Abstract
Viticulture is characterized by substantial pesticide applications, impacting natural enemies. New pest control strategies and management of plant diversity into agrosystems acting as reservoirs of natural enemies are assumed to limit pesticide use. Various studies support this hypothesis but gaps exist on the effect of diversification on Phytoseiidae mites, generalist predators reported as prevalent and efficient natural enemies in vineyards. This study focuses on the effect of cover crop management (no cover crop, spontaneous cover crops with or without agroforestry) and grape variety (resistant cv. Artaban and cv. Syrah) on predatory mites and prey communities, in a newly planted experimental vineyard in South-East France. Samplings were carried out three times a year on vine, cover crops, and co-planted trees. Phytoseiidae, Tydeiidae, Eriophyidae mites and thrips were characterized. Nine Phytoseiidae species were identified on vine, the main ones being Kampimodromus aberrans, Typhlodromus exhilaratus, Phytoseius finitimus and Euseius gallicus. Kampimodromus aberrans was prevalent on the cv. Syrah, highlighting a strong effect of variety. The low unexpected effect of system management observed outcome could be due to several factors, such as the experimental plot size or the influence of vine stress on Phytoseiidae communities in vines with cover crops. All phytoseiid species present on vine were identifed at least once on cover crops and co-planted trees, suggesting their potential role as reservoirs. Further studies should be performed investigating the evolution of communities in this newly-planted experimental system, as well as potential differences in trophic network interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agrosystem diversification via a greater diversity of spontaneous or managed vegetation can (i) favour beneficial organisms and thereby pest regulation services, by providing refuges and foods and (ii) unfavour pests (especially monophagous) by limiting dispersion/settlement (Altieri and Letourneau 1982; Andow 1991; Altieri and Nicholls 2002; Togni et al. 2019; Blassioli-Moraes et al. 2022). Such correlations are supported in various meta-analyses in different crop systems and at different scales (e.g. Landis et al. 2000; Paredes et al. 2013; Lichtenberg et al. 2017; Venzon et al. 2019). However, these trends are not always consistent across pests and crops and further knowledge is required to identify which plant diversity can be managed and how, for applied advice to farmers in a context of ecological transition (Vialatte et al. 2023).
Among the natural enemies in vineyards, predatory mites of the family Phytoseiidae are known to be the most frequent and efficient natural enemies of phytophagous mites (e.g. Duso et al. 2010, 2012). Most of them are generalist predators, feeding on prey (mite pests and small insects) and other food resources, as pollen, nectar and mycelium (Duso et al. 2004; McMurtry and Croft 1997; McMurtry et al. 2015). Several studies have shown that their natural occurrence in neighbouring vegetation around vineyards positively impacts pest biological control (i.e. Boller et al. 1988; Duso 1993; Tixier et al. 1998, 2000a, 2000b). Other works dealing with vine agroforestery management showed a slight impact on Phytoseiidae mite communities, depending on co-planted trees and vine varieties (Altieri and Nicholls 2002; Barbar et al. 2006; Liguori et al. 2011; Tixier et al. 2015).
However, only a few studies focused on cover crop effect as a reservoir of Phytoseiidae mites (Girolami et al. 2000; Vogelweith and Thiery 2017; Sáenz-Romo et al. 2019; Rosado et al. 2021; Möth et al. 2021, 2023). Sáenz-Romo et al. (2019) reported the occurrence of Typhlodromus pyri Scheuten both on vine and ground cover vegetation, suggesting that cover crop could shelter this predator. In addition, natural vegetation could also act as a reservoir of alternative food (prey and pollen). Some studies report positive correlations between flowering season, pollen air borne density and Phytoseiidae mite abundance (e.g. Sentenac et al. 2018; Duso et al. 2004; Malagnini et al. 2022). All these elements as well as other studies dealing with cover crops in orchards (e.g. citrus, apple trees, e.g. Nyrop et al. 1998; Mailloux et al. 2010; Aguilar-Fenollosa et al. 2011; Sahraoui et al. 2014) show that cover crop globally positively impacts Phytoseiidae mite densities. However, additional studies are required to determine more accurately the functional interactions between vineyard cover crops for regulation services, that might depend on (i) the Phytoseiidae species and associated resource requirements (habitat and foods) and (ii) the ability of the naturally present plants to supply these resources.
This study aims at evaluating the Phytoseiidae communities in a Mediterranean vineyard with contrasted management vegetation management (no cover crops, spontaneous cover crops, spontaneous cover crops + agroforestery management) with low application of pesticides due to the use of a new variety resistant to powdery and downy mildew (cv. Artaban). In addition, Phytoseiidae communities were evaluated on another traditional vine variety (cv. Syrah) in the control modality without cover crop. The hypotheses tested are thus as follow: (i) soil management influences the diversity and abundance of Phytoseiidae mites and their prey on vines, (ii) the vine variety affect diversity and density of Phytoseiidae mites, and (iii) plants in cover crops and co-planted trees act as reservoir of Phytoseiidae mites and potential prey.
Material and methods
The vine plots studied
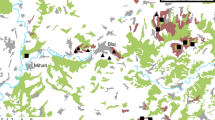
The vine plots studied, planted in 2019, are located at Villeneuve-les-Maguelone (43° 31′ 50.46ʺ N 3° 52′ 05.95ʺ E), near Montpellier, South of France. They belong to an experimental domain (Domaine du Chapitre) where agricultural practices are homogenously controlled. The three soil management systems considered are included in a 3 ha-vineyard to limit effects of different climate and surrounding natural vegetation. They were similarly conducted, except for soil and herbicide management (see below). Because vines are young, suffering competition for water and nutrients, irrigation was carried out on vine plots with cover crops. No Phytoseiidae introduction/releases have been performed. The communities of predatory mites found in the vineyards have thus naturally colonized the vineyards. The three modalities are as follow and illustrated on Fig. 1:
Schematic diagram of the studied plot. Grey lines represent rows of the cv. Artaban while dark line represent rows of the cv. Syrah. Green rectangles correspond to the replicates of the modality “Aviti” (cover crop modality), Blue rectangles correspond to the replicates of the modality “Dviti” (cover crop + agroforestry modality) and Red rectangles correspond to the replicates of the modality “Tviti” (control modality). Violet lines represent rows of co-planted trees (fig and pomegranate trees) in the “Dviti” system
(i) “Tviti”: control modality (3 replicates corresponding each to 12 rows of 60 m long with 6 rows of cv. Artaban and 6 rows of cv. Syrah, plantation density: 3333 vinestocks/ha): no covercrop, plants mechanically destroyed four times a year between the rows. “Tviti – Artaban” and “Tviti – Syrah” will be used to specify the grape variety considered. No fungicide was applied on the cv. Artaban as it is resistant to mildew and powdery mildew. Fungicide treatments were applied (fluopyram and trifloxystrobine on 15 June 2022, Copper and sulfur on 07 July 2022) on the cv. Syrah. Both cultivars were treated in a same way to control the cicadellid Scaphoideius titanus Ball on the 22 June 2022 (using Esfenvalerate).
(ii) “Aviti”: covercrop modality (3 replicates corresponding each to 12 rows of 60 m long, plantation density: 3,571 vinestocks/ha with the cv. Artaban), spontaneous vegetation between the rows mowed three times a year (30 March 2022, 15 June 2022 and 01 August 2022), rice and coconut fiber mulch used along the row to avoid grass.
(iii) “Dviti”: covercrop + agroforestry modality (3 replicates corresponding each to 8 vine rows of 60 m long + two rows of trees—one row of Ficus carica L. (Moraceae) and one row of Punica granatum (Lythraceae), plantation density: 3,571 vinestocks/ha with the cv. Artaban). The spontaneous vegetation was managed as in “Aviti”. The three species were chosen because of drought resistance (adapted to the Mediterranean climate) and potential additional income for growers.
Samplings, countings and identifications
Samplings were carried out three times a year in 2022 (3 May 2022, 8 June 2022, 30 August 2022) to study whether the effect of plant diversification is stable over the season, as mite densities and sometimes species change through the season. At each sampling date, for each replicate of each modality and vine variety, 10 vine leaves were randomly collected. At each sampling date and for the three replicates of the modality “Dviti”, 10 leaves of F. carica and 10 shoots of 10 cm of P. granatum were randomly sampled. The plant species in the cover crops (in "Aviti" and "Dviti" modalities) were identified and their abundance assessed two times a year (27 April 2022, 31 May 2022), placing six and five quadrats (0.25 m2) for “Dviti” and “Aviti”, respectively. The most frequent plant species (24) were then randomly sampled in the cover crop of the three replicates of “Aviti” and “Dviti”. Samples were maintained in iceboxes and brought back to the laboratory. Then, the ‘soaking- checking-washing-filtering’ method (Boller 1984) was applied, using a double filtration on 200 µm and 25 µm mesh- sieves to extract Phytoseiidae mites and their potential prey categorised as (i) tydeiid mites, (ii) eriophyid mites (mainly Colomerus vitis) and (iii) thrips. No Tetranychidae mite was observed in the samples, for this they were not considered. Phytoseiidae and potential prey were counted under a stereoscopic microscope (× 25 magnification). For eriophyid mite countings, a few drops of methylene blue were added to distinguish between vegetative material (blue color) and arthropods (white-yellow color) (Duffner 1999). Phytoseiidae mites were mounted on slides in Hoyer’s medium and identified with a phase contrast and Differential interference contrast (DIC) optical microscope (Leica DMR) based on the generic classification of Chant and McMurtry (2007) and specific literature (e.g. Ferragut et al. 2009; Papadoulis et al. 2009).
