Abstract
The interaction of grape erineum mite (GEM), Colomerus vitis Pagenstecher (Acari: Eriophyidae), with grape was investigated in the laboratory. We studied some plant morphological biochemical features potentially related to vine resistance/tolerance of eight native grapevine cultivars, extensively cultivated in western Iran, and two non-native cultivars. Free-choice experiments indicated that the cultivars Shahani, Flame seedless and Yaghuti were colonized by lower levels of GEM, whereas Muscat Gordo, Gazne and White Thompson seedless hosted denser populations. These differences between cultivars may be due to differential attractiveness to GEM, possibly associated with plant biochemical and morphological traits. In no-choice assays with six grapevine cultivars, mite population development and some cultivar features were assessed. Mite populations grew fastest on Gazne and Muscat Gordo, and slowest on Yaghuti and Shahani. The degree of mite infestation was associated with reduction of leaf area, increase of leaf weight, shortening of shoots and more numerous erinea: these features were larger on the most infested Gazne, whereas morphological features of Shahani and Yaghuti were scarcely affected by GEM infestation. Also trichome type and density of the assayed cultivars appeared to be related to mite density: the most infested cultivars (Gazne and Muscat Gordo) displayed higher ranks of blade and vein hairs and lower ranks of blade and vein bristles and domatia. No correlation was found between mite density and leaf thickness of mature leaves. The amount of leaf waxes was highest in Shahani and Yaghuti, which displayed the lowest mite density, the fewest erinea and the largest leaves. Carbohydrate amount of uninfested leaves was lowest on the least infested Shahani and highest on the most infested Gazne; phenols increased in leaves of Shahani and decreased in those of Gazne after mite infestation. Finally, cultivars also appeared to influence some morphological traits of the mites: larger specimens were detected on White Thompson seedless, Flame seedless and Gazne, whereas smaller mites were found on leaves of the less infested Yaghuti and Shahani. These results indicate that leaf hairiness, leaf wax and carbohydrate contents may be useful tools for a preliminary screening among vine cultivars and help predict resistance/tolerance to GEM. Shahani and Yaghuti seem quite promising for developing grape resistance programs against GEM in western Iran.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Various phytophagous arthropods feed on grapevine, Vitis vinifera L., but a few can actually threaten its production in commercial vineyards. Among these pests, Colomerus vitis (Pagenstecher) (Acari: Eriophyidae) is fairly common and its harmfulness appears to be quite variable within the grape-growing regions of the world (e.g., Duso and de Lillo 1996; Avgin and Bahadiroğlu 2004; Bernard et al. 2005; Linder et al. 2006; Walton et al. 2007; Luchian et al. 2008; Tomoioaga and Comsa 2010; Craemer and Saccaggi 2013). Based on the feeding symptoms induced, C. vitis comprises three strains with different lifestyle but apparently identical morphology: a bud strain, a leaf gall-inducing erineum strain (here indicated as grape erineum mite, GEM), and a leaf curl strain (Smith and Stafford 1948; Duso and de Lillo 1996). GEM is much more frequently and widely recorded than the other strains; it causes conspicuous white, later brown, felt patches (erinea) on the lower leaf surface, which appear blister-like on the upper surface, and it affects grape production (Avgin and Bahadiroğlu 2004). The bud strain can induce malformation of leaves, abnormal shoot growth, premature falling of flower clusters and bud death. It has been suggested to be a closely related but separate species from GEM based on Internal Transcribed Spacer 1 (ITS1) sequences (Carew et al. 2004); this species status has not been supported by morphological and reproductive evidence. Finally, the leaf curl strain is associated with leaf abnormalities and stunted and scarred shoots (Smith and Stafford 1948; Duso and de Lillo 1996). GEM is a real threat to the vine plantations in Iran (Khanjani and Hadad Irani-Nejad 2009) and its population dynamics were studied only in the western part of the country (Gholami et al. 2005b). However, the relevance of the grapevine cultivation for grape and leaf production, and the severity of GEM infestations recorded also in other parts of Iran (Tafazoli et al. 1992) make their control necessary.
In general, the adverse effects of pesticide use and the increased restrictions for pesticide applications have stimulated the exploration of eco-friendly pest control tactics. Plant genotypes strongly influence density, distribution and fitness of pests, and the degree of injury caused by them (Peterson and Higley 2001; Hochwender et al. 2005). Accordingly, the survey and selection of genotypes provided with antixenosis, antibiosis and tolerance properties can contribute to integrated pest management (IPM) tactics (Smith 2005; Hoy 2011). Antixenosis influences the plant feeders’ behavior and makes the plant less attractive for feeding, sheltering and egg-laying (non-preference effect). Antibiosis affects the pest’s biology and often reduces its survivorship, fecundity, longevity and developmental rate, so that its impact is reduced compared to that expected on a susceptible host. Tolerance is the plant’s ability to withstand pest attack and to recover from damage without loss in fitness or yield. Plant morphology (e.g., leaf trichomes, thickened plant epidermal layers, waxy deposits on plant surfaces) and plant physiology (e.g., primary and secondary plant metabolites) are involved in these defense reactions (Johnson 1975; Beck and Maxwell 1976; Gibson and Valencia 1978; Dhaliwal and Dilawari 1993; Panda and Khush 1995; Commenil et al. 1997; Walling 2000; Khanjani and Khalghani 2008). Plant defensive factors are also implied in the interaction between eriophyoid mites and their host plants, but they have been investigated in detail in only few cases (Duso et al. 2010), even though host specificity and plant susceptibility have been studied at species and cultivar level for weeds and few other plants of economic interest (e.g., Herr 1991; Smith et al. 2009).
