Abstract
The novel associations between invasive plants and their natural enemies in the introduced range have recently received increasing attention; however, the effects of novel enemies on exotic plant performance and competition with native species remain poorly explored. Here, we tested the impact of herbivory by a native beetle, Cassida piperata, on the performance of the exotic species Alternanthera philoxeroides and competition with a native congener, Alternanthera sessilis, using common garden experiments in central China. We found A. philoxeroides was able to fully compensate for intense herbivory by C. piperata. Herbivory by C. piperata that released at the average density in this region had no impact on competition between the native and exotic plant species. Our results indicate that herbivory by novel enemies may not reduce exotic plant performance due to plant compensation. However, high tolerance to herbivory may not confer a competitive advantage for exotic species compared to less tolerant native competitors if the herbivore damage is below a certain threshold. Thus, it is necessary to assess the impact of novel enemies on exotic plant performance and competition with native plants along gradients of insect densities. This may lead to a better understanding of how best to exploit the role of native herbivores in facilitating or slowing plant invasions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Release from co-evolved natural enemies is frequently invoked to explain the success of exotic species in their new ranges (Keane and Crawley 2002). However, native herbivores, including both generalists and specialists, may accumulate on and establish novel associations with exotic naïve species (Bertheau et al. 2010). Results from host choice experiments indicate that some native herbivores prefer novel hosts over native hosts (Parker and Hay 2005; Morrison and Hay 2011). In addition, a field survey found that exotic plants may harbor a similar or even more diverse herbivore community than native species (Agrawal and Kotanen 2003). It has been hypothesized that native herbivores could suppress the population of exotic plants, and exert resistance to invasions (biotic resistance hypothesis) (review by Maron and Vilà 2001). Though an increasing number of studies have tested the impact of native herbivores on exotic plant performance (Parker et al. 2006; Pearson et al. 2012), most of them focused on mammalian herbivory, while the impacts of native insects on exotic plant species and their competition with native plant species have been little explored (Verhoeven et al. 2009).
The impacts of herbivory on plant fitness and competitive outcomes are affected by plant compensation (Maschinski and Whitham 1989; Callaway et al. 2001), which have been proposed to be common in invasive plants and to be an important mechanism underlying biological invasion and the failure of some biological control programs (Müller-Schärer et al. 2004; Li et al. 2012). Plant compensation, the ability of a plant to mitigate negative impacts of herbivory by re-growth or increased reproduction (Strauss and Agrawal 1999), may increase a plant’s competitive ability in the presence of herbivory (Callaway et al. 2001). Nonetheless, this strategy might depend on the level of damage received. For instance, plant compensation enables a plant to recover from herbivory below a certain threshold, with higher rates of herbivory potentially causing a linear decrease in plant fitness once that threshold is passed (Oksanen et al. 1981; Persson et al. 2005).
As a mechanism of plant defense, compensatory response to a specific herbivore may be affected by the co-evolutionary history between the plant and the herbivore. Based on a meta-analysis, Parker et al. (2006) argued that plants are more susceptible to non-coevolved novel enemies than to co-evolved enemies. Moreover, the diversity and density of native enemies affecting an exotic species may increase during the invasion process (Siemann et al. 2006; Gendron et al. 2012). As a result of these trends, it is expected that non-native plant species may experience decreased performance in the novel range. Yet, non-native invasive species should have overcome these limitations to succeed. To our knowledge, no studies have yet tested the impacts of novel enemies on non-native plants and their competition with native plant species under different herbivory regimes, but such experiments are desirable for a better understanding of the mechanisms underlying plant invasion (Colautti et al. 2004; Chun et al. 2010).
