixed-effects models for competitive effect and response) Additional statistical analyses are presented in Table 1
The negative effect exerted by I. glandulifera on the above-ground biomass of U. dioica was significantly affected by plant origin and was 19 % greater in native compared to invasive plants (Fig. 2a). Similarly, the response of I. glandulifera to competition with U. dioica was significantly affected by plant origin as invasive plants exhibited a 114 and 43 % greater negative response in their above-ground biomass compared to native plants (Fig. 2a). This association between greater competitive effect and reduced competitive response of native compared to invasive plants is indicated also by the significant interaction between competition setting and origin (Table 1).
The greater competitive effect and decreased competitive response exhibited by native compared to invasive I. glandulifera corresponded with their 58 % greater above-ground biomass under control competition-free conditions (Table 2; Fig. 3a). Although invasive plants produced 38 % more pods compared to native plants (Table 2; Fig. 3c), this did not translate to greater seed production (Table 2; Fig. 3d). Invasive plants also exhibited an earlier onset of flowering compared to native plants (Pearson’s χ2 test: χ2 = 20.073, df = 10, P = 0.029).
Growth and reproductive parameters of Impatiens glandulifera plants from native and invasive origin (mean ± SE) under competition-free conditions. Additional statistical analyses are presented in Table 2
Within-population genetic correlations were not found between the performance of genotypes under competition-free conditions, their competitive effect and competitive response, except for the competitive response of native genotypes which, in contrast with our predictions, correlated negatively with their performance under control conditions (Table 3).
Allelopathic effects
Both native and invasive I. glandulifera genotypes exerted strong allelopathic effects on the production of above-ground biomass in U. dioica (Table 4; Fig. 4a), but did not differ in these effects (Fig. 4a). Under competition-free conditions there was no difference between control plants and those treated with activated carbon (Fig. 4a), suggesting its use did not elicit any confounding effects on the performance of U. dioica. However, no effects were found for either plant height (Table 4; Fig. 4b) or total inflorescence length (Kruskal–Wallis test, control: χ2 = 1.241, df = 1, P = 0.265; native: χ2 = 0.099, df = 1, P = 0.753; invasive: χ2 = 0.647, df = 1, P = 0.421).
Effects of activated carbon on growth parameters of Urtica dioica grown under competition-free (control) conditions or with I. glandulifera from native or invasive origin. Values are Relative Allelopathy Index (mean ± SE) [RAI = (no carbon − carbon)/(no carbon + carbon)]. Significant differences between competition-treatment means and zero are indicated above bars (**P < 0.01; one-sample t tests). Additional statistical analyses are presented in Table 4
Discussion
In this study we examined the use of a new approach to test for predicted competitive superiority of invasive plants. By using an appropriate experimental setup, which independently examines both competitive effect and response, we were able to confirm the competitive dominance of Impatiens glandulifera over a native resident species. Our results indicate that both invasive and native I. glandulifera genotypes exerted greater competitive effects on the co-occurring species, Urtica dioica, than vice versa, despite the fact that U. dioica is a vigorous perennial herb, which is highly dominant in habitats occupied by I. glandulifera (Pyšek and Prach 1995; Tickner et al. 2001). These results highlight the need for using both components of competitive ability in studies of invasive species. Had we measured only their competitive effect we could have concluded that, compared to their native conspecifics, invasive I. glandulifera exert a weaker competitive pressure on their recipient species. However, by examining both components of competitive ability we were able to show that invasive genotypes are still competitively superior to their co-occurring species at their invasive range. We therefore suggest that the assessment of both competitive effect and response can effect the interpretation of results and advocate that it can provide a more comprehensive examination of the competitive superiority of invasive plants, as previously proposed also by Vilà and Weiner (2004).