Bergh and Weiss (1993) identified antibiosis effects on the oviposition and development rate of the pear rust mite, Epitrimerus pyri (Nalepa), infesting pear cultivars and other Pyrus species under laboratory conditions, but relationships with plant features were not described. Similarly, effects on fecundity and survival of the apple rust mite, Aculus schlechtendali (Nalepa), were detected in laboratory experiments on differently resistant apple varieties (Kozlowski 1995). A differential increase of proteins, phenols and other chemicals in infested leaf tissues seemed to be quite common and it was hypothesized to be an adaptive response of the plant’s physiology towards stress factors and/or to be involved with antibiosis (Balasubramanian and Purushothamam 1972; Tandon and Arya 1980; Tandon 1984; Bronner et al. 1991; Ning et al. 1996; Goncalves et al. 1998; Tomczyk and Boczek 2006; Petanović and Kielkiewicz 2010; Kielkiewicz et al. 2011).
When looking at morphology alone, coconut varieties producing large fruits appeared to be less susceptible to the coconut mite, Aceria guerreronis Keifer, than varieties producing small fruits (Mariau 1977). Yet, leaf morphology, trichome types and trichome density on tomato (Solanum lycopersicum L. [=Lycopersicon esculentum Mill.] and Solanum habrochaites S. Knapp & D.M. Spooner [=Lycopersicon hirsutum f. glabratum C.H. Muell.]) appeared to be largely responsible for its resistance to the tomato russet mite, Aculops lycopersici (Tryon) (Leite et al. 1999). Similarly, morphological features of the leaves of tea varieties resistant to the tea pink mite, Acaphylla theae (Watt), were related to feeding inhibition of the mite (Ning et al. 1996). Further examples of cultivar resistance to eriophyoid mites can be found for black currant (Herr 1991), olive (Mohiseni et al. 2011), wheat (Li et al. 2007) and a few other crops.
The interactions between plants and eriophyoids could also concern (phenotypic) variation of the mites, which may be the result of adaptation to micro-environmental conditions created by the host and last throughout mite ontogeny (Dres and Mallet 2002). In particular, Kozlowski (1998) ascertained differences in body size and ventral seta length of protogyne females in apple rust mite collected from different apple tree cultivars. Skoracka et al. (2002) pointed out differences in some phenotypic traits of the grass rust mite complex, Abacarus hystrix (Nalepa), living on different host plants which were later, in part, demonstrated to discriminate cryptic species (Skoracka 2009).
Interactions between GEM and grapevine cultivars have been investigated poorly (Dellei and Szendrey 1991; Gholami et al. 2005a; Luchian et al. 2008), whereas knowledge of factors influencing plant responses to this mite can surely assist in implementing IPM by using resistant cultivars. The present study was aimed at developing an experimental method for evaluating the resistance/sensitiveness of Iranian-native and non-native grapevine germplasm to the grape erineum (GEM) strain of C. vitis under controlled laboratory conditions. Based on previous field observations (Gholami et al. 2005a) eight native grapevine cultivars, extensively cultivated in western Iran, were selected for this study, as well as two non-native cultivars, originally from largely cultivated Australian and North American germplasm, but crossed with oriental cultivars that are widely used in Iran. In addition, plant morphological and biochemical features potentially related to the host plant’s resistance were identified and could also be useful for provisional resistance screening.
Materials and methods
Plant material
The selected native cultivars, from amongst the most common in vineyards of western Iran, were: Fakhri, Yaghuti, Asgari and Khalili (used for fresh consumption), White Thompson seedless and Red Thompson seedless (cultivated for both fresh and dried—raisin—markets), and Gazne and Shahani (used for juice production). The non-native Flame seedless is used for fresh usage, whereas the non-native Muscat Gordo is cultivated for fresh and dried consumption, and wine making. The native cultivars were obtained from the Vine Research Institute of Malayer (Iran), whilst the non-native ones were provided by the Vine Collection of Bu-Ali Sina University, Hamedan (Iran).