Alternanthera philoxeroides (Mart.) Griseb. (Amaranthaceae), native to South America, is an invasive plant in the USA, Australia, New Zealand, and China (Julien et al. 1995). The weed invades both terrestrial and aquatic habitats in China (Ma 2001), although in the USA, Australia, and New Zealand it mostly occurs in aquatic habitats (Sainty et al. 1998). In a greenhouse experiment using introduced genotypes, Wilson et al. (2007) found that when damaged, A. philoxeroides allocates more resources to below-ground tissues to increase its resource acquisition ability in the introduced range. This trait enables A. philoxeroides to compensate for herbivory or mechanical damage such as mowing in terrestrial habitats (Lu and Ding 2010; Lu et al. 2010). In China, A. philoxeroides often co-occurs with its native congener A. sessilis (L.) R. Br. ex DC. in terrestrial habitats, and both species are prominently damaged by a widespread indigenous tortoise beetle Cassida piperata Hope (Coleoptera: Cassididae). However, the impacts of this beetle on A. philoxeroides and its competition with A. sessilis have not yet been studied.
Here, we tested the impacts of C. piperata on performance of A. philoxeroides and its competition with A. sessilis. Specifically, given that A. philoxeroides allocates more resources to below-ground tissues following herbivore damage than its native congener following simulated herbivory via clipping (2010), suggesting a higher tolerance to herbivory than A. sessilis, we predicted that (1) A. philoxeroides would compensate for herbivory by C. piperata even under intense herbivore damage; and (2) herbivory by C. piperata would increase the competitive ability of A. philoxeroides over A.sessilis.
Materials and methods
Study species
Alternanthera philoxeroides, an herbaceous perennial, was first introduced into China in the 1930s. Since then it has spread rapidly and is now widely distributed as far north as 36.8°N in China (Lu et al. 2013). It can grow in aquatic and terrestrial habitats, but in China it has occurred mainly in terrestrial habitats since the 1950s (Ma 2001), where it rarely sets seeds but rather reproduces by vegetative means from apical stem buds and axillary stem and root buds.
Alternanthera sessilis is an annual or short-lived perennial herb native to China. It propagates both by vegetative means from stem buds and sexually via seeds. Alternanthera sessilis produces large numbers of small seeds in August–October, which are transported by wind and germinate in the following April in moist soils.
Cassida piperata is an oligophagous tortoise beetle native to China and is the most widespread native enemy of both A. philoxeroides and A. sessilis in China. The beetle can have up to four generations each year from early May to early October. In China, adults overwinter in the soil or under the litter of host plants (Tang 1994). Both beetle larvae and adults feed exclusively on leaves of several plants of the Amaranthaceae and Chenopodiaceae families, including A. philoxeroides, Chenopodium album L., C. serotinum L., and A. sessilis (Lin et al. 1990). Of these, it prefers the native hosts over A. philoxeroides (Dai et al. 2013). The beetle often produces tiny-feeding scars on plant leaves, and its eggs are laid individually on the underside of leaves.
In Wuhan where we carried out this research, the densities of this beetle in the field range from 0 to 48 adults and/or larvae/m2, with an average of 9.1 ± 1.3 beetles/m2. The level of damage on individual plants from this beetle ranged from 0 to 50–60 % defoliation, with an average of 18.8 ± 2.5 % defoliation. To test the impact of C. piperata on A. philoxeroides and competition with A. sessilis, we included a range of insect densities/defoliation levels that are likely capture a good range of the variation present in beetle densities/defoliation levels across the entire geographic distribution of the plant/beetle species.
Experiment 1: compensatory response of A. philoxeroides to a gradient of defoliation intensities
A common garden experiment was conducted in Wuhan Botanical Garden in Hubei Province, China, from early June to December 2007. Cut plant stems and insects were collected in a field near Wuhan Botanical Garden in early June, and the insects were reared for one generation in the laboratory on A. philoxeroides before the experiment started to minimize possible trans-generational effects of maternal environments (i.e., host plant species) on insect host use (i.e., diet preferences) (Mousseau and Dingle 1991). Ten randomly selected stems were planted vertically into a 25-cm diameter pot (filled with topsoil collected from the same field) and immediately enclosed within a nylon cage (decreasing available light by about 20 %). All plants (both those in the herbivory and the control treatments) were caged for the entire experiment to account for cage effects. After that, all pots were placed randomly (25 cm apart) in a field that had been previously mowed. Plants were watered every 2 days, and pots were weeded as necessary. Twenty days later, we thinned the plants to six similar-sized plants per pot to minimize plant size variation among pots.