Data analyses
The Shannon index was used to compare the diversity and distribution of Phytoseiidae species on vine between the systems considered (Barbault 1992). Statistical analyses were performed using R software (version 4.2.2). Figures were done with ‘ggplot2’ package (Wickham et al. 2016). To compare the abundance, Shannon index and richness of each organism considered (Phytoseiidae, Tydeiidae, Eriophyidae, thrips) in the systems and vine varieties, Generalized Linear Mixed Models (GLMM) with a negative binomial link function were used (‘lme4’ package, Bates et al. 2015). Replicates were considered as a random effect. When the assumptions of normality and homoscedasticity were validated, Analyses of variance (Anova) were performed on these models (‘car’ package, Fox et al. 2012), followed by multiple comparison tests (Tukey test, ‘multcomp’ package, Hothorn et al. 2016). When these conditions were not validated, non-parametric tests (Kruskal Wallis, 'rstatix' package, Kassambara 2021) were performed followed by multiple comparison tests (Dunn’s tests, ‘dunn.test’ package, Dinno and Dinno 2017). The p-values were then adjusted with the Holm method. In some cases, the Kruskal–Wallis tests indicated significant differences between the modalities, but the non-parametric post hoc Dunn tests with Holm correction did not show statistically significant results. In these cases, we concluded that there was no significant difference between the modalities tested. To determine the impact of plant diversity and prey on Phytoseiidae communities, Principal Component Analyses were performed using the packages ‘FactoMineR’ and ‘factoextra’ (Lê et al. 2008; Kassambara 2016).
To compare the Phytoseiidae abundance between the cover crops and the plant species, because the amount of vegetation per plant species was not equally calibrated, it was not possible to directly compare Phytoseiidae abundance between plants. Thus, we used the Phytoseiidae frequency as a proxy of Phytoseiidae abundance, considering the percentage of time that Phytoseiidae were found on a plant species (named Phytoseiidae occurrence frequency). Furthermore, to assess the potential recovery rate of each Phytoseiidae species on each plant species in cover crops of each system, we proposed an index calculated as follows:
Rʹ(Phytoseiidae species, Plant species) = Plant species recovery rate × Phytoseiidae species occurrence frequency
Where, “Plant species recovery rate” represents the average percentage of the plant species (obtained from quadrat data) and “Phytoseiidae species occurrence frequency” represents the number of times a Phytoseiidae species was found on a plant species. To estimate the overall abundance of a Phytoseiidae species within the covercrop, a total recovery rate (named Rtotal) was then calculated by summing for each Phytoseiidae species the R’ indices of each plant species present in each cover crop. Both indicators were calculated for May and June samplings, as no data on plant occurrence was available in August. These indices were calculated for the three replicates of the two systems “Aviti” and “Dviti”.
Results
Phytoseiidae on vine
1409 Phytoseiidae specimens belonging to nine species were collected. The most frequent species were Kampimodromus aberrans (Oudemans) (49,8%), Euseius gallicus Kreiter & Tixier (19,45%), Phytoseius finitimus Ribaga (13,91%) and Typhlodromus (T.) exhilaratus Ragusa (8,68%). The other species, less frequently observed, were Euseius stipulatus (Athias-Henriot), Typhlodromus (Anthoseius) recki Wainstein, Paraseiulus soleiger (Ribaga), P. triporus (Chant & Yoshida-Shaul) and P. talbii (Athias-Henriot). The mean number of Phytoseiidae/leaf (Ph/l) was significantly different between the three sampling dates (P = 2.16e-2), with lower densities in May than in June and August (0.24 ± 0.37 Ph/l in May, 2.27 ± 2.50 Ph/l in June and 1.86 ± 1.45 Ph/l in August). The number of Phytoseiidae species was significantly different through time (P = 1.49e-4), being lower in May (0.74 ± 0.79) than in June (2.17 ± 1.08) and August (2.74 ± 1.35).
Effect of grape vine variety on predatory mite communities
Effect of vine variety was studied only considering the control modality to prevent interactions with cropping management. The average number of Phytoseiidae was significantly lower on the cv. Artaban (1.55 ± 1.68 Ph/l) than on the cv. Syrah (2.99 ± 2.67 Ph/l) (P = 2e-47). In contrast, species richness was significantly higher on the cv. Artaban (2.35 ± 1.67) than on the cv. Syrah (1.30 ± 0.72) (P = 1e-32). Similar results were observed for the Shannon index (cv. Artaban, H’ = 0.80 ± 0.73; cv. Syrah, H’ = 0.16 ± 0.26) (P = 2.86e-4), especially because of the predominance of K. aberrans on the cv. Syrah for the three sampling dates (Fig. 2). On the cv. Artaban, the species differ across time, T. exhilaratus being the sole species in May, while E. gallicus and P. finitimus were dominant in June and August.
a Proportion (%) and b total number of each Phytoseiidae species (number/leaf) collected on vine for each system considered (“Aviti”: cover crop modality, “Dviti”: cover crop + agroforestry modality, “Tviti-Artaban”: control modality planted with the cv. Artaban, “Tviti-Syrah”: control modality planted with the cv. Syrah) and at the three collection dates
Effect of cropping systems on predatory mite communities on vine
The data here considered are those from the sampling carried out on the cv. Artaban only in the three management systems, to avoid variety effects. Merging data for all the dates, the mean Phytoseiidae density was significantly different between the three systems (P = 1.93e-2), with the highest densities observed in the control modality. This is exclusively due to June observations, as only at this date, differences in mean densities of Phytoseiidae were observed (Fig. 3a). No statistical differences were noted in species richness (P = 0.24) and Shannon index (P = 0.50) between the three systems when pooling data of the three dates. The species richness was not significantly different either for each sampling date (Fig. 3b), whereas the Shannon index was significantly different between the systems at each sampling date (higher in “Aviti” than in the other systems in May (P = 0.03), lower on “Aviti” than “Tviti-Artaban” in August (P = 0.03)) (Fig. 3c). Globally, the Shannon index increased over time (Hʹ = 0.13 ± 0.36 in May, Hʹ = 0.93 ± 0.63 in June, Hʹ = 1.30 ± 0.59 in August) (P = 4.57e-8). Considering each cropping system separately, the Shannon index was not statistically different between the three dates for the modality “Aviti”, where as it was statistically lower in May than June in the modality “Dviti”, and different between the three collections dates for the control modality “Tviti – Artaban” (Supplementary file 1).
a Mean numbers of Phytoseiidae per vine leaf, b Richness of Phytoseiidae (mean numbers of species) and c Mean Shannon index of Phytoseiidae communities in the three managing systems considered (“Aviti”: cover crop modality, “Dviti”: cover crop + agroforestry modality, “Tviti-Artaban”: control modality planted with the cv. Artaban), at the three sampling dates. Different letters above the bars correspond to statistical differences between the systems
Phytoseiidae species differ over time, regardless of the system, especially between May and the other two dates (Fig. 2a; Table 1). In May, T. exhilaratus was the main species whereas in June and August even if present, its proportion was very low compared to P. finitimus and E. gallicus. It is worth noting the presence of E. stipulatus in August in the three systems, whereas this species was absent in May and June (Fig. 2). The proportion of T. (A.) recki remained consistently low through time and in all the systems, with the highest proportion observed in June in "Dviti". In most cases, nor the proportion nor the abundance of the main Phytoseiidae species were different between the systems for each date; only in June "Tviti-Artaban" exhibited a higher proportion and abundance of E. gallicus compared to "Aviti" and "Dviti" (63.47% vs. 32.46% and 24.93%, respectively, Table 2). Even if no significant difference was noted, some elements can be pointed out (i) in May: E. gallicus and P. finitimus were exclusively present in "Aviti" while T. exhilaratus was the sole species found in "Tviti-Artaban"; K. aberrans and P. soleiger were only observed in "Dviti"; (ii) in June: a lower proportion of P. finitimus was observed on “Tviti-Artaban” than in the other modalities (13.04% vs. 28.44% and 25.97%, respectively), and the highest proportion of K. aberrans was found on "Dviti", and (iii) in August: the proportion of P. finitimus was slightly higher in "Aviti" (39.63%) compared to "Dviti" (26.28%) and "Tviti-Artaban" (17.92%).
Potential prey on vine
Eriophyidae
3010 Eriophyidae were observed on vine. Significantly higher densities were found in June than at the two other dates (P = 2.2e-16). Considering vine variety effect, differences were only observed in June (P = 0.016), with a significantly higher number on the cv. Artaban (11.11 ± 10.64 Eriophyidae per leaf) than on the cv. Syrah (3.34 ± 2.06 Eriophyidae per leaf). No effect of the managing system was observed for all the dates pooled (P = 0.15) nor for each date considered separetely (Table 1).
Tydeidae
1383 Tydeidae were observed on vine. The density was significantly higher in June than at the other sampling dates (P = 7.23e-6). No difference between grape varieties was observed in May (P = 0.15) and August (P = 0.53). In June, the Tydeidae density was higher on the cv. Artaban (4.30 ± 2.50 Tydeidae per leaf) than on the cv. Syrah (1.36 ± 1.31 Tydeidae per leaf) (P = 0.03). Significantly higher numbers of Tydeidae were observed on ‘Tviti-Artaban’ (2.07 ± 2.21 Tydeidae per leaf) than on “Aviti” (0.89 ± 1.05), “Dviti” showing intermediate values (1.25 ± 0.98) (P = 0.01). Results are slightly different when focusing on each collection date. In May, greater densities were observed on “Dviti” than on “Aviti”, whereas densities in “Tviti-Artaban” were intermediate (Table 1). In June, higher densities of Tydeidae were observed on “Tviti-Artaban” than on “Aviti” and no statistical difference between the three systems was noted in August (Table 1).