The cuttings (40–50 per cultivar) were treated with the fungicide Benomyl (Agrocit®, Bayer CropScience) and stored in the field to stimulate their rooting. Once rooted (in January–February 2010), they were planted singly in 2.5-kg pots containing sand and sheep manure, and kept in a garden at the Faculty of Agriculture, Bu-Ali Sina University, Hamedan (Iran) until September 20, 2010.
Before beginning the experiments, the new plants were treated by Tebuconazole (Folicur®, Bayer CropScience) against powdery mildew and by Abamectin (Vertimec®, Syngenta Crop Protection) against possible mite infestation. After the treatments, all plants were kept in a garden at the Faculty of Agriculture, Bu-Ali Sina University, Hamedan (Iran). About 5 plants per cultivar were randomly selected and checked at the beginning of the assays (bud break) by means of a washing and sieving procedure (de Lillo 2001; Monfreda et al. 2008) in order to ensure the lack of GEM infestation and other mites, including predators. The assays on these plants started 6 months after the miticide spray and no fertilizers or pesticides were further applied.
Mite population source
Two days before each assay, leaves infested by C. vitis were collected from white Sahebi vineyard cultivated in the area of Heydarh village (Hamadan, Iran; 34°48’N, 48°28′E, 1,830 m asl). Leaf samples were stored in plastic bags within a cooler and transported to the laboratory (about 2 km from the collection site) for being used as infesting source; organisms different from GEM were carefully removed from these leaves. A few erinea were detached from leaves randomly selected within the samples. The mites were extracted through the above-mentioned washing and sieving method and the mite density (i.e., mite number per unit area) was calculated.
The Sahebi cultivar was excluded among the assayed genotypes in favor of other cultivars, in order to preclude effects of rearing history on the biological parameters of GEM.
Free-choice assay (antixenosis)
A free-choice assay was carried out to assess the cultivar preferences of GEM. The assay was set up in a white cylindrical plastic cage (1 m diameter, 15 cm high), with 10 round holes (5 cm diameter, 1.5 cm in between) in a circular arrangement in the bottom plate (see Fig. 1). Ten potted plants (one per cultivar) with similar vegetative growth (mean size of new canes ca. 40 cm) were randomly selected. One apical leaf of each plant was introduced through a hole in the bottom plate, allowing the simultaneous comparison of all cultivars (Fig. 1).
Free-choice assay: a top view of the cylindrical plastic device showing the arrangement of the holes in the bottom plate and the position of pots and poles underneath (dotted circles); b side view of apical leaves passing through the holes in the bottom plate; c detail of the assayed cultivars and infesting erinea; d detail of the central part of the device
For each replicate, 20 erinea from Sahebi leaves were placed in the center of the ring of test leaves (Fig. 1). Each erineum had a surface of 2 ± 0.03 cm2 and a mean density of 180 ± 7.4 mites. Polypropylene film covered the bottom plate and the empty spaces in each of the ten holes, thus preventing the escape of mites onto other leaves of the assayed plants. During an assay, the cage was covered by a thin plastic lid to keep the air still, so that mites could not move on air currents. An assay lasted 48 h and was carried out at 16L: 8D photoperiod, 300 lux light intensity on the leaf surface, and 25 ± 1 °C. Then, the apical leaves of the potted plants were examined under a dissecting microscope and all mites (juveniles and adults) were counted. Assays were replicated 10 times, following a completely randomized design, each plant representing a replicate.
No-choice assay (antibiosis)
A no-choice bioassay was carried out to assess cultivar effects on GEM biology. Six cultivars (Shahani, Yaghuti, Flame seedless, Muscat Gordo, White Thompson seedless and Gazne) were selected among the original pool based on the results of the free-choice assay; the four cultivars displaying intermediate mite preferences were discarded.
Ten potted plants with similar vegetative growth (mean size of new canes ca. 30 cm) were selected per cultivar. Each plant was artificially infested by two ‘eriocards’ (i.e., an ‘eriocard’ is a carton card of about 4 cm2 with a piece of leaf glued to it, with a leaf erineum surface of 4 ± 0.12 cm2 and a mean density of 800 ± 8.42 mites) which were tied to the plant’s stem, near the tenth node counting from the bottom of the plant. Each separate plant was fully surrounded by a net (mesh size ≤50 μm) during the assay to avoid mites moving between plants. Plants were kept in a growth room under controlled conditions (25 ± 1 °C, 65 ± 5 % RH and 16L: 8D photoperiod) during the whole period of the assay. The assay was carried out on 10 groups of 6 plants (one for each cultivar) which were mixed randomly and each plant represented a replicate.
Two months after the assay starting, all leaves, buds and the entire branches were used to extract all mite stages, eggs included, according to the above-mentioned washing and sieving procedure. All mites were counted and 50 individuals per cultivar were mounted onto slides, following Amrine and Manson (1996). Individual measurements of adult females were taken under an Olympus BX 51 phase contrast microscope: body width (at widest size) and length of body (from the gnathosoma tip to the anal lobe end), idiosoma (from the gnathosoma base to the anal lobe end), gnathosoma (from the gnathosoma base to the tip of the palps) and chelicerae.