In late June, potted A. philoxeroides plants were randomly assigned to one of four possible treatments: undamaged controls or 20–30 %, 40–50 %, or 100 % of plant leaf area removed by the beetle. These damage treatments corresponded to about one, two, and five times the average field defoliation level, respectively. To achieve the necessary levels of defoliation within 7 days, we manipulated the number of insects released into each cage. We visually estimated the defoliation level for all plants for each pot. We then removed the insects and allowed the plants to grow for an additional 80 days. Each treatment was repeated 6–8 times and a total of 30 pots and 180 individual plants were used, based on insect and plant material availability. We finished the experiment and harvested all the plants in early December. The plants were washed with tap water and brought back to the laboratory, where they were divided into above- and below-ground parts and dried separately at 80 °C for 48 h before weighing.
Experiment 2: compensatory response of A. philoxeroides to a gradient of beetle densities
Another common garden experiment was conducted during the same period with the same source of plants and insects as Experiment 1, to test the response of plants to varying densities of C. piperata. To begin, we mowed a portion of the field and spaced plastic tubs evenly apart (0.5 m between tubs) in the field on June 8, 2007. These plastic tubs were 0.5 m long, 0.4 m wide, and 0.3 m deep, and were filled with topsoil collected from the same field. We randomly selected cut stems of A. philoxeroides (7 cm long with one node) and planted them vertically in tubs at a density of 12 stems per tub. Stems were immediately caged as above. Plants were watered and tubs were weeded every 2 days. After 20 days, we thinned the plants to 8 similar-sized plants per tub to minimize plant size variation among tubs. Ten days later, tubs were randomly selected to be treated either as an undamaged control or to receive 2, 4, or 8 adult C. piperata, which is about one, two, and four times the average field density, respectively. Each treatment was repeated 9–13 times and a total of 43 tubs and 344 plants were used, based on availability of the insect and plant. Throughout the trial, the number of insects in each tub was maintained by adding adults or removing eggs when necessary. At the end of the trial, we visually estimated overall defoliation level for plants in each pot, and found that C. piperata had removed 21.9 ± 1.8 %, 40 ± 2.2 %, and 87.2 ± 3.5 % of leaf area when released at densities of 2, 4 and 8 individuals per pot, respectively. We finished the experiment on December 5 and carried out the same harvesting, drying, and weighing steps as for Experiment 1.
Experiment 3: impact of C. piperata on the competition between A. philoxeroides and A. sessilis
To test the impact of C. piperata on competition between A. philoxeroides and A. sessilis, we conducted a common garden experiment with A. philoxeroides from 20 different populations to assess potential geographic variation in plant competitive ability, given that they are known to differ in compensatory ability (Lu and Ding 2012). In early April 2008, we gathered 10–15 cm cut stems of A. philoxeroides from 20 populations across a wide area of China (see supporting information Table S1). The stems were placed in coolers with dry ice during shipment. In the laboratory, we further cut the stems to 4–5 cm, each containing a single node. The stem pieces were then planted vertically in plastic containers (0.5 m long, 0.4 m wide, and 0.3 m deep) containing a homogenized mixture of peat, topsoil (collected from the same field), and sand. Containers were then placed in a naturally lighted greenhouse. Stems of A. sessilis were collected from one population in Wuhan and received the same treatment as A. philoxeroides. After two and a half months, we clipped 4–5 cm stem pieces (one node for each) from their newly grown shoots for the experiment to reduce variation in size based on environmental maternal effects, which tend to manifest during the early ontogenetic stages of growth (Roach and Wulff 1987). Cassida piperata were collected from one population on A. philoxeroides in a suburb of Wuhan and cultured in the laboratory for one generation, and the adults of the second generation were used for the experiment.
On June 1, 2008, we established five experimental blocks (0.5 m apart) in a field of 8 × 20 m2, and further divided each block into two equal-sized sub-blocks (9 × 1 m2 each and 1 m apart). In each sub-block, 80 pots (25 cm in diameter and filled with top soil collected from the same field) were buried in the ground to a depth of 10 cm, spaced 0.3 m apart. Plant stems of A. philoxeroides from each population were planted individually or paired with one similarly-sized A. sessilis seedling. Each treatment was repeated twice in each sub-block, generating a total of 10 replicates per treatment for the experiment. The positions of seedlings of each population for each planting treatment (planted individually or paired with A. sessilis) were randomized in the field. After planting, all the sub-blocks were immediately caged with 9 × 1 × 1 m3 nylon cages.