Thrips
202 thrips were observed on vine. Their densities differed between the three dates (P = 1.39e-7) with the highest numbers observed in June (0.33 ± 0.26 thrips/leaf), then in May (0.17 ± 0.21 thrips/leaf) and finally in August (0.02 ± 0.04 thrips/leaf). No statistical difference was observed globally between the two grape varieties (P = 0.88), nor considering each date separately (P = 0.40 in May, P = 0.59 in June, P = 0.23 in August). The thrips densities were not significantly different between the systems, when compared globally nor at each date separately (Table 1).
Relationship between Prey and Phytoseiidae communities
On the cv. Artaban
Principal Component Analyses (PCA) were performed to determine relationships between the abundance of main Phytoseiidae species (T. exhilaratus, K. aberrans, E. gallicus, P. finitimus and T. recki) and of prey in the three systems. In May, the main three axes explain 73.3% of the variability (Fig. 4a, b). The biplots show that the densities of E. gallicus, T. exhilaratus and P. finitimus were well correlated and globally no clear relation was observed between these Phytoseiidae species and prey abundance (thrips, Tydeiidae and Eriophyidae). The three systems were not well separated. In June, the three main axes explain 69% of the variability (Fig. 4c, d). The biplots show positive correlation between densities of P. finitimus and E. gallicus, as well between these two species and densities of Eriophyidae mites. A positive correlation was also observed between the densities of K. aberrans, thrips and Tydeiidae. The two diversified systems were grouped together, whereas the modality “Tviti-Artaban” stands out, being positively related to both predator and potential prey abundance. In August, the three main axes explain 63.35% of the variability (Fig. 4e, f). The biplots show positive correlations between the densities of Eriophyidae, P. finitimus and K. aberrans in a lesser extent; whereas this prey was not well related to the densities of E. gallicus. Furthermore, the densities of K. aberrans and Tydeiidae were also correlated. No differentiation of the systems was observed at that date.
Biplots of the Principal Component Analyses carried out for data in May (a, b), June (c, d) and August (e, f) considering all variables (prey, main Phytoseiidae species abundance and total abundance of ‘Phytoseiidae’) for the three systems (“Aviti” in red: cover crop modality, “Dviti” in green: cover crop + agroforestry modality, “Tviti-Artaban” in blue: control modality planted with the cv. Artaban). For each date, the three main axes are considered and variables only represented if its cos2 > = 0.4
On the cv. Syrah
Similar Principal Component Analyses (PCA) were conducted for the cv. Syrah (Supplementary information 2). In May, the biplot (axes 1 & 2 explaining 80.5% of the variability) shows (i) positive correlations between the abundance of K. aberrans and Tydeidae and (ii) negative correlations between the abundance of K. aberrans and thrips, and the density of P. finitimus and Eriophyidae. In June, the biplot (axes 1 and 2 explaining 73.2% of the variability) shows (i) positive correlations between the abundances of K. aberrans and Tydeidae, and (ii) negative correlations between the density of Eriophyidae and that of the three main Phytoseiidae species (P. finitimus, K. aberrans, E. gallicus) and between the density of thrips and P. finitimus. Moreover, a positive correlation was noted between densities of thrips and E. gallicus. In August, the biplot (axes 1 & 2 explaining 83.3% of the variability) shows positive correlations between the abundances of K. aberrans, Tydeidae and Eriophyidae. Conversely, negative correlations were observed between the density of K. aberrans and thrips.
Phytoseiidae communities on trees of “Dviti” system
641 Phytoseiidae were collected on F. carica and P. granatum. On F. carica, K. aberrans was the prevalent species (52.75%), followed by T. recki (17.44%) and P. finitimus (16.95%) (Fig. 5b). On pomegranate, E. gallicus was the main species (95.24% in June), and T. recki was also present (4.76%) (Fig. 5b). The three species found on the two co-planted trees were among the five most abundant species retrieved on vine. Phytoseiidae densities on each tree species were significantly different between the three sampling dates (fig: P = 6.3e-2, pomegranate: P = 6.5e-2). The abundance of Phytoseiidae was higher on fig leaves (6.87 ± 7.9/leaf) than on pomegranate shoots (0.18 ± 0.32/shoot) where data are pooled (P = 3.69e-3) (Fig. 5a). When comparing the richness of Phytoseiidae on fig and pomegranate (Fig. 5a), no difference was observed (P = 0.25).
Phytoseiidae species on cover crop
Twelve Phytoseiidae species were observed on the 24 plants considered in the cover crops: K. aberrans, T. exhilaratus, E. gallicus, P. finitimus, T. (A.) recki, Neoseiulus alpinus (Karg), Graminaseius graminis (Chant), Amblyseius obtusus (Koch), Amblyseiella setosa Muma, P. triporus and P. soleiger. The main species found on vine were thus also retrieved in the cover crops. Neoseiulus alpinus, G. graminis, A. obtusus and A. setosa were not found on vine. Paraseiulus triporus and P. soleiger were found in the two compartiments but in both cases very few specimens were observed.
In May, Phytoseiidae occurrence was low. Only six and four species were observed on the cover crops of “Aviti” and “Dviti”, respectively (Fig. 6a, b). Among these species, T. exhilatatus and T. recki occurred in both systems, and P. finitimus only in the cover crop of “Aviti”. Phytoseiidae were found on 31% and 37% of the plant sampled in the cover crops of “Aviti” and “Dviti”, respectively. In June, the covercrops show a much higher Phytoseiidae diversity and frequency on a higher number of plant species (Fig. 6c, d). They were detected on 76% and 58% of the plants sampled in the cover crop of "Aviti" and "Dviti", respectively. Among the 9 and 8 Phytoseiidae species observed in the cover crops of "Aviti," and "Dviti”, respectively, the two most frequent and abundant species were T. recki and T. exhilaratus for the two systems. Typhlodromus recki was found on 58% and 46% plant species in "Aviti" and “Dviti” cover crops, respectively, whereas T. exhilaratus was found on 53% and 26% plant species in “Aviti” and “Dviti" cover crops, respectively. Kampimodromus aberrans and E. gallicus only found on the cover crops in June were present at low frequency, on three and two plants in “Aviti” and “Dviti” for K. aberrans and one and two plants in “Aviti” and “Dviti” for E. gallicus. Phytoseius finitimus was poorly frequent, being observed on only two plants in each system. This species observed in May on Medicago sativa L. in the cover crop of “Aviti” was not retrieved on the same plant in this system in June. Most of the plant species harboured two or three species of Phytoseiidae. Plants sheltering T. exhilaratus were globally the same as those harbouring T. recki. Two plant species sheltered a higher Phytoseiidae diversity whatever the system considered, Helminthotheca echioides and Erigeron sumatrensis, and the main Phytoseiidae reported on vine were retrieved on both. Euseius gallicus was only found on these two cover crop plants.
Occurrence frequency (in %) of Phytoseiidae species on each plant species of the cover crop for the systems: “Aviti” in red (cover crop modality) in May and June (a, c) and “Dviti” in blue (cover crop + agroforestry modality) in May and June (b, d). The darker the color, the higher the occurrence frequency is
Phytoseiidae reservoir capacity of covercrops
The abundance of each plant species harbouring Phytoseiidae mites in “Aviti” and “Dviti” was very similar, with H. echioides, Vicia faba, E. sumatrensis, Medicago sativa and Picris hieracioides being the dominant plant species (Supplementary file 3). The two plant species harbouring the highest Phytoseiidae diversity and occurrence were thus among the plants most frequenly present in the cover crops. The highest R’ indices were observed for H. echioides and E. sumatrensis for all Phytoseiidae species, even if the former accounts for better scores (Supplementary file 4). Globally, higher R’ indexes were recorded in “Aviti” than in “Dviti”, suggesting that in “Aviti” the reservoir potential would be higher. The Rtotal indices, corresponding to an estimate for each system of the amount of each Phytoseiidae species, are presented on Fig. 7. These indices are globally higher in June than in May. In May, no clear difference was observed between the two systems, but the main Phytoseiidae retrieved on vine were only reported on “Aviti”. In June, the Rtotal indices were globally higher in “Aviti” than in “Dviti” especially for T. recki, T. exhilaratus and P. finitimus but not for K. aberrans and E. gallicus (the two species prevailing on co-planted trees in “Dviti”).