Symptoms and grape growth evaluation
This assay was performed with the same six cultivars selected for the no-choice assay. Twenty potted plants (mean size of canes was ca. 40 cm) per cultivar were transferred to a growth room under controlled conditions (25 ± 1 °C, 65 ± 5 % RH and 16L: 8D photoperiod). Ten out of 20 plants were infested by two ‘eriocards’ each (see no-choice assay), the other 10 stayed uninfested (i.e., control). All plants were singly surrounded by a thin net (mesh size ≤50 μm) and mixed randomly. Two weeks later, the net was removed to avoid that plant growth would be affected by the modified microenvironment and infested plants were kept apart from the uninfested one. Four months after starting the infestation, individual measurements were taken from all plants: area and weight of a sub-sample of leaves (nodes 1 [basal node], 5, 9, 15, 19, and 25 along the cane), number of infested leaves per plant, and internode and shoot length. The leaves were detached and their area was measured using a planimeter (Koizumi AJP Model). Leaf blade weight (without the stalk) was measured with a precision balance (readability of 0.001 g). After measuring leaf area and weight, the infested and non-infested plants were transferred to outdoor natural conditions until leaf fall, in order to allow mites to move into overwintering sites. After bud break at early growth (April 2011), the total shoot length and the internode distance of the previous season’s growth were measured. Internode distances were measured from nodes 1–2 up to 24–25 (hence, 25 distances per shoot). Each plant represented a replicate.
Leaf trichomes and thickness
Density and types of trichomes of uninfested grapevine leaves were assessed according to Loughner et al. (2008) from the cultivars previously used in the no-choice assay. Trichomes were classified on the basis of length and position (bristles <250 μm and upright; hairs >250 μm and prostrate). Pubescence was evaluated on three distal-shoot leaves per plant. Per each leaf, trichomes were measured on four vein sections (each section 5 mm long, selected randomly) (Fig. 2b) and on four circular areas between veins on the blade (each 10 mm diameter, selected randomly) (Fig. 2c). Hair and bristle density ranks of either vein or blade were assessed under 25× magnifications. Bristle density was assessed also on four vein axils (selected randomly) per leaf (Fig. 2a) and their mean density generated a domatium rank (cf. Loughner et al. 2008). Measurements were taken on 10 plants per cultivar in June 2011 and each plant represented a replicate.
Location of the various vine leaf parts for measuring (a) domatium size and rank, b leaf vein bristles and hairs, and c leaf blade bristles and hairs (modified from Loughner et al. 2008)
Leaf thickness of uninfested leaves was assessed by a digital micrometer on four mid-shoot and four distal-shoot leaves per cultivar previously used in the no-choice assay. Four segments of each leaf were measured and the mean of these values generated the thickness of each leaf. Measurements were taken on 10 grapes per cultivar in June 2011 and each plant represented a replicate.
Leaf epicuticular waxes
About 100 g of fresh vine leaf material was randomly selected from mid- and terminal-shoot leaves of each uninfested plant previously used in the no-choice assay. This plant material was washed in distilled water and dried on filter paper. The cuticular waxes were extracted by chopping the leaves, immersing the pieces in pure chloroform for 60 s (100 g leaf in 100 ml chloroform). After removing the leaf material, the organic solvent was left to evaporate at room temperature under a fume hood and the wax residues were measured with a precision balance (readability of 0.001 g) according to Casado and Heredia (1999). Amounts of epicuticular wax were expressed in mg/100 g leaf fresh weight. The measurements were replicated on five grapes per cultivar in June 2011 and each plant represented a replicate.
Total soluble carbohydrates in leaves
Fresh vine leaves were randomly selected from mid- and terminal-shoot leaves of each uninfested plant previously used in the no-choice assay. A colorimetric method was applied to determine the total soluble carbohydrates according to Irigoyen et al. (1992). About 0.5 g of leaves was treated with 15 ml of ethanol (83 %). Then, 0.1 ml of alcoholic extract was added to 3 ml of a fresh anthron solution (150 mg anthron + 100 ml sulfuric acid at 72 %) and placed in a boiling water bath for 10 min. After cooling, the absorbance was measured at 625 nm by a UV–visible spectrophotometer (Carry model 100, Bio UV–Visible, USA). A calibration curve was obtained measuring the absorbance of a series of standard solutions at 20, 40, 60, 80, 100 and 120 mg of glucose/l. Soluble carbohydrate amount was expressed in mg/g leaf fresh weight based on the following formula:
The measurements were replicated on five grapes per cultivar in June 2011 and each plant represented a replicate.