Twenty days later, one sub-block in each block was randomly selected to receive herbivory treatment, with the other sub-block serving as an undamaged control. Ninety C. piperata adults (10 adult beetles/m2), about equal to the average beetle density found in the field in Wuhan, where the experiment was conducted, were released into each selected sub-block and allowed to reproduce and feed on the plants during the trial.
We finished the experiment and harvested all the plants in early December 2008. Plants were washed with tap water and then were brought back to the laboratory where they were dried and weighed as in Experiment 1.
Data analysis
The effects of defoliation level and insect density on total plant biomass and the root-to-shoot mass ratio were analyzed using one-way ANOVAs at the pot level. When any main effect was significant (e.g., defoliation level or insect density), further tests among different levels of the main effect were applied using a Bonferroni correction. Given that above- and below-ground tissues are not independent from each other, the impacts of defoliation level or insect density on plant root and shoot mass were analyzed with MANOVA followed by one-way ANOVAs and post hoc tests using a Bonferroni correction when main effects were significant.
To test the role of C. piperata herbivory on total biomass and the root-to-shoot mass ratio of A. philoxeroides from different populations in the presence or absence of the native plant A. sessilis, we conducted a four-way mixed ANOVA with the full dataset (including single and paired data) with the model including herbivory and competition as fixed factors and plant population, its interactions with herbivory and/or competition, and block as random factors. Mixed MANOVAs were carried out to test the impacts of competition and herbivory on root and shoot mass of A. philoxeroides, with the model including competition and herbivory as fixed factors and plant population, its interaction with competition and/or herbivory, and block as random factors.
A mixed three-way ANOVA was conducted to test the effects of herbivory and population source of A. philoxeroides on A. sessilis total biomass with paired data. The model included herbivory as a fixed factor and plant population, its interaction with herbivory, and blocks as random factors. Since during the course of the experiment some individuals died, we excluded data for a pair if one individual plant died; therefore, only data for 291 pairs were used for the analyses.
Before analysis, total plant biomass, root and shoot mass, and the root-to-shoot mass ratio for all the experiments were log10-transformed to improve normality and reduce heterogeneity of variances. All analyses were conducted with SAS version 8.1 (SAS Institute Inc., NC, USA).
Results
Experiment 1: compensatory response of A. philoxeroides to a gradient of defoliation intensities
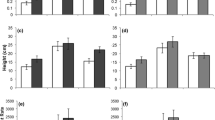
Defoliation level significantly influenced A. philoxeroides total biomass (F 3, 26 = 3.09 and P = 0.0445), but had no impact on plant root-to-shoot mass ratio (F 3, 26 = 2.17, P = 0.1153). MANOVA analysis showed defoliation level had a significant multivariate effect on plant root and shoot mass as a group (Value of Wilks’ landa was 0.5558, F 6, 50 = 2.84 and P = 0.0184). ANOVA analysis showed that defoliation level significantly affected plant root mass (F 3, 26 = 3.71 and P = 0.024), but had no impact on plant shoot mass (F 3, 26 = 2.52 and P = 0.0796) (Fig. 1). In particular, only the highest defoliation treatment (100 % of leaf area been removed) decreased plant total and root mass compared to plants defoliated at a low level (20–30 % of leaf area been removed), but no differences were detected among any other treatments (Fig. 1).