The results of this study also support the prediction that I. glandulifera’s competitive superiority and invasive characteristics could be promoted by its strong allelopathic effects on co-occurring native species. The novel weapons hypothesis (NWH) asserts that the production of allelochemicals could confer a competitive advantage for invasive plants, to which associated species at the invasive range have not evolved defences (Callaway and Aschehoug 2000). The fact that both native and invasive genotypes exhibited strong allelopathic effects suggests that this attribute is indeed common also in native genotypes. However, the change in the response of the recipient community predicted by the NWH requires a comparison of allelopathic effects at both the native and invasive range and thus could not be inferred from the results of this study. Further studies are therefore needed in order to compare the allelopathic effects of native and invasive I. glandulifera on co-occurring species at both the native and invasive range.
The notion that the competitive superiority of I. glandulifera could be the result of allelopathic effects rather than rapid exploitation of limiting resources might be supported by the lack of genetic correlations between the size of individuals and their competitive effect. The fact that allocation to biomass did not equip I. glandulifera plants of either origin with greater competitive effect and even hindered the competitive response of native plants suggests that their competitive advantage might be attributed to factors that are traded-off with biomass, such as the ability to tolerate low resource levels (Goldberg and Landa 1991; Cahill et al. 2005; He et al. 2010) or the production of allelochemicals (Lankau and Kliebenstein 2009). These results stress the importance of examining genetic correlations rather than only correlation between population means when studying the adaptive implications of life-history traits.
Unlike the support of the competitive superiority of I. glandulifera in its invasive range, our results failed to support the hypothesis that this competitive advantage is the result of evolution of increased competitive ability (EICA) at the invasive range (Blossey and Nötzold 1995). Although the use of only two native populations of I. glandulifera might not inform much about genetic divergence between the invasive and native range, the reduced competitive ability of the invasive genotypes, both in terms of competitive effect and response, suggests the potentially reduced role of competition as a selection pressure in invasive I. glandulifera. For example, Bossdorf et al. (2004) suggested that invasive plants might be selected for reduced competitive ability if they experience reduced competition at the introduced compared to native range. This idea might be supported by our findings, as even the reduced competitive effect of the invasive I. glandulifera plants was sufficient to out-compete the dominant U. dioica. However, genetic divergence between invasive and native populations might be attributed to processes other than adaptive post-invasion evolution. Particularly, many invasive species have been shown to be small non-random samples of their source native-range population, often leading to founder effects and genetic bottlenecks (Sakai et al. 2001; Dlugosch and Parker 2008). As very little is known about either the number of I. glandulifera source populations or the number of their introductions into Europe, and as only two native populations were sampled in this study, we cannot discern between adaptive evolution, founder effects or non-random sampling of native populations in causing the observed genetic divergence.
The genetic differentiation exhibited between native and invasive I. glandulifera genotypes coincides with the results of Kollmann and Bañuelos (2004), who found a latitudinal trend of decreased growth and onset of reproduction in northern populations across the invasive range of I. glandulifera, which they associated with variations in the length of the growing season. Although Kollmann and Bañuelos (2004) studied only invasive-range populations of I. glandulifera, the reduced biomass allocation and onset of reproduction exhibited also by our invasive compared to native genotypes might be accounted for local adaptations merely to a shorter growing season rather than to selection pressures specific to the invasive range. Further studies with additional replications of native populations are thus needed to substantiate the potential role of evolution in the invasiveness of this species.
Conclusion
This study is the first to demonstrate the competitive superiority of the highly invasive species I. glandulifera, and attribute it to its allelopathic effects on a co-occurring native species. Our results demonstrate essential methodological aspects that should be implemented when studying the common prediction of increased competitive ability of invasive plants. We suggest that an appropriate experimental setup, which examines competitive effect and response independently, can provide a more factual evaluation of the competitive ability of invasive plants. Furthermore, the use of a dominant co-occurring species as a target plant rather than a random species from only one range or one with poor competitive ability, renders our results more general, implying that I. glandulifera might exert greater competitive effect on the less robust co-occurring species.