Total phenols in leaves
A colorimetric method was applied to determine the total phenolic content according to Singleton and Rossi (1965). Fresh grape leaves were randomly selected from mid- and terminal-shoot leaves of infested and control plants previously used in the no-choice assay. About 0.5 g of leaf lamina was powdered with methanol 85 % on a pounder. The suspension was filtered on a filter paper, and 300 μl was taken and mixed with 1,500 μl of diluted Folin Ciocalteu reagent (1:10 with distilled water). After 8 min at room temperature, 1,200 μl of Na2Co3 7 % was added and the solution was placed on a Burrill shaker and treated for 90 min at room temperature in darkness. Sample absorbance was measured at 765 nm by a UV–visible spectrophotometer (Carry 100). A calibration curve was obtained measuring the absorbance of a series of standards solutions at 0, 4, 8, 16, 24 and 48 mg of gallic acid/100 ml. Amount of total phenols was expressed as mg of gallic acid equivalents per 100 g of leaf fresh weight. Measurements were replicated on five grapes per cultivar in June 2011 and each plant represented a replicate.
Statistical analysis
All experiments were carried out according to a complete random design. All results, except the plant growth features, were subjected to one-way analysis of variance (ANOVA) using Proc GLM (SAS 2003), after checking normal distribution and equal variance of data. Plant growth features were analyzed with t-tests using SPSS 13.0 (2004). Percentages of infested leaves were arcsine transformed before analysis. After ANOVA indicated significant differences between treatments, mean values were separated using Tukey’s test for post hoc comparison (α = 0.05).
Pearson’s correlation coefficient was determined to assess the relationship between mite population density and GEM impact on grape growth features (leaf area and weight, shoot and internode length, percentage of leaves with erinea), between mite population density and cultivar features (leaf trichome density, leaf thickness, amount of waxes, total carbohydrates and total phenols), and between mite impact on grape growth features and morphological as well as biochemical cultivar features, using SPSS 13.0 (2004).
Based on the C. vitis morphological measurements after the no-choice assays, cluster analysis was performed among the various vine cultivars, following Ward’s (1963) method using SPSS 13.0 (2004).
All graphs were produced by Sigma Plot version 11.0 (Systat Software 2008).
Results
Free-choice assay (antixenosis)
The mean density of juveniles and adults of GEM was unevenly distributed over the apical leaves of the various vine cultivars and it was significantly different among cultivars (F9,90 = 99.32, P < 0.0001) (Fig. 3). The lowest densities were detected on Shahani (mean = 20.0 mites per leaf), Flame seedless (24.2) and Yaghuti (24.7). The highest densities were found on leaves of Muscat Gordo (100.4), Gazne (95.5) and White Thompson seedless (87.4) (Fig. 3).
Free-choice assay: Colomerus vitis density (mean + SE number of mites per leaf; n = 10) after 48 h on each of ten vine cultivars, when offered simultaneously (see Fig. 1 for test set-up). Means capped by different letters are significantly different (P < 0.05, Tukey’s test)
No-choice assay (antibiosis)
When feeding of GEM was limited to leaves of one of six vine cultivars, mite density after two months was highly different among cultivars (F5,54 = 23852.9, P < 0.0001) (Fig. 4). The lowest number of eggs, juveniles + adults was recorded on Shahani (mean = 44.7 specimens per plant), the most severe mite infestation was detected on Gazne (822.7) (Fig. 4).
The body size analysis of female mites on each of the six vine cultivars indicated that the largest mites were found on White Thompson seedless and Flame seedless, whereas the smallest mites were found on Shahani and Yaghuti (Table 1). Significant differences among cultivars were found for all body size parameters: body length (F5,294 = 352.87), idiosoma length (F5,294 = 357.23), body width (F5,294 = 301.29), chelicera length (F5,294 = 121.98) and gnathosoma length (F5,294 = 75.84; all P < 0.0001).
Cluster analysis based on the mite body measurements clearly pointed out the existence of two groups of cultivars: White Thompson seedless, Flame seedless and Gazne vs Shahani, Yaghuti and Muscat Gordo (Fig. 5).
Symptoms and grape growth evaluation
The occurrence of erineum leaf patches after direct mite infestation (tolerance assay) differed among the six selected cultivars (F5,54 = 1462.98, P < 0.0001). The highest percentage of affected leaves was found on Gazne, whereas no erinea were recorded on Shahani (Table 2).
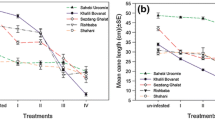
The mite population that developed after the artificial infestation affected various vegetative growth features in most of the six vine cultivars, in comparison with those of uninfested plants (Table 3). Overall, the mites had a pronounced effect on leaf area and weight, whereas their influence on shoot growth was quite weak. Infested leaves were significantly smaller than uninfested ones in all cultivars, except Shahani where no difference was found (F1,18 = 1.35, P = 0.26) (Table 3). Similarly, infested leaves were always heavier than those collected from control plants and these differences were significant on White Thompson seedless, Flame seedless and Gazne. Shoot and/or internode lengths were slightly longer on uninfested than on infested plants and these differences were significant on Gazne and Muscat Gordo (Table 3).