Proportion of Phytoseiidae species on vines and co-planted trees, and Rtotal of the cover crops for each Phytoseiidae species for the two systems: “Aviti” (cover crop modality) in May and June (a, c), “Dviti” (cover crop + agroforestry modality) in May and June (b, d). For cover crop and co-planted trees, only the plant species on which a Phytoseiidae species were found at least once in the vines were considered. Digits on each proportion of Phytoseiidae species on vines represent the number of different plant species in the cover crop or trees harbouring the considered Phytoseiidae species at least once. Digits next to each plant species of the cover crop and trees represent the number of Phytoseiidae species found at least once on the plant species considered
Relationships between Phytoseiidae on vine, cover crops and trees
Phytoseiidae communities on vine, cover crops and trees are illustrated on Fig. 7 for both dates and systems. In May on “Aviti”, two species (T. exhilaratus and P. finitimus) observed on the vine were also present in the cover crops on M. sylvestris and M. sativa, respectively (Fig. 7a). However, whereas the prevalence of these two Phytoseiidae species in the cover crops was similar, only T. exhilaratus prevailed on the vine. Euseius gallicus, found on vine, was not retrieved on the cover crops. In May on “Dviti”, T. exhilaratus prevailing on vine was not observed on the cover crop nor on the co-planted trees (Fig. 7b). Kampimodromus aberrans was the unique species found on both vine, fig co-planted tree and cover crop (on only one plant) with a very low Rtotal index, suggesting its scarcety in this environment. In June on “Aviti”, the five species observed on vine were also found in the cover crop (Fig. 7c). No relation was observed between the proportion of each species on vine and the number of plants in the cover plants harbouring this species. As an example, whereas T. exhilaratus and E. gallicus accounted for similar proportion on vine, T. exhilaratus was observed on 9 plant species whereas E. gallicus only on one. The same was observed considering the Rtotal, T. exhilaratus and T. recki were globally the most abundant species in the cover crops, whereas their proportion was not the highest on vine leaves. In June on “Dviti”, all the species found on the vine leaves were retrieved on the cover crops and on the co-planted trees (Fig. 7d). No relation between the proportion of Phytoseiidae species on vine and the number of plants where they were retrieved on the cover crops was noted. No clear relation was either observed between the abundance of each Phytoseiidae species on the covercrop and their proportion on the vine leaves. However, the Rtotal of E. gallicus was higher than in May, whereas that of T. recki and T. exhilaratus were lower suggesting that these two species were not so prevalent in June in the cover crops.
Discussion
General considerations on diversity of Phytoseiidae mites on vine
Nine Phytoseiidae species were identified on the vine plots in this one-year survey. Five species (E. stipulatus, T. recki, P. soleiger, P. talbii and P. triporus) were poorly observed as already reported in other vineyards in South of Europe (Kreiter et al. 2000; Tixier et al. 2013). It is interesting to note that E. stipulatus occurred only in August in quite high densities, without any hypothesis to explain this occurrence. Three other species (K. aberrans, T. exhilaratus, P. finitimus) were much more abundant which is also in line with previous reports of Phytoseiidae in southern Europe vineyards (e.g. Kreiter et al. 2000; Papaioannou-Souliotis et al. 2000; Tixier et al. 2013, 2018). Kampimodromus aberrans is the prevailing species in vineyards in South of France, but this prevalence was here only observed on the cv. Syrah (see below for further discussion). Phytoseius finitimus is a common Phytoseiidae in vineyards in Italy and Corsica (Kreiter et al. 2000; Duso and Fontana 2002; Peverieri et al. 2009; Tixier et al. 2013; Demite et al. 2023). It is the first time that this species is reported so frequently and in so high densities in South of France and several combined hypotheses can put forward to explain this: (i) effect of climatic change and warmer temperatures favoring its presence and development in upper latitudes, (ii) vine variety effect as this species was mainly observed on the cv. Artaban, a new variety that was never considered in vineyard surveys carried out in France. Typhlodromus exhilaratus is commonly found in some vineyards in South of Europe, especially in newly planted crops of different grape varieties (Barbar et al. 2006; Tixier et al. 2006; Peverieri et al. 2009; Duso et al. 2010). Furthermore, it is reported to be adapted to low humidity conditions (Liguori and Guidi 1995; Duso et al. 2010). Finally, it is the the first time that E. gallicus is reported in such high frequency in vineyards. This species, probably a synonym of E. amissibilis Meshkov, might be an invasive species from Asia in expansion in various crops in Europe (dos Santos and Tixier 2018; Döker et al. 2024).
Does the vine variety affect diversity and density of Phytoseiidae mites and their prey?
The Phytoseiidae density was higher on the cv. Syrah compared to the cv. Artaban, whereas the opposite was observed for Phytoseiidae diversity. Kampimodromus aberrans prevailed on the cv. Syrah which is in line with Phytoseiidae fauna in vineyards of South of France (Kreiter et al. 2000, 2002; Tixier et al. 2002). This species is known to be adapted to vine crop conditions in the south of France and to be affected by hairy leaves of vine cultivars (Kreiter et al. 2002). It is a generalist predator able to feed on pollen, and mite pests as C. vitis or Eotetranychus carpini (Oudemans) (e.g., Lorenzon et al. 2012). On the cv. Artaban, T. exhilaratus was the unique species retrieved in May, and E. gallicus and P. finitimus were dominant in June and August. Typhlodromus exhilaratus is a Mediterranean species adapted to low relative humidity, known to feed on mite pests as E. carpini, Tetranychus urticae Koch and pollen (Ragusa 1981; Liguori and Guidi 1990). High populations have been recorded in vineyards infested with eriophyid mites (Liguori 1987, 1988). The biology of P. finitimus is not well known. It is a quite common species in vineyards of south of Europe. As a generalist predator, it probably feeds on mite pests belonging to Tetranychidae and Eriophyidae families and pollen and seems to be most found in cultivars with hairy leaves (Duso and Vettorazzo 1999). The early occurence of T. exhilaratus can be associated to an absence of diapause as reported by Veerman (1992). Its displacement afterwards can be associated to competition due to vine leaf characteristics, climatic conditions, or prey occurrence, but the present study does not allow to dicepher between these factors. It is worth to note that a so different Phytoseiidae species composition between the two varities occured in a same plot, these two cultivars being only separated by an inter-row. Several factors might affect both diversity and densities of Phytoseiidae. First the hairier leaves of the cv. Syrah might be more favorable for Phytoseiidae development and especially for K. aberrans acting on Phytoseiidae competition (e.g. Duso and Vettorazzo 1999; Kreiter et al. 2002; Roda et al. 2003; Duso et al. 2010; Schmidt 2014; Lorenzon et al. 2018; Tixier 2018). Tixier et al. (2006) and Liguori et al. (2011) showed that in a same plot, densities of Phytoseiidae on the cv. Syrah are much higher than on the cv. Grenache (glabrous leaves). However, this is not the unique factor, because in other vineyards planted with other vine varieties including glabrous ones, K. aberrans also prevails and because P. finitimus is also reported on pilose plants as kiwi and pilose vine varieties (Duso and Vettorazzo 1999; Papaioannou-Souliotis et al. 2000; Miñarro and Kreiter 2012; Tixier et al. 2017; Demite et al. 2023). Phytoseiidae mite communities might be also affected by pesticide application. In the present study, the only difference in pesticide application between the plots planted with the two varieties concerns fungicides. The cv. Artaban, resistant to mildew and powdery mildew, received no treatment, whereas fungicides were applied twice on the cv. Syrah. Fungicides are known to affect Phytoseiidae mite via lethal and sub-lethal effects (e.g. Bernard et al. 2010; Gadino et al. 2011). Furthermore, Möth et al. (2021) showed for instance that use of pesticide (without deciphering between pesticide type) was the main factor affecting Phytoseiidae density in vineyards. However, in the present case, densities are higher on treated plots than in not treated ones. We can thus hypothesize that as the species found on the cv. Artaban and the cv. Syrah are different, they might be differently affected by fungicide application. Fungicide resistance in K. aberrans, the main species found on the cv. Syrah but also the dominant in the vineyards of South of France, may be suspected (Auger et al. 2004; Tirello et al. 2012; Lorenzon et al. 2018). The main species found on the cv. Artaban might be less adapated than K. aberrans, or simply physiological susceptibility to pesticide varies according to the species considered (Bergeron and Schmidt-Jeffris 2020). Among the fungicide applied, studies show low to medium impact on Phytoseiidae mites for Fluopyram and trifloxystrobine, both on female and immature survival (e.g. Kishimoto et al. 2020). For sulfur, the results are much more controversial (e.g. Asalf et al. 2012; Tacoli et al. 2020). Auger et al. (2003) reported that toxicity on T. urticae depends on temperature and humidity, and resistance to sulfur has been reported in Phytoseiidae mites (Hoy and Standow 1981). Finally, prey might affect Phytoseiidae communities. Among the prey reported, thrips densities were not different between the two varieties and densities of Eriophyidae and Tydeidae were significantly higher on the cv. Artaban only in June. It can be assumed that on the cv. Syrah, K. aberrans better controlled Eriophyidae than the Phytoseiidae species present on the cv. Artaban and/or that the cv. Artaban might be more susceptible to those pests. Several authors showed indeed different susceptibility levels of grape varieties to C. vitis, the most common Eriophyidae here present (Khederi et al. 2014, 2018). In addition, the greater occurrence of Eriophyidae on the cv. Artaban could have affect the diversity of Phytoseiidae species, especially the presence of E. gallicus and P. finitimus. However, to our knowledge, Eriophyidae predation by these two species has not been directly studied (Duso et al. 2010). Euseius gallicus is known feed on pollen, spider mites, tarsonemid mites and thrips eggs (Pijnakker et al. 2014). Phytoseius finitimus is reported to feed on Tetranychidae, thrips and whiteflies (Papaioannou-Souliotis et al. 2000; Pappas et al. 2013). However, as generalist predators type III and IV, they might also eat Eriophyidae as reported for other species of those two genera (Abdallah et al. 2001; McMurtry et al. 2013; Pappas et al. 2013).
Does the management system affect the diversity and abundance of Phytoseiid mites and their prey on vines canopy?