Experiment 2: compensatory response of A. philoxeroides to a gradient of beetle densities
Insect density significantly influenced A. philoxeroides total mass (F 3, 39 = 4.12 and P = 0.0124), and root-to-shoot mass ratio (F 3, 39 = 3.36 and P = 0.0282). MANOVA analysis showed that insect density had a significant multivariate effect on plant root and shoot mass as a group (Value of Wilks’ landa was 0.5978, F 6, 76 = 3.72 and P = 0.0027). While insect density had a significant impact on plant shoot mass (F 3, 39 = 6.050 and P = 0.0017), it had no impact on plant root mass (F 3, 39 = 2.12 and P = 0.1127) (Fig. 2). Beetles at a high density (8 individuals/pot) significantly decreased plant total mass, but beetles at a low (2 individuals/pot) and medium density (4 individuals/pot) had no impact on total plant mass. Likewise, beetles at a high density decreased plant shoot mass (Fig. 2), but increased plant root-to-shoot mass ratio compared to undamaged plants (t test and P = 0.016), while beetles at low and medium densities had no impacts on plant shoot mass and root-to-shoot mass ratio (for both, P > 0.05).
Experiment 3: impact of C. piperata on the competition between A. philoxeroides and A. sessilis
While total biomass of A. philoxeroides was significantly affected by competition, it was not affected by herbivory, plant population, or their interactions, though there was a variation among blocks (Table 1). Similarly, only competition (Value of Wilks’ landa was 0.5732, F 2, 542 = 201.75, and P < 0.0001), plant population (Value of Wilks’ landa was 0.8500, F 38, 1084 = 2.42, and P < 0.0001) and block (Value of Wilks’ landa was 0.9392, F 8, 1084 = 4.31, and P = 0.0001) significantly affected plant root and shoot mass as a group, as indicated by Mixed MANOVA analysis. Plant root-to-shoot mass was not affected by any of the factors or their interactions (Table 1). Alternanthera philoxeroides planted with A. sessilis accumulated less total mass, and root, and shoot mass than when planted individually by the end of the experiment, whether or not the herbivore was present (Fig. 3).
The native species accumulated significantly more biomass than the paired invasive species whether or not the native herbivore was present (for both, t test, P < 0.0001, Fig. 4). Total biomass of A. sessilis accumulated at the end of the experiment was not affected by herbivory (F 1, 43.049 = 0.07 and P = 0.7937), the population source of the neighboring A. philoxeriodes (F 19, 19.021 = 1.37 and P = 0.2475), or their interaction (F 19, 217 = 1.09 and P = 0.3615), and showed no variation among blocks (F 4, 217 = 0.64 and P = 0.6346).
Discussion
With these common garden experiments, we demonstrated that compensation enables the invasive plant A. philoxeroides to fully recover from herbivory by the novel enemy C. piperata in terms of biomass, as predicted. Such high compensatory ability enables A. philoxeroides to endure damage caused by C. piperata that released across a gradient of densities. The results indicate that this compensation might enable A. philoxeroides to escape from top-down control by the novel enemy C. piperata in the field. However, contrary to our prediction, the native congener A. sessilis, which is thought to be less tolerant to herbivory based on previous work, suppressed the growth of A. philoxeroides whether or not C. piperata was present.
Plant resource allocation strategy, such as altering root-to-shoot ratios following damage, is a common trait that allows plants to compensate for herbivory (Strauss and Agrawal 1999). Two lines of evidence suggest that maintain high root mass is an important mechanism underlying compensatory response of A. philoxeroides to artificial and actual herbivory. First, once above-ground tissues were mowed, A. philoxeroides quickly re-allocated root resources to shoot growth to insure photosynthetic capacity (Schooler et al. 2007). Second, we found that decreased root growth due to flooding impaired this compensatory response of A. philoxeroides in a greenhouse experiment (Lu and Ding 2010). In this study, the root mass of A. philoxeroides was not affected by herbivory across varying beetle densities/defoliation levels, although the beetle at high density suppressed shoot mass in our garden experiments. This result suggests the high root mass may also underpin the compensatory response of A. philoxeroides to C. piperata. However, plants damaged at a high intensity had accumulated less root mass at the end of the experiment than those damaged at a lower intensity, suggesting a threshold beyond which the beetle could suppress the growth of A. philoxeroides.