Correlation analysis indicated that mite density (number of eggs, juveniles + adults) was negatively related with infested leaf area (Pearson’s coefficient = −0.86), shoot length (−0.87; both P < 0.01) and internode length (−0.28, P < 0.05), and it was positively related with leaf weight (0.57) and percentage of affected leaves (0.81; both P < 0.01).
Cluster analysis based on the vegetative growth features pointed out the existence of three groups of cultivars: (1) White Thompson seedless and Flame seedless, (2) Yaghuti and Shahani, and (3) Muscat Gordo and Gazne (Fig. 6).
Leaf trichomes and leaf thickness
Rank density of the five plant trichome types differed significantly among grape cultivars (Table 4): blade bristle (F5,54 = 259.04), blade hair (F5,54 = 72.27), vein bristle (F5,54 = 1778.69), vein hair (F5,54 = 1449.30) and domatia (F5,54 = 469.90; all P < 0.0001). Blade bristle rank ranged from (mean ± SE) 4.6 ± 0.2 (Shahani) to 0.05 ± 0.02 bristles per mm2 (Flame seedless, Gazne); blade hair rank ranged from 2.7 ± 0.1 (Muscat Gordo) to 0.01 ± 0.01 hairs per mm2 (Shahani); vein bristle rank ranged from 13.5 ± 0.2 (Yaghuti) to 0.06 ± 0.04 bristles per mm2 (Flame seedless); vein hair rank ranged from 14.7 ± 0.2 (Muscat Gordo) to 0.03 ± 0.03 hairs per mm2 (Shahani); and domatia rank ranged from 37.4 ± 0.5 (Yaghuti) to 16.7 ± 0.3 trichomes per mm2 (Gazne) (Table 4). Leaf thickness differed among cultivars (F5,54 = 25.75, P < 0.0001); uninfested leaves of Shahani, Yaghuti and Gazne were thickest (Table 5).
Correlation analysis of mite population density (number of eggs, juveniles + adults) and leave thickness and trichome features, assessed on leaves of uninfested plants of the same grapevine cultivars, indicated that mite density was negatively related with blade bristle rank (Pearson’s coefficient = −0.73), vein bristle rank (−0.65), and domatium rank (−0.81), and it was positively related with blade hair rank (0.76) and vein hair rank (0.56; all P < 0.01). No significant correlation was found between mite density and leaf thickness (−0.05, P > 0.05).
Biochemical features
Significant differences among vine cultivars were found in the amount of leaf epicuticular waxes (F5,24 = 8.33, P = 0.0001) and total carbohydrate content (F5,24 = 215.42, P < 0.0001). The amount of leaf epicuticular waxes ranged from 0.17 (Shahani) to 0.11 mg/100 g (White Thompson seedless, Flame seedless), total carbohydrate content ranged from 295.4 (Gazne) to 187.0 mg/g (Shahani) (Table 6).
The amount of phenolic compounds in uninfested leaves did not differ among the six vine cultivars (F5,24 = 1.26, P = 0.31) (Table 6). When infested by GEM, the differences in phenol content among cultivars were still small (range: 0.10 ± 0.02–0.13 ± 0.04 mg/100 g gallic acid), but significant (F5,24 = 15.83, P < 0.0001) (Table 6).
Correlation analysis indicated that mite density (number of eggs, juveniles + adults) was negatively related with leaf wax contents (Pearson’s coefficient = −0.42) and phenol concentration in infested leaves (−0.97), and it was positively related with carbohydrate concentration of uninfested leaves (0.93; all P < 0.01). The correlation between mite density and phenol concentration in uninfested leaves was not significant (−0.25; P > 0.05).
Correlation of vegetative growth vs leaf biochemical and trichome features
Correlation analysis indicated negative relations between leaf area and shoot length vs carbohydrate content, and between leaf weight and % leaves with erinea vs phenol content in infested leaves and amount of epicuticular waxes (Table 7). Significant positive relations were found between leaf weight and % leaves with erinea vs carbohydrate content, between leaf area and shoot length vs phenol content in infested leaves, and between leaf area, shoot length and internode length vs epicuticular wax amount; phenol content of uninfested leaves were not significantly related to any vegetative growth feature (Table 7).
Correlation analysis indicated strong positive relations between leaf area and shoot length vs blade bristle, vein bristle and domatium ranks, and between leaf weight and % leaves with erinea vs blade hair and vein hair ranks (Table 8). Strong negative relations were found between leaf weight and % leaves with erinea vs blade bristle, vein bristle and domatium ranks, and between leaf area, shoot length and internode length vs to blade hair and vein hair ranks (Table 8).