Phytoseiidae density was globally not statistically different between the systems for all the Phytoseiidae species retrieved, except in June with a higher density of E. gallicus but in the control modality without cover crop. In addition, the diversity and species found were also similar, showing no clear effect of the management system on Phytoseiidae diversity. Those results are in contradiction with current hypotheses stating that plant diversification positively affects predator diversity and density in vineyards (Duso et al. 2010; de Villiers and Pringle 2011; Letourneau et al. 2011; Burgio et al. 2016; Sáenz-Romo et al. 2019; Möth et al. 2021, 2023). Several hypotheses could be put forward to explain such results. First, the vine plot is newly planted (2019), and effects on diversification might be not yet visible such as suggested by Tixier et al. (2015) and Barbar et al. (2006) studying agroforestry effects on Phytoseiidae in vineyards. Second, the systems studied are close to each other bordered by same uncultivated vegetation, which can have masked cover crop effects due to Phytoseiidae colonization by aerial dispersal from neighbouring uncultivated plants (Tixier et al. 1998, 2000a, 2006; Duso et al. 2004; Gavinelli et al. 2020). However, some trends can be noted. In May, a higher Phytoseiidae diversity was observed in both “Aviti” and “Dviti” compared to the control with no cover crop. The presence of cover crops could act as a reservoir for some species during the winter, such it is known for Neoseiulus fallacis and N. californicus in apple orchards (Veerman 1992; Croft and Jung 2001; de Villiers and Pringle 2011), but further samplings would be necessary to confirm this hypothesis for T. exhilaratus, the dominant species in May. The multifactorial analyses showed that the two diversified modalities (“Aviti” and “Dviti”) are more similar to each other than to the control, suggesting an impact of cover crop, but not of co-planted trees. Such results can be associated to a direct reservoir effect of the cover crop but also to an indirect effect due to the vine stress in the diversified modality, where vine plants were less vigourous than in the control modality, affecting thus mite habitat (Liguori et al. 2011). Malison (1994) reported for example lower densities of K. aberrans on water stressed vines, and several studies highlighted such an impact on other arthropods (Gely et al. 2020). Finally, Tydeidae were more abundant in the control modality than in the two diversified ones, which agrees with Vogelweith and Thiéry (2017) who reported higher densities in bare soil vineyards comparing to the ones with covercrops. It is interesting to note that K. aberrans and Tydeiidae abundance were often correlated both on the cv. Artaban and Syrah, as observed by Peverieri et al. (2009).
Do co-planted trees act as reservoirs of Phytoseiidae mites?
Phytoseiidae were observed on the two co-planted trees, but higher abundance and occurrence frequency was observed on fig tree than on pomegranate. Those results are probably associated to the leaf structure, large and pilose leaves on fig trees being more favorable than thin and glabrous leaves of pomegranate. The Phytoseiidae species retrieved on these two trees were also present on vine. Thus, they might constitute Phytoseiidae reservoirs. However, despite the high quantity of K. aberrans observed on fig tree, the density of this species was not higher on the vine of the “Dviti” modality. Some slight effect of the co-planted fig tree might be nevertheless hypothesized because: (i) in May, “Dviti” was the sole modality where K. aberrans was found and (ii) in June, “Dviti” showed a higher proportion of this latter species than in the other modalities. However, these effects are not strong, and we can also hypothetized that even if K. aberrans disperses to the adjacent vine plants, the cv. Artaban might not be suitable for this species. The same applies for P. finitimus: even if high density are observed on fig trees, the density of this latter species was not different between the management modalities. Such results have also been noted in an agroforestry vineyard in south of France, where the densities of Phytoseiidae were not significantly different between agroforestry or monoculture systems (Barbar et al. 2006, 2010; Tixier et al. 2015) and where the co-planted trees did not significantly affect Phytoseiidae abundance on adjacent vine rows (Barbar 2007). Furthermore, since all Phytoseiidae species found on trees are also present on vine in the three systems, the role of the co-planted trees as Phytoseiidae reservoirs for all the plots can also be questionned. Sentenac et al. (2018) and Möth et al. (2021, 2023) reported that intraplot diversification poorly affect Phytoseiidae communities, compared to the close landscape effects. Several studies have shown that Phytoseiidae colonization occurs mainly through aerial dispersal from nearby vegetation, up to 90 m (Hoy et al. 1984; Tixier et al. 1998, 2000a, 2000b).
Do cover crops act as reservoirs of Phytoseiidae mites?
Twelve Phytoseiidae species were observed in the cover crops. Among them, some are not observed on vine (G. graminis, N. alpinus, A. obtusus, A. setosa). This is in line with what is known on the distribution of these species, mainly reported in soil and/or herbaceous habitats (McMurtry et al. 2013; Demite et al. 2023). Their biology is globally poorly known, and they are assumed to have no direct effect on pest biological control. Among the other species reported both on vine and cover crops, P. soleiger and P. triporus are not frequently observed in the two habitats. These species are reported on different plant families, usually on trees and their biology is not known (Tuovinen and Rokx 1991; Tixier et al. 2013). Finally, the five most commonly found species on vine are also reported in the cover crops. This result is consistent with Sahraoui et al. (2014) and Sáenz-Romo et al. (2019), who observed similar Phytoseiidae species on weeds and citrus trees and vine, respectively. In the present experiment, the most frequently observed species were T. recki and T. exhilaratus for the two systems, suggesting that the cover crops represent a good reservoir for these two species. They are reported on a large number of plants including trees, shrubs and grasses (Tixier et al. 2020; Demite et al. 2023), and already reported in cover crops and surrounding vegetation in vineyards (Castagnoli et al. 1999; Barbar et al. 2006; Sáenz-Romo et al. 2019). Kampimodromus aberrans, P. finitimus and E. gallicus were only found on some plants at low frequency (K. aberrans on O. vicifolia, M. sativa and E. sumatrensis; P. finitimus on E. sumatrensis, H. echioides, P. hieracioides). This is not a surprising result, as these three species are usually reported on shrubs and trees (Tixier et al. 2013; Döker et al. 2014; Demite et al. 2023; Novljan et al. 2023). Thus, even if cover crops harbour Phytoseiidae mites, the main species found on vine are not the most abundant and frequently observed species in the cover crops and vice versa. It thus seems that the cover crops do not constitute an important reservoir for the Phytoseiidae retrieved on vine, but this small amount might be sufficient to allow vine colonisation. It would be interesting to further focus on dispersal from the cover crop to the vine canopy via ambulatory dispersal, as already observed for several Phytoseiidae species (Moraes et al. 1986; Tixier et al. 1998, 2000b; Auger et al. 1999; Kreiter et al. 2006; Duso et al. 2010).
Overall insights of cropping system effects on pest regulations
Tetranychidae were very low during all the experiment duration, in the three cropping systems considered (so low that they were not taken into account in the present study). This suggests a good regulation by the predatory mites reported in the three systems. The same was observed for thrips and C. vitis, with no difference between the systems. The effects of cropping system was different according to the dates on the population of Tydeiidae. As no strong effect of cropping system was observed on Phytoseiidae mites, no clear effects on prey are not a surprising result. However, we can note that the uncommon predatory mite species found in the three systems on the cv. Artaban seem able to maintain the populations of pests. Some differences are observed between the cultivar only in June and only concerning C. vitis and Tydeiidae mites. Higher densities were observed on the cv. Artaban. This resistant cultivar was not treated with sulfur. Yet, sulfur is known to have miticide effects (Hoy and Standow 1981; Auger et al. 2003; Gadino et al. 2011; Asalf et al. 2012), which might explain the highest densities in not treated modalities. However, this explanation can not be retained as the sulfur application (7 July) was done after the June sampling (8 june). Other factors as the different Phytoseiidae communities on the two cultivars and their predation effects, might be one of them.
Conclusion
Overall, this study highlights the significant influence of grape variety on predatory mite, suggesting an effect of both vine leaf characteristics and fungicide treatments. The high proportion of P. finitimus and E. gallicus on vines in southern France is unprecedented and may be due to a new grape variety commercialised only since 2018, global warming and/or the emergence of the invasive species E. gallicus. While crop diversification did not positively affect Phytoseiidae density and diversity in this three -years-old vineyard, all Phytoseiidae species on vines were present in co-planted trees and cover crops, indicating their role as potential reservoirs. Ongoing surveys will be crucial to monitor changes in mite communities over time, as well as new investigations to determine whether exchanges between the non-crop plants and vines are actually taking place.