We detected no impacts of herbivory on plant root-to-shoot mass ratio at varying densities/defoliation levels 80 days after herbivory treatments. This was inconsistent with the finding of Wilson et al. (2007) that mowing increased plant root-to-shoot mass after 5 weeks. This inconsistency may be explained by (1) the differing duration of the two experiments. We measured plant root and shoot mass 80 days after herbivory treatments when damaged plants had fully recovered, while Wilson et al. do the same measurements just 5 weeks after herbivory treatments when damaged plants had not yet fully recovered. (2) The varying responses of plant to herbivory may also results in differing herbivory treatments (Tiffin and Inouye 2000). We used actual herbivores in our study, while herbivory in their study were artificially imposed.
Tolerance to herbivory is an important trait of invasive species, and is proposed to confer competitive superiority to invasive plants in the presence of herbivores (Müller-Schärer et al. 2004). However, when it occurs and how it is affected by the density and diversity of herbivores remains unclear. A. philoxeroides had shown to allocate a larger proportion (up to 60 %) of biomass to below-ground tissues than A. sessilis (up to 30 %) (Lu and Ding, unpub. data), and is more tolerant than A. sessilis when 50 and 100 % of the leaf area was clipped (Sun et al. 2010). In our field experiment, the beetle removed an average of 18.8 % of the leaf area similar to that we observed in the field in Wuhan. The beetle had no impact on either invasive or native plants, indicating that both species were fully able to recover from defoliation and thus may not subject to top-down control by C. piperata in this region. This may explain why the beetle had no impact on the competition between the two species in our study.
We detect no difference in competitive ability among A. philoxeroides populations, though they had shown to be vary in tolerance to herbivory (Lu and Ding 2012). This may be explained by the competitor we chosen in this study. In experiments of inter-specific competition, competitor identity can strongly affect the ability to detect intra-specific variation in competitive ability (Bossdorf et al. 2004). Within a single growth season, A. sessilis individuals produce many more stems and ramets, and grow more rapidly than A. philoxeroides (Lu and Ding, unpub. data). These traits may enable A. sessilis to use above-ground resources (i.e., light) more efficiently than A. philoxeroides, and thus making A. sessilis more competitive as evidenced in this study. The higher competitive ability of A.sessilis may have masked the difference in competitive ability among A. philoxeroides populations.
Plant compensatory response to herbivory is regulated by a series of biotic factors, including timing of herbivory and insect species (Strauss and Agrawal 1999). Beside C. piperata, the insects Hymenia recurvalis Fabricius (Lepidoptera: Pyralidae) and Spodoptera litura Fabricius (Lepidoptera: Noctuidae), along with a root-knot nematode Meloidogyne incognita (Kofold and White) Chitwood also attack A. philoxeroides (Ma 2001; Mao et al. 2011). They may interact antagonistically or additively with C. piperata on A. philoxeroides though in most cases the damage they caused is negligible. Moreover, the presence of other currently unknown herbivores or pathogens might be of importance in this novel range. The compensatory ability of a plant is also affected by abiotic factors, such as resource availability (Maschinski and Whitham 1989). Resource availability may increase plant growth rate and herbivory intensity by altering plant palatability (i.e., nitrogen content), and thus may directly or indirectly influence plant compensation. Therefore, further studies evaluating the impact of native herbivores on the performance of invasive species and their competition with native species need to be conducted across varying environmental conditions (i.e., resource availability and composition of herbivores).
The negative impacts of herbivory on plant performance and competitive ability have been uncritically assumed to explain the failure or success of invasive species. Our study demonstrated that herbivory by novel enemies may not directly translate into reduced plant performance because plants are capable of compensation. This implies that novel herbivores in their new ranges may not provide resistance to exotic plant invasion despite the fact that they may accumulate on exotic plants. In addition, our results indicate that although the invasive species may be more tolerant to herbivory, attack by native herbivores may not facilitate exotic plant invasion in the field, since susceptible natives may also be able to sustain a certain degree of herbivory in natural habitats. Thus, it is necessary to assess the impact of novel enemies on exotic plant performance and competition with natives to elucidate the conditions under which novel herbivores may facilitate or slow the invasion process.