Discussion
Host selection by eriophyoid mites is probably the combined result of differential attractiveness of a plant (in this case, grapevine cultivars towards GEM) and its biochemical and morphological features opposing mite infestation. Strategies and mechanisms applied by eriophyoids in selecting their host plants are still mostly vague and unexplained, but it appears likely that the few known chemosensilla on legs and mouthparts of these tiny mites (de Lillo and Aldini 2001; de Lillo et al. 2002, 2005) are involved in perceiving and discriminating plant chemical cues (short distance volatiles and contact compounds) among thousands of compounds on plant surfaces, mostly with unknown roles (Michalska et al. 2010). Considering the homogeneity of the environmental conditions during the experiments described in this study, the differences in mite size and population density observed on the various grapevine cultivars might be consequences of mite adaptation to the biochemical and morphological features of the hosts, which are involved in mite nourishment and/or plant defense mechanisms.
Intraspecific variation in morphology has rarely been investigated in eriophyoids. Some studies concerned the morphological variability in natural populations inhabiting closely related host plant species, e.g., of Aculus fockeui (Nalepa & Trouessart) protogyne females on Prunus species (Boczek et al. 1984) and Aceria tosichella Keifer on grass species (Skoracka and Kuczyński 2006), or in geographically separated populations, e.g., of A. guerreronis females on Cocos nucifera L. (Navia et al. 2006). Discontinuous morphological variation allowed the discrimination of cryptic entities, already genetically and biologically separated, within the generalist A. hystrix complex (Skoracka 2009) or Cirsium-associated Aceria spp. (Vidović et al. 2010) and Ribes-Cecidophyopsis species (Amrine et al. 1994). In addition, effects of plant cultivars on mite morphology have been reported in the free-living Aculus schlechtendali, pointing out differences in body size and length of the ventral setae within protogyne females (Kozlowski 1998).
Interestingly, GEM population density on Flame seedless in the no-choice assay was higher than expected based on the free-choice assays. Relative attractiveness, repellence and physical features of Flame seedless plants will have played a role during free-choice assay. But when the mite was forced to feed on less-preferred plants, no negative effect on the mite physiology became apparent, as indicated by the large size of the mite. In other words, the intrinsic features of Flame seedless appear not detrimental to the development of the mite population. Similar apparently contradictory results of free-choice vs no-choice assays have been found for Aceria salsolae de Lillo and Sobhian (Smith et al. 2009) and Aceria solstitialis de Lillo et al. (Stoeva et al. 2012) in host specificity trials in the laboratory.
Reduction of leaf surface, leaf weight increase, shoot shortening and erineum formation were related to mite density and were greatest on Gazne, while Shahani and Yaghuti were scarcely affected by GEM infestation. Plant feeding effects (distortive and non-distortive) are largely the result of eriophyoid piercing and injecting of salivary compounds, which re-direct the plant’s physiology and cell development, the details of which are far from clear (Oldfield 1996; de Lillo and Monfreda 2004; Monfreda and Spagnuolo 2004; Monfreda and de Lillo 2006; Petanović and Kielkiewicz 2010). Leaf erinea mites, such as GEM, are known to induce cell re-differentiation, which alters the size and the chemical content of infested organs (Petanović and Kielkiewicz 2010).
Mite density appeared to be largely related to the leaf physical structures (trichome types and density) of the assayed cultivars. Ning et al. (1996) found that some tea varieties resistant to A. theae had denser pubescent leaves than susceptible varieties. GEM can induce erineum leaf patches on very young leaves, which are smaller and have denser hairs (i.e., more trichomes per unit area) than mature leaves which were submitted during the current experiments. Perhaps these young leaves form favorable microhabitats that allow GEM to hide away from predators during the initial colonization of leaves and can offer a more suitable microclimatic and nutritional environment. Also long leaf hairs can favor escaping or hiding of free-living eriophyoids: the mites may climb into the hairs and stay unnoticed by predators wandering on the leaf surface (Michalska 2003; Michalska et al. 2010; Simoni and Sabelis 2010).
No significant correlation was found between mite density and leaf thickness of mature leaves; leaves of the least infested cultivars (Shahani and Yaghuti) were the thickest, along with Gazne which was largely infested. Measurements should be extended to the blade thickness of the youngest leaves, i.e. the most preferred by the mites and the most susceptible to the induction of erinea at the initial phase of the leaf infestation (Petanović and Kielkiewicz 2010).
Interactions between mite density and (non-glandular) trichome arrangement of grapevine leaves were previously observed in the field for GEM and grape rust mite, Calepitrimerus vitis (Nalepa), along with other phytophagous and predatory mites, on strongly haired leaves of Canaiolo in comparison to the weakly haired leaves of Sangiovese (Castagnoli et al. 1997). Similar results were obtained, still in the field, by Dellei and Szendrey (1991) on Hungarian and international cultivars. The complexity of the investigated microenvironments, the simultaneous presence of mites with different feeding behaviors and the various aims of the studies did not allow determining correlations of leaf physical structures vs eriophyoid population development. In addition, erinose species seem to take advantage of the leaf domatia stimulating the further development of dense hair-like structures inside them (O’Dowd and Willson 1997). Our observations in the current study regarded uninfested leaves.