References
Abdallah AA, Zhang Z-Q, Masters GJ, Mcneill S (2001) Euseius finlandicus (Acari: Phytoseiidae) as a potential biocontrol agent against Tetranychus urticae (Acari: Tetranychidae): life history and feeding habits on three different types of food. Exp Appl Acarol 25:833–847. https://doi.org/10.1023/A:1020431531446
Aguilar-Fenollosa E, Ibáñez-Gual MV, Pascual-Ruiz S, Hurtado M, Jacas JA (2011) Effect of ground-cover management on spider mites and their phytoseiid natural enemies in clementine mandarin orchards (I): bottom-up regulation mechanisms. Biol Contr 59:158–170. https://doi.org/10.1016/j.biocontrol.2011.06.013
Altieri MA, Letourneau DK (1982) Vegetation management and biological control in agroecosystems. Crop Prot 1:405–430. https://doi.org/10.1016/0261-2194(82)90023-0
Altieri MA, Nicholls CI (2002) The simplification of traditional vineyard based agroforests in northwestern Portugal: some ecological implications. Agroforest Syst 56:185–191. https://doi.org/10.1023/A:1021366910336
Andow DA (1991) Yield loss to arthropods in vegetationally diverse agroecosystems. Environ Entomol 20:1228–1235. https://doi.org/10.1093/ee/20.5.1228
Asalf B, Trandem N, Stensvand A, Wekesa VW, de Moraes GJ, Klingen I (2012) Influence of sulfur, powdery mildew, and the predatory mite Phytoseiulus persimilis on two-spotted spider mite in strawberry. Biol Contr 61:121–127. https://doi.org/10.1016/j.biocontrol.2012.01.015
Auger P, Tixier M-S, Kreiter S, Fauvel G (1999) Factors affecting ambulatory dispersal in the predaceous mite Neoseiulus californicus (Acari: Phytoseiidae). Exp Appl Acarol 23:235–250. https://doi.org/10.1023/A:1006019014708
Auger P, Guichou S, Kreiter S (2003) Variations in acaricidal effect of wettable sulfur on Tetranychus urticae (Acari: Tetranychidae): effect of temperature, humidity and life stage. Pest Manag Sci 59:559–565. https://doi.org/10.1002/ps.665
Auger P, Bonafos R, Kreiter S (2004) Mancozeb resistance patterns among Kampimodromus aberrans and Typhlodromus pyri (Acari: Phytoseiidae) strains from French vineyards. Can Entomol 136:663–673. https://doi.org/10.4039/n03-115
Barbar Z (2007) Structure inter- et intra-spécifique des guildes d’acariens prédateurs (Acari : Phytoseiidae) dans un agrosystème viticole du sud de la France conduit en agroforesterie. Phd thesis, Montpellier Supagro
Barbar Z, Tixier M-S, Cheval B, Kreiter S (2006) Effects of agroforestry on phytoseiid mite communities (Acari: Phytoseiidae) in vineyards in the south of France. Exp Appl Acarol 40:175–188. https://doi.org/10.1007/s10493-006-9044-z
Barbar Z, Tixier M-S, Cheval B, Kreiter S (2010) Does agroforestry affect phytoseiid mite communities in vineyards in the South of France? In: Sabelis MW, Bruin J (eds) Trends in acarology. Springer, Dordrecht, pp 409–412
Barbault R (1992) Ecologie des peuplements: structure, dynamique et évolution. Masson, Barcelone, p 88
Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B, Grothendieck G, Green P, Bolker MB (2015) Package ‘lme4.’ Convergence 12(1):2
Bergeron PE, Schmidt-Jeffris RA (2020) Not all predators are equal: miticide non-target effects and differential selectivity. Pest Manag Sci 76:2170–2179. https://doi.org/10.1002/ps.5754
Bernard MB, Cole P, Kobelt A, Horne PA, Altmann J, Wratten SD, Yen AL (2010) Reducing the impact of pesticides on biological control in australian vineyards: pesticide mortality and fecundity effects on an indicator species, the predatory mite Euseius victoriensis (Acari: Phytoseiidae). J Econ Entomol 103:2061–2071. https://doi.org/10.1603/EC09357
Blassioli-Moraes MC, Venzon M, Silveira LCP, Gontijo LM, Togni PHB, Sujii ER, Haro MM, Borges M, Michereff MFF, de Aquino MFS, Laumann RA, Caulfield J, Birkett M (2022) Companion and smart plants: scientific background to promote conservation biological control. Neotrop Entomol 51:171–187. https://doi.org/10.1007/s13744-021-00939-2
Boller E (1984) Eine einfache Ausschwemm-methode zur schnellen erfassung, von raubmilben, Thrips und anderen kleinarthropoden im Weinbau. Schweiz Z Obst Weinbau 120:16–17
Boller EF, Remund U, Candolfi MP (1988) Hedges as potential sources of Typhlodromus pyri, the most important predatory mite in vineyards of Northern Switzerland. Entomophaga 33:249–255. https://doi.org/10.1007/BF02372661
Burgio G, Marchesini E, Reggiani N, Montepaone G, Schiatti P, Sommaggio D (2016) Habitat management of organic vineyard in northern Italy: the role of cover plants management on arthropod functional biodiversity. Bull Entomol Res 106:759–768. https://doi.org/10.1017/S0007485316000493
Castagnoli M, Liguori M, Nannelli R (1999) Influence of soil management on mite populations in a vineyard agroecosystem. In: Bruin J, van der Geest LPS, Sabelis MW (eds) Ecology and evolution of the acari: proceedings of the 3rd symposium of the european association of acarologists 1–5 July 1996, Amsterdam, The Netherlands. Springer Netherlands, Dordrecht, pp 617–623.
Chant DA, McMurtry JA (2007) Illustrated keys and diagnoses for the genera and subgenera of the Phytoseiidae of the world (Acari: Mesostigmata). Indira Publishing House, West Bloomfield, Michigan, p 220
Croft BA, Jung C (2001) Phytoseiid dispersal at plant to regional levels: a review with emphasis on management of Neoseiulus fallacis in diverse agroecosystems. Exp App Acarol 25:763–784
da Rosado M, C, Araújo GJ de, Pallini A, Venzon M, (2021) Cover crop intercropping increases biological control in coffee crops. Biol Control 160:104675. https://doi.org/10.1016/j.biocontrol.2021.104675
de Villiers M, Pringle KL (2011) The presence of Tetranychus urticae (Acari: Tetranychidae) and its predators on plants in the ground cover in commercially treated vineyards. Exp Appl Acarol 53:121–137. https://doi.org/10.1007/s10493-010-9391-7
Demite PR, Mcmurtry JA, De Moraes GJ (2023) Phytoseiidae Database: a website for taxonomic and distributional information on phytoseiid mites (Acari). Zootaxa 3795:571
Dinno A, Dinno MA (2017) Package ‘dunn. test.’ CRAN Repos 10:1–7
Döker I, Witters J, Pijnakker J, Kazak C, Tixier MS, Kreiter S (2014) Euseius gallicus Kreiter and Tixier (Acari: Phytoseiidae) is present in four more countries in Europe: Belgium, Germany, the Netherlands and Turkey. Acarologia 54:245–248. https://doi.org/10.1051/acarologia/20142132
Döker I, Khaustov VA, Joharchi O, Khaustov A, Kazakov DV, Meshkov YI (2024) Integrative taxonomy demonstrates synonymy between Euseius amissibilis Meshkov and Euseius gallicus Kreiter & Tixier (Acari: Phytoseiidae). Syst App Acarol 29:60–77
Dos Santos VVD, Tixier M-S (2018) Integrative taxonomy approach for analysing evolutionary history of the tribe Euseiini Chant & McMurtry (Acari: Phytoseiidae). Syst Biodiv 16:302–319. https://doi.org/10.1080/14772000.2017.1401562
Duffner K (1999) Untersuchungen zur biologie, morphologie und bekämpfung der kräuselmibe Calepitrimerus vitis Nalepa 1905 (Acari, Eriophyoidea). Albert-Ludwigs-Universität Freiburg im Breisgau, p 162
Duso C (1993) La vegetazione spontanea come riserva di ausiliari: considerazioni sulla diffusione degli Acari Fitoseidi (Acari Phytoseiidae) in un vigneto e sulle piante spontanee contigue. Boll Zool Agr Bachicol 25:183–203
Duso C, Fontana P (2002) On the identity of Phytoseius plumifer (Canestrini and Fanzago, 1876) (Acari: Phytoseiidae). Acarologia 42:127–136
Duso C, Vettorazzo E (1999) Mite population dynamics on different grape varieties with or without phytoseiids released (Acari: Phytoseiidae). Exp Appl Acarol 23:741–763. https://doi.org/10.1023/A:1006297225577
Duso C, Malagnini V, Paganelli A, Aldegheri L, Bottini M, Otto S (2004) Pollen availability and abundance of predatory phytoseiid mites on natural and secondary hedgerows. Biocontrol 49:397–415. https://doi.org/10.1023/B:BICO.0000034601.95956.89
Duso C, Kreiter S, Tixier M-S, Pozzebon A, Malagnini V (2010) Biological control of mites in European vineyards and the impact of natural vegetation. In: Sabelis MW, Bruin J (eds) Trends in Acarology. Springer, Netherlands, Dordrecht, pp 399–407
Duso C, Pozzebon A, Kreiter S, Tixier M-S, Candolfi M (2012) Management of phytophagous mites in European vineyards. In: Bostanian NJ, Vincent C, Isaacs R (eds) Arthropod management in vineyards. Springer, Dordrecht, pp 191–217
Ferragut F, Perez-Moreno I, Iraola V, Escudero-Colomar L-A (2009) Ácaros depredadores en las plantas cultivadas. Familia Phytoseiidae. Ediciones Agrotecnias
Fox J, Weisberg S, Adler D, Bates D, Baud-Bovy G, Ellison S, Heiberger R (2012) Package ‘car.’ R Foundation for statistical computing, Vienna