References
Agrawal AA, Kotanen PM (2003) Herbivores and the success of exotic plants: a phylogenetically controlled experiment. Ecol Lett 6:712–715. doi:10.1046/j.1461-0248.2003.00498.x
Bertheau C, Brockerhoff EG, Roux-Morabito G, Lieutier F, Jactel H (2010) Novel insect-tree associations resulting from accidental and intentional biological ‘invasions’: a meta-analysis of effects on insect fitness. Ecol Lett 13:506–515. doi:10.1111/j.1461-0248.2010.01445.x
Bossdorf O, Prati D, Auge H, Schmid B (2004) Reduced competitive ability in an invasive plant. Ecol Lett 7:346–353. doi:10.1111/j.1461-0248.2004.00583.x
Callaway RM, Newingham B, Zabinski CA, Mahall BE (2001) Compensatory growth and competitive ability of an invasive weed are enhanced by soil fungi and native neighbours. Ecol Lett 4:429–433. doi:10.1046/j.1461-0248.2001.00251.x
Chun YJ, Kleunen MV, Dawson W (2010) The role of enemy release, tolerance and resistance in plant invasions: linking damage to performance. Ecol Lett 13:937–946. doi:10.1111/j.1461-0248.2010.01498.x
Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7:721–733. doi:10.1111/j.1461-0248.2004.00616.x
Dai H, Lu X, Zhang J, Ding J (2013) Responses of a native beetle to novel exotic plant species with varying invasion history. Ecol Entomol 39:118–124. doi:10.1111/een.12072
Gendron AD, Marcogliese DJ, Thomas M (2012) Invasive species are less parasitized than native competitors, but for how long? The case of the round goby in the Great Lakes-St. Lawrence Basin. Biol Invasions 14:367–384. doi:10.1007/s10530-011-0083-y
Julien MH, Skarratt B, Maywald GF (1995) Potential geographical distribution of alligator weed and its biological control by Agasicles hygrophila. J Aquat Plant Manage 33:55–60
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170. doi:10.1016/S0169-5347(02)02499-0
Li Y-P, Feng Y-L, Barclay G (2012) No evidence for evolutionarily decreased tolerance and increased fitness in invasive Chromolaena odorata: implications for invasiveness and biological control. Plant Ecol 213:1157–1166. doi:10.1007/s11258-012-0073-x
Lin G, Yang Y, Hu J (1990) Studies on biology and control of Alternanthera philoxeroides. J Jiangsu Agric Coll 11:57–63 (in Chinese)
Lu X-M, Ding J (2010) Flooding compromises compensatory capacity of an invasive plant: implications for biological control. Biol Invasions 12:179–189. doi:10.1007/s10530-009-9441-4
Lu X, Ding J (2012) History of exposure to herbivores increases the compensatory ability of an invasive plant. Biol Invasions 14:649–658. doi:10.1007/s10530-011-0106-8
Lu X, Dai H, Ding J (2010) Con-specific neighbours may enhance compensation capacity in an invasive plant. Plant Biol 12:445–452. doi:10.1111/j.1438-8677.2009.00247.x
Lu X-M, Siemann E, Shao X, Wei H, Ding J-Q (2013) Climate warming affects biological invasions by shifting interactions of plants and herbivores. Glob Change Biol 19:2339–2347. doi:10.1111/gcb.12244
Ma R-Y. 2001 Ecological adaptation for the introduced biocontrol agent, Agasicles hygropghila, for Alligatorweed, Alternanthera philoxeroides, in China. PhD Thesis, Chinese Academy of Agricultural Sciences, Beijing, p 120 (in Chinese)
Mao R, Lu X, Ding J (2011) Effects of a nematode Meloidogyne incognita and its interaction with above-ground herbivory on an invasive wetland plant, alligator weed (Alternanthera philoxeroides). Plant Spec Biol 26:73–83. doi:10.1111/j.1442-1984.2010.00309.x
Maron JL, Vilà M (2001) When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 95:361–373. doi:10.1034/j.1600-0706.2001.950301.x
Maschinski J, Whitham TG (1989) The continuum of plant responses to herbivory: the influence of plant association, nutrient availability, and timing. Am Nat 134:1–19. doi:10.1086/284962