References
Alpert P (2006) The advantages and disadvantages of being introduced. Biol Invasions 8(7):1523–1534
Armas C, Ordiales R, Pugnaire FI (2004) Measuring plant interactions: a new comparative index. Ecology 85(10):2682–2686
Baskin CC, Baskin JM (1998) Seeds: ecology, biogeography and evolution of dormancy and germination. Academic Press, San Diego
Beaton LL, Van Zandt PA, Esselman EJ, Knight TM (2011) Comparison of the herbivore defense and competitive ability of ancestral and modern genotypes of an invasive plant, Lespedeza cuneata. Oikos 120(9):1413–1419
Beerling DJ, Perrins JM (1993) Impatiens-glandulifera royle (Impatiens-roylei walp). J Ecol 81(2):367–382
Blair AC, Wolfe LM (2004) The evolution of an invasive plant: an experimental study with Silene latifolia. Ecology 85(11):3035–3042
Blossey B, Nötzold R (1995) Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. J Ecol 83(5):887–889
Blumenthal DM, Hufbauer RA (2007) Increased plant size in exotic populations: a common-garden test with 14 invasive species. Ecology 88(11):2758–2765
Bossdorf O, Prati D, Auge H, Schmid B (2004) Reduced competitive ability in an invasive plant. Ecol Lett 7(4):346–353
Bossdorf O, Auge H, Lafuma L, Rogers WE, Siemann E, Prati D (2005) Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia 144(1):1–11
Cahill JF, Kembel SW, Gustafson DJ (2005) Differential genetic influences on competitive effect and response in Arabidopsis thaliana. J Ecol 93(5):958–967
Callaway RM, Aschehoug ET (2000) Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science 290(5491):521–523
Dlugosch KM, Parker IM (2008) Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol 17(1):431–449
Doorduin LJ, Vrieling K (2011) A review of the phytochemical support for the shifting defence hypothesis. Phytochem Rev 10(1):99–106
Dostal P (2011) Plant competitive interactions and invasiveness: searching for the effects of phylogenetic relatedness and origin on competition intensity. Am Nat 177(5):655–667
Franks SJ, Pratt PD, Dray FA, Simms EL (2008) Selection on herbivory resistance and growth rate in an invasive plant. Am Nat 171(5):678–691
Godoy O, Valladares F, Castro-Diez P (2011) Multispecies comparison reveals that invasive and native plants differ in their traits but not in their plasticity. Funct Ecol 25(6):1248–1259
Goldberg DE, Landa K (1991) Competitive effect and response: hierarchies and correlated traits in the early stages of competition. J Ecol 79(4):1013–1030
He WM, Feng YL, Ridenour WM, Thelen GC, Pollock JL, Diaconu A, Callaway RM (2009) Novel weapons and invasion: biogeographic differences in the competitive effects of Centaurea maculosa and its root exudate (±)-catechin. Oecologia 159(4):803–815
He WM, Thelen GC, Ridenour WM, Callaway RM (2010) Is there a risk to living large? Large size correlates with reduced growth when stressed for knapweed populations. Biol Invasions 12(10):3591–3598
Hejda M, Pyšek P (2006) What is the impact of Impatiens glandulifera on species diversity of invaded riparian vegetation? Biol Conserv 132(2):143–152
Inderjit, Callaway RM (2003) Experimental designs for the study of allelopathy. Plant Soil 256(1):1–11
Inderjit EvansH, Crocoll C, Bajpai D, Kaur R, Feng YL, Silva C, Carreon JT, Valiente-Banuet A, Gershenzon J, Callaway RM (2011) Volatile chemicals from leaf litter are associated with invasiveness of a neotropical weed in Asia. Ecology 92(2):316–324
Kollmann J, Bañuelos MJ (2004) Latitudinal trends in growth and phenology of the invasive alien plant Impatiens glandulifera (Balsaminaceae). Divers Distrib 10(5–6):377–385
Lankau RA, Kliebenstein DJ (2009) Competition, herbivory and genetics interact to determine the accumulation and fitness consequences of a defence metabolite. J Ecol 97(1):78–88