Gadino AN, Walton VM, Dreves AJ (2011) Impact of vineyard pesticides on a beneficial arthropod, Typhlodromus pyri (Acari: Phytoseiidae), in laboratory bioassays. J Econ Entomol 104:970–977. https://doi.org/10.1603/EC10330
Gavinelli F, Fusaro S, Ivan D, Ragusa S, Paoletti MG (2020) Unpredicted ecological and ecosystem services of biodiversity. Spontaneous vegetation, hedgerows, and maple trees as useful landscape components to increase predatory mite population in agroecosystems. Appl Soil Ecol 154:103584. https://doi.org/10.1016/j.apsoil.2020.103584
Gely C, Laurance SGW, Stork NE (2020) How do herbivorous insects respond to drought stress in trees? Biol Rev 95:434–448. https://doi.org/10.1111/brv.12571
Girolami V, Borella E, di Bernardo A, Malagnini V (2000) Positive influence on phytoseiid mites of allowing the grassy interrow to flower. Informatore Agrario 56:71–73
Hothorn T, Bretz F, Westfall P, Heiberger RM, Schuetzenmeister A, Scheibe S, Hothorn MT (2016) Package ‘multcomp’. Simultaneous inference in general parametric models. Project for statistical computing Vienna, Austria, pp 1–36
Hoy MA, Standow KA (1981) Resistance to sulfur in a vineyard spider mite predator. Calif Agr 35:8–10
Hoy MA, Van De Baan HE, Groot RJJ, Field RP (1984) Aerial movements of mites in almonds: implications for pest management. Calif Agr 38:21–24
Kassambara A (2016) Factoextra: extract and visualize the results of multivariate data analyses. R package version 1
Kassambara A (2021) rstatix: pipe-friendly framework for basic statistical tests. R package version 0.7
Khederi SJ, de Lillo E, Khanjani M, Gholami M (2014) Resistance of grapevine to the erineum strain of Colomerus vitis (Acari: Eriophyidae) in western Iran and its correlation with plant features. Exp Appl Acarol 63:15–35. https://doi.org/10.1007/s10493-014-9778-y
Khederi SJ, Khanjani M, Gholami M, Lillo ED (2018) Sources of resistance to the erineum strain of Colomerus vitis (Acari: Eriophyidae) in grapevine cultivars. Syst App Acarol 23:405–425
Kishimoto H, Yaginuma K, Furihata S, Ito T, Yaegashi H, Toyama M (2020) Effects of fungicides on four native generalist phytoseiid species (Acari: Phytoseiidae). Jpn J Appl Entomol Zool 64:175–182. https://doi.org/10.1303/jjaez.2020.175
Kreiter S, Tixier MS, Auger P, Muckensturm N, Sentenac G, Doublet B, Weber M (2000) Phytoseiid mites of vineyards in France (Acari: Phytoseiidae). Acarologia 41:77–96
Kreiter S, Tixier M-S, Croft BA, Auger P, Barret D (2002) Plants and leaf characteristics influencing the predaceous mite Kampimodromus aberrans (Acari: Phytoseiidae) in habitats surrounding vineyards. Environ Entomol 31:648–660. https://doi.org/10.1603/0046-225X-31.4.648
Kreiter S, Tixier MS, Barbar Z (2006) The importance of the vegetation surrounding the agrosystems on predatory mites associated to vineyards. Proceed Intern Conf La difesa Integrata della vite dagli artropodi dannosi Marsala (Italia), 10–11. 2005:97–147
Landis DA, Wratten SD, Gurr GM (2000) Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu Rev Entomol 45:175–201. https://doi.org/10.1146/annurev.ento.45.1.175
Lê S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. J Stat Softw 25:1–18
Letourneau DK, Armbrecht I, Rivera BS, Lerma JM, Carmona EJ, Daza MC, Escobar S, Galindo V, Gutiérrez C, López SD, Mejía JL, Rangel AMA, Rangel JH, Rivera L, Saavedra CA, Torres AM, Trujillo AR (2011) Does plant diversity benefit agroecosystems? A synthetic review. Ecol Appl 21:9–21. https://doi.org/10.1890/09-2026.1
Lichtenberg EM, Kennedy CM, Kremen C, Batáry P, Berendse F, Bommarco R, Bosque-Pérez NA, Carvalheiro LG, Snyder WE, Williams NM, Winfree R, Klatt BK, Åström S, Benjamin F, Brittain C, Chaplin-Kramer R, Clough Y, Danforth B, Diekötter T, Eigenbrode SD, Ekroos J, Elle E, Freitas BM, Fukuda Y, Gaines-Day HR, Grab H, Gratton C, Holzschuh A, Isaacs R, Isaia M, Jha S, Jonason D, Jones VP, Klein A-M, Krauss J, Letourneau DK, Macfadyen S, Mallinger RE, Martin EA, Martinez E, Memmott J, Morandin L, Neame L, Otieno M, Park MG, Pfiffner L, Pocock MJO, Ponce C, Potts SG, Poveda K, Ramos M, Rosenheim JA, Rundlöf M, Sardiñas H, Saunders ME, Schon NL, Sciligo AR, Sidhu CS, Steffan-Dewenter I, Tscharntke T, Veselý M, Weisser WW, Wilson JK, Crowder DW (2017) A global synthesis of the effects of diversified farming systems on arthropod diversity within fields and across agricultural landscapes. Global Change Biol 23:4946–4957. https://doi.org/10.1111/gcb.13714
Liguori M (1987) Population trends of phytophagous and predatory mites in two vineyards of the Chianti area. Redia 70:141–149
Liguori M (1988) Effect of different pest control treatments on the population of the phytoseiid predator Typhlodromus exhilaratus Ragusa and on phytophagous mites in a vineyard in Siena. Redia 71:455–466
Liguori M, Guidi S (1990) Influence of food conditioning of Typhlodromus exhilaratus Ragusa (Acari: Phytoseiidae) on its consumption of prey. Redia 73:201–211
Liguori M, Guidi S (1995) Influence of different constant humidities and temperatures on eggs and larvae of a strain of Typhlodromus exhilaratus Ragusa (Acari Phytoseiidae). Redia 78:321–329
Liguori M, Tixier M-S, Hernandes AF, Douin M, Kreiter S (2011) Agroforestry management and phytoseiid communities in vineyards in the South of France. Exp Appl Acarol 55:167–181. https://doi.org/10.1007/s10493-011-9450-8
Lorenzon M, Pozzebon A, Duso C (2012) Effects of potential food sources on biological and demographic parameters of the predatory mites Kampimodromus aberrans, Typhlodromus pyri and Amblyseius andersoni. Exp Appl Acarol 58:259–278. https://doi.org/10.1007/s10493-012-9580-7
Lorenzon M, Pozzebon A, Duso C (2018) Biological control of spider mites in North-Italian vineyards using pesticide resistant predatory mites. Acarologia 58:98–118
Mailloux J, Le Bellec F, Kreiter S, Tixier M-S, Dubois P (2010) Influence of ground cover management on diversity and density of phytoseiid mites (Acari: Phytoseiidae) in Guadeloupean citrus orchards. Exp Appl Acarol 52:275–290. https://doi.org/10.1007/s10493-010-9367-7
Malagnini V, Pozzebon A, Facchin P, Paganelli A, Duso C (2022) Airborne pollen can affect the abundance of predatory mites in vineyards: implications for conservation biological control strategies. Pest Manag Sci 78:1963–1975. https://doi.org/10.1002/ps.6815
Malison M (1994) Influenza dello stress idrico della pianta ospite sulle popolazioni del predatore Ambyseius aberrans (Oudemans)(Acarina: Phytoseiidae). Atti XVII congresso nazionale italiano di entomologia Udine:13–18
McMurtry JA, Croft BA (1997) Life-styles of phytoseiid mites and their roles in biological control. Annu Rev Entomol 42:291–321. https://doi.org/10.1146/annurev.ento.42.1.291
McMurtry JA, Moraes GJD, Sourassou NF (2013) Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst Appl Acarol 18(4):297–320
McMurtry JA, Sourassou NF, Demite PR (2015) The Phytoseiidae (Acari: Mesostigmata) as biological control agents. In: Carrillo D, de Moraes GJ, Peña JE (eds) Prospects for biological control of plant feeding mites and other harmful organisms. Springer International Publishing, Cham, pp 133–149
Miñarro M, Kreiter S (2012) Phytoseiids in vineyards of the appellation cangas quality wine (Asturias, NW Spain). Boletín De Sanidad Vegetal, Plagas 38:73–82
Moraes GJ de, McMurtry JA, Denmark HA (1986) A catalog of the mite family Phytoseiidae: references to taxonomy, synonymy, distribution and habitat. EMBRAPA, Brasilia
Möth S, Walzer A, Redl M, Petrović B, Hoffmann C, Winter S (2021) Unexpected effects of local management and landscape composition on predatory mites and their food resources in vineyards. InSects 12(2):180. https://doi.org/10.3390/insects12020180
Möth S, Richart-Cervera S, Comsa M, Herrera RA, Hoffmann C, Kolb S, Popescu D, Reiff JM, Rusch A, Tolle P, Walzer A, Winter S (2023) Local management and landscape composition affect predatory mites in European wine-growing regions. Agr Ecosyst Environ 344:108292. https://doi.org/10.1016/j.agee.2022.108292
Novljan M, Bohinc T, Kreiter S, Döker I, Trdan S (2023) The indigenous species of predatory mites (Acari: Phytoseiidae) as biological control agents of plant pests in Slovenia. Acarologia 63:1048–1061
Nyrop J, English-Loeb G, Roda A (1998) Chapter 17—conservation biological control of spider mites in perennial cropping systems. In: Barbosa P (ed) Conservation biological control. Academic Press, San Diego, pp 307–333