Morrison WE, Hay ME (2011) Herbivore preference for native vs. exotic plants: generalist herbivores from multiple continents prefer exotic plants that are evolutionarily naïve. PLoS ONE 6:e17227. doi:10.1371/journal.pone.0017227
Mousseau TA, Dingle H (1991) Maternal effects in insect life histories. Annu Rev Entomol 36:511–534. doi:10.1146/annurev.en.36.010191.002455
Müller-Schärer H, Schaffner U, Steinger T (2004) Evolution in invasive plants: implications for biological control. Trends Ecol Evol 19:417–422. doi:10.1016/j.tree.2004.05.010
Oksanen L, Fretwell SD, Arruda J, Niemela P (1981) Exploitation ecosystems in gradients of primary productivity. Am Nat 118:240–261. doi:10.1086/283817
Parker JD, Hay ME (2005) Biotic resistance to plant invasions? Native herbivores prefer non-native plants. Ecol Lett 8:959–967. doi:10.1111/j.1461-0248.2005.00799.x
Parker JD, Burkepile DE, Hay ME (2006) Opposing effects of native and exotic herbivores on plant invasions. Science 311:1459–1461. doi:10.1126/science.1121407
Pearson DE, Potter T, Maron JL (2012) Biotic resistance: exclusion of native rodent consumers releases populations of a weak invader. J Ecol 100:1383–1390. doi:10.1111/j.1365-2745.2012.02025.x
Persson I-L, Danell K, Bergström R (2005) Different moose densities and accompanied changes in tree morphology and browse production. Ecol Appl 15:1296–1305. doi:10.1890/04-0499
Roach DA, Wulff RD (1987) Maternal effects in plants. Annu Rev Ecol Evol S 18:209–235. doi:10.1146/annurev.es.18.110187.001233
Sainty G, McCorkelle G, Julien M (1998) Control and spread of alligator weed Alternanthera philoxeroides (Mart.) Griseb., in Australia: lessons for other regions. Wetl Ecol Manage 5:195–201. doi:10.1023/A:1008248921849
Schooler SS, Yeates AG, Wilson JRU, Julien MH (2007) Herbivory, mowing, and herbicides differently affect production and nutrient allocation of Alternanthera philoxeroides. Aquat Bot 86:62–68
Siemann E, Rogers WE, Dewalt SJ (2006) Rapid adaptation of insect herbivores to an invasive plant. Proc R Soc Lond B Biol Sci 273:2763–2769. doi:10.1098/rspb.2006.3644
Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends Ecol Evol 14:179–185. doi:10.1016/S0169-5347(98)01576-6
Sun Y, Ding J, Frye MJ (2010) Effects of resource availability on tolerance of herbivory in the invasive Alternanthera philoxeroides and the native Alternanthera sessilis. Weed Res 50:527–536. doi:10.1111/j.1365-3180.2010.00822.x
Tang Y-G (1994) Study on the bionomies of Cassida piperata Hope (Coleoptera: Cassididae). Entomol Knowl 31:158–160 (in Chinese)
Tiffin P, Inouye BD (2000) Measuring tolerance to herbivory: accuracy and precision of estimates made using natural versus imposed damage. Evolution 54:1024–1029. doi:10.1111/j.0014-3820.2000.tb00101.x
Verhoeven KJF, Biere A, Harvey JA, van der Putten WH (2009) Plant invaders and their novel natural enemies: who is naïve? Ecol Lett 12:107–117. doi:10.1111/j.1461-0248.2008.01248.x
Wilson JRU, Yeates A, Schooler S, Julien MH (2007) Rapid response to shoot removal by the invasive wetland plant, alligator weed (Alternanthera philoxeroides). Environ Exp Bot 60:20–25. doi:10.1016/j.envexpbot.2006.06.003
Acknowledgments
We would like to thank Wenfeng Guo, Hongjun Dai, Yan Sun, and Sunliang Feng for their field and laboratory assistance. This work was funded by the Knowledge Innovation Program of the Chinese Academy of Sciences and the National Science Foundation of China (31100302 and 31370547) while preparing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Martin Nunez.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, X., Shao, X. & Ding, J. No impact of a native beetle on exotic plant performance and competitive ability due to plant compensation. Plant Ecol 215, 275–284 (2014). https://doi.org/10.1007/s11258-014-0296-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-014-0296-0