Lau JA, Puliafico KP, Kopshever JA, Steltzer H, Jarvis EP, Schwarzlander M, Strauss SY, Hufbauer RA (2008) Inference of allelopathy is complicated by effects of activated carbon on plant growth. New Phytol 178(2):412–423
Maron JL, Vila M, Arnason J (2004) Loss of enemy resistance among introduced populations of St. John’s Wort (Hypericum perforatum). Ecology 85:3243–3253
Moloney KA, Holzapfel C, Tielbörger K, Jeltsch F, Schurr FM (2009) Rethinking the common garden in invasion research. Perspect Plant Ecol Evol Syst 11(4):311–320
Müller-Schärer H, Schaffner U, Steinger T (2004) Evolution in invasive plants: implications for biological control. Trends Ecol Evol 19(8):417–422
Prati D, Bossdorf O (2004) Allelopathic inhibition of germination by Alliaria petiolata (Brassicaceae). Am J Bot 91(2):285–288
Pyšek P, Prach K (1995) Invasion dynamics of Impatiens glandulifera - a century of spreading reconstructed. Biol Conserv 74(1):41–48
Richards CL, Bossdorf O, Muth NZ, Gurevitch J, Pigliucci M (2006) Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol Lett 9(8):981–993
Ridenour WM, Vivanco JM, Feng YL, Horiuchi J, Callaway RM (2008) No evidence for trade-offs: Centaurea plants from America are better competitors and defenders. Ecol Monogr 78(3):369–386
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, Kimberly A, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive specie. Annu Rev Ecol Evol Syst 32:305–332
Scharfy D, Funk A, Venterink HO, Gusewell S (2011) Invasive forbs differ functionally from native graminoids, but are similar to native forbs. New Phytol 189(3):818–828
Schlaepfer DR, Glattli M, Fischer M, van Kleunen M (2010) A multi-species experiment in their native range indicates pre-adaptation of invasive alien plant species. New Phytol 185(4):1087–1099
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. W.H. Freeman, New York
Šrůtek M (1993) Distribution of the stands with Urtica dioica L. along the Lužnice River floodplain on the border between Austria and Czechoslovakia and land management. Vegetatio 106(1):73–87
Suding KN, LeJeune KD, Seastedt TR (2004) Competitive impacts and responses of an invasive weed: dependencies on nitrogen and phosphorus availability. Oecologia 141(3):526–535
Taylor K (2009) Biological Flora of the British Isles: Urtica dioica L. J Ecol 97(6):1436–1458
Thebaud C, Simberloff D (2001) Are plants really larger in their introduced ranges? Am Nat 157(2):231–236
Tickner DP, Angold PG, Gurnell AM, Mountford JO, Sparks T (2001) Hydrology as an influence on invasion: experimental investigations into competition between the alien Impatiens glandulifera and the native Urtica dioica in the UK. Plant invasions: species ecology and ecosystem management. Backhuys Publishers, Leiden
van Kleunen M, Weber E, Fischer M (2010) A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol Lett 13(2):235–245
Vilà M, Weiner J (2004) Are invasive plant species better competitors than native plant species? evidence from pair-wise experiments. Oikos 105(2):229–238
Vilà M, Gomez A, Maron JL (2003) Are alien plants more competitive than their native conspecifics? A test using Hypericum perforatum L. Oecologia 137(2):211–215
Acknowledgments
We wish to thank Ortrun Ebinger, Mohammad Golkary, Ernst Schwärzli, Ralf Wegerer and Sinja Zieger for their technical assistance, Hana Skálová for providing Impatiens glandulifera seeds from the Czech Republic and Zafar Reshi for providing seeds from Kashmir. This study was supported by a grant of the German Research Foundation (DFG) to KT (TI338/8-1), and by the Minerva Foundation to MG.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gruntman, M., Pehl, A.K., Joshi, S. et al. Competitive dominance of the invasive plant Impatiens glandulifera: using competitive effect and response with a vigorous neighbour. Biol Invasions 16, 141–151 (2014). https://doi.org/10.1007/s10530-013-0509-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-013-0509-9