Papadoulis G, Emmanouel N, Kapaxidi E (2009) Phytoseiidae of Greece and Cyprus (Acari Mesostigmata). Indira Publishing House, West Bloomfield, p 200
Papaioannou-Souliotis P, Markoyiannaki-Printziou D, Rumbos I, Adamopoulos I (2000) Phytoseiid mites associated with vine in various provinces of Greece: a contribution to faunistics and biogeography, with reference to eco-ethological aspects of Phytoseius finitimus (Ribaga) (Acari: Phytoseiidae). Acarologia 40:113–125
Pappas ML, Xanthis C, Samaras K, Koveos DS, Broufas GD (2013) Potential of the predatory mite Phytoseius finitimus (Acari: Phytoseiidae) to feed and reproduce on greenhouse pests. Exp Appl Acarol 61:387–401. https://doi.org/10.1007/s10493-013-9711-9
Paredes D, Cayuela L, Campos M (2013) Synergistic effects of ground cover and adjacent vegetation on natural enemies of olive insect pests. Agr Ecosyst Environ 173:72–80. https://doi.org/10.1016/j.agee.2013.04.016
Peverieri GS, Simoni S, Goggioli D, Liguori M, Castagnoli M (2009) Effects of variety and management practices on mite species diversity in Italian vineyards. Bull Insectol 62:53–60
Pijnakker J, de Souza A, Wäckers F (2014) Euseius gallicus, a bodyguard for roses. IOBC/WPRS Bull 102:191–195
Ragusa S (1981) Influence of different kinds of food substances on the developmental time in young stages of the predacious mite Typhlodromus exhilaratus Ragusa (Acarina: Phytoseiidae). Redia
Roda A, Nyrop J, English-Loeb G (2003) Leaf pubescence mediates the abundance of non-prey food and the density of the predatory mite Typhlodromus pyri. Exp Appl Acarol 29:193–211. https://doi.org/10.1023/A:1025874722092
Sáenz-Romo MG, Martínez-García H, Veas-Bernal A, Carvajal-Montoya LD, Martínez-Villar E, Ibáñez-Pascual S, Marco-Mancebón VS, Pérez-Moreno I (2019) Effect of ground-cover management on predatory mites (Acari: Phytoseiidae) in a Mediterranean vineyard. J Grapevine Res 58:25–32. https://doi.org/10.5073/VITIS.2019.58.SPECIAL-ISSUE.25-32
Sahraoui H, Tixier M-S, Lebdi-Grissa K, Kreiter S (2014) Diversity and abundance of Phytoseiidae (Acari: Mesostigmata) in three crop management strategies of citrus orchards in Tunisia. Acarologia 54:155–169. https://doi.org/10.1051/acarologia/20142123
Schmidt RA (2014) Leaf structures affect predatory mites (Acari: Phytoseiidae) and biological control: a review. Exp Appl Acarol 62:1–17. https://doi.org/10.1007/s10493-013-9730-6
Sentenac G, Rusch A, Kreiter S, Bouvier J-C, Thiery J, Delbac L, Thiery D, Lavigne C, Madejski M, Guilbault P, Guisset M, Tixier M (2018) Functional biodiversity: effect of landscape context around vineyards on natural pest control services. Innov Agron 63:139–161
Tacoli F, Cargnus E, Zandigiacomo P, Pavan F (2020) Side effects of sulfur dust on the european grapevine moth Lobesia botrana and the predatory mite Kampimodromus aberrans in vineyards. InSects 11:825. https://doi.org/10.3390/insects11110825
Tirello P, Pozzebon A, Duso C (2012) Resistance to chlorpyriphos in the predatory mite Kampimodromus aberrans. Exp Appl Acarol 56:1–8. https://doi.org/10.1007/s10493-011-9486-9
Tixier MS (2018) Predatory mites (Acari: Phytoseiidae) in agro-ecosystems and conservation biological control: a review and explorative approach for forecasting plant-predatory mite interactions and mite dispersal. Front Ecol Evol 6:192. https://doi.org/10.3389/fevo.2018.00192
Tixier MS, Kreiter S, Auger P, Weber M (1998) Colonization of Languedoc vineyards by phytoseiid mites (Acari: Phytoseiidae): influence of wind and crop environment. Exp Appl Acarol 22:523–542. https://doi.org/10.1023/A:1006085723427
Tixier MS, Kreiter S, Auger P (2000a) Colonization of vineyards by phytoseiid mites: their dispersal patterns in the plot and their fate. Exp Appl Acarol 24:191–211. https://doi.org/10.1023/a:1006332422638
Tixier MS, Kreiter S, Auger P, Sentenac G, Salva G, Weber M (2000b) Phytoseiid mite species located in uncultivated areas surrounding vineyards in three French regions. Acarologia 41:127–140
Tixier MS, Kreiter S, Croft BA, Auger P (2002) Colonization of vineyards by Kampimodromus aberrans (Oudemans) (Acari: Phytoseiidae): dispersal from surrounding plants as indicated by random amplified polymorphism DNA typing. Agr Forest Entomol 4:255–264. https://doi.org/10.1046/j.1461-9563.2002.00154.x
Tixier MS, Kreiter S, Cheval B, Guichou S, Auger P, Bonafos R (2006) Immigration of phytoseiid mites from surrounding uncultivated areas into a newly planted vineyard. Exp Appl Acarol 39:227–242. https://doi.org/10.1007/s10493-006-9010-9
Tixier MS, Baldassar A, Duso C, Kreiter S (2013) Phytoseiidae in European grape (Vitis vinifera L.): bio-ecological aspects and keys to species (Acari: Mesostigmata). Zootaxa 3721:101–142
Tixier M-S, Arnaud A, Douin M, Kreiter S (2015) Effects of agroforestry on Phytoseiidae communities (Acari: Mesostigmata) in vineyards. A synthesis of a 10-year period of observations. Acarologia 55:361–375
Tixier M-S, dos Santos VV, Douin M, Duso C, Kreiter S (2017) Great molecular variation within the species Phytoseius finitimus (Acari: Phytoseiidae): implications for diagnosis decision within the mite family Phytoseiidae. Acarologia 57:493–515. https://doi.org/10.1051/acarologia/20174168
Tixier M-S, Douin M, Kreiter S (2020) Phytoseiidae (Acari: Mesostigmata) on plants of the family Solanaceae: results of a survey in the south of France and a review of world biodiversity. Exp Appl Acarol 81:357–388. https://doi.org/10.1007/s10493-020-00507-0
Togni PHB, Venzon M, Souza LM, Santos JPCR, Sujii ER (2019) Biodiversity provides whitefly biological control based on farm management. J Pest Sci 92:393–403. https://doi.org/10.1007/s10340-018-1021-x
Tuovinen T, Rokx JAH (1991) Phytoseiid mites (Acari: Phytoseiidae) on apple trees and in surrounding vegetation in southern Finland. Densities and species composition. Exp Appl Acarol 12:35–46. https://doi.org/10.1007/BF01204398
Veerman A (1992) Diapause in phytoseiid mites: a review. Exp Appl Acarol 14:1–60. https://doi.org/10.1007/BF01205351
Venzon M, Amaral DSSL, Togni PHB, Chiguachi JAM (2019) Interactions of natural enemies with non-cultivated plants. In: Souza B, Vázquez LL, Marucci RC (eds) natural enemies of insect pests in neotropical agroecosystems: biological control and functional biodiversity. Springer International Publishing, Cham, pp 15–26
Vialatte A, Martinet V, Tibi A, Alignier A, Angeon V, Bohan D, Bougherara D, Carpentier A, Castagneyrol B, Cordeau S, Courtois P, Deguine J-P, Enjalbert J, Fabre F, Femenia F, Fréville H, Grateau R, Grimonprez B, Gross N, Hannachi M, Launay M, Lelievre V, Lemarié S, Martel G, Navarrete M, Plantegenest M, Ravigné V, Rusch A, Suffert F, Thoyer S (2023) Protéger les cultures en augmentant la diversité végétale des espaces agricoles. In: Rapport de l’expertise scientifique collective. INRAE ed. https://doi.org/10.17180/Q7WM-Q442
Vogelweith F, Thiéry D (2017) Cover crop differentially affects arthropods, but not diseases, occurring on grape leaves in vineyards: cover crop effect on grapevine leaf communities. Aust Grape Wine R 23:426–431. https://doi.org/10.1111/ajgw.12290
Wickham H, Chang W, Wickham MH (2016) Package ‘ggplot2’. Create elegant data visualisations using the grammar of graphics. Version 2(1):1–189
Aknowledgements
This project was made possible thanks to the SALSA Project (2018-2023) within the ECOPHYTO plan. We also thank all the people that helped during the samplings, especially Stephen Roulin, Philippe Auger and Isis Oliveira and for technical assistance for vine plot management, especially Raphael Metral. Finally, we thank Maïna Bon for plant identification. We really thank the three reviewers for their valuable comments that improved the scientific quality of the manuscript.
Funding
This study was funded by the ‘Défi Clé Vinid'Occ’ project and the Vigne et Vin chair of Institut Agro Montpellier.
Author information
Authors and Affiliations
Contributions
M-S. T. and D.N. supervised the studies. L.T. carried out the statistical analysis. L.T., D.N. and M-S. T. wrote the manuscript. R.S., M.D., D.N., M.-S. T and L.T. all participated to field and laboratory work. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tabary, L., Navia, D., Steele, R. et al. Impact of agroecological practices on Phytoseiidae communities in a vineyard of South of France: effect of covercrops and agroforestry. Exp Appl Acarol 93, 369–395 (2024). https://doi.org/10.1007/s10493-024-00938-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-024-00938-z