The amount of leaf waxes was the highest in Shahani and Yaghuti, which hosted the lowest mite density and showed the least erinea on infested leaves. Higher wax content corresponded to lower leaf erineum induction and larger leaf area. It is well known that epicuticular waxes of the plant surfaces could affect the feeding behavior of herbivores and physically prevent their movement across the leaf surface (Howe and Schaller 2008). Wax compounds could interfere with mite feeding and, consequently, affect survival rates, developmental duration, egg production and other biological parameters. Thus, wax may be a valuable indicator for screening cultivar resistance. A potential correlation exists between the leaf wax layer and the thickness of leaf cuticle and epidermal cell walls. Eriophyoids pierce the cuticle and the epidermal cell walls by means of their short stylets, while injecting saliva into the cells, or, as some researchers believe, stylet piercing follows the digestion of epidermal cell wall components by means of mite salivary secretion (Schmeits and Sassen 1978; Thomsen 1988; Hoy 2011). The interference of the wax layer with cell piercing and the actual thickness of the leaf cuticle has already been related to resistance of pigeon pea, Cajanus cajan (L.) Millsp., to Aceria cajani Channabasavanna (Reddy et al. 1995).
Mite impact on the assayed cultivars proved to be strongly correlated with total soluble carbohydrates and phenols: mites appeared to thrive better on cultivars with higher leaf carbohydrate content, and infestation was lower on cultivars which do not increase leaf phenol content. Carbohydrate amount in uninfested leaves was lowest on the least mite-infested Shahani and highest on the heavily infested Gazne, whereas phenols increased in leaves of Shahani and decreased in leaves of Gazne after mite infestation. Similarly, lower contents of reducing and water-soluble sugars were found in shoots of tea varieties resistant to A. theae and this was supposed to have an antibiotic effect (Ning et al. 1996). Carbohydrates and proteins are known to (1) be required as nutrients by herbivorous arthropods (Bernays and Chapman 1994), (2) act as cues that affect plant susceptibility (Sadasivam and Macickam 1992; Dhaliwal and Dilawari 1993), and (3) affect the success of phytophagous organisms (Shi and Tomczyk 2001). Plant phenols are well known to interact with plant pests/pathogens and they play a role as antioxidants and modulators of the activity of other phytochemicals (Martin-Tanguy 1997). How eriophyoids may induce modifications in a plant’s phenol profile is still unclear and studies on other biochemical responses of host plants to eriophyoid feeding are scarce (Royalty and Perring 1996; Shi and Tomczyk 2001; Petanović and Kielkiewicz 2010). An increase of phenols after mite infestation has been recorded in olives fruits infested by Aculus olearius Castagnoli and Aceria oleae (Nalepa) (Cetin et al. 2011), in lemon buds infested by Aceria sheldoni (Ewing) (Ishaaya and Sternlicht 1969), in leaves of blackberry cultivars infested by Epitrimerus gibbosus (Nalepa) (Shi and Tomczyk 2001), but its detailed relationship with plant resistance to the mites was not always investigated. Some previous research pointed out that eriophyoid infestations (E. gibbosus on the evergreen blackberry cultivar Thornless, A. schlechtendali on a susceptible apple cultivar) can increase the ratio between total phenol compounds to carbohydrates in infested leaves (Kozlowski 1998; Shi and Tomczyk 2001); this higher ratio can depend both on the increase of phenols and the reduction of sugars (Kozlowski and Kozlowska 1996; Tomczyk and Boczek 2006).
In conclusion, the grapevines can be divided in (relatively) resistant, sensitive and intermediate cultivars. Shahani and Yaghuti can be considered resistant, because few mites were attracted by them, mite population development was poor and few or no erineum leaf patches were induced. In addition, infested and non-infested vines appeared to tolerate mite occurrence well. Muscat Gordo and Gazne were the most sensitive cultivars, as they sustained high populations of GEM and their leaves and shoots were affected considerably by the feeding mites. The seedless cultivars Flame and White Thompson showed intermediate responses concerning mite attraction and mite-plant interaction.
Leaf hairiness, leaf surface waxes and leaf carbohydrate contents could be used as tools to predict resistance/tolerance to GEM in preliminary screenings among vine cultivars.
Further experiments should be conducted, on these selected cultivars and also others with a larger distribution in the world, to investigate whether GEM affects gas exchange, photosynthesis, stomatal conductance, transpiration and other parameters more closely related to vine physiology and grape yield (carbohydrate reserves of grapes and harvest quality), such as carried out in part on Muscat in Swiss vineyards (Linder et al. 2006). The effects of cultivars on the reproductive fitness of the mites should be compared along with morphological details of the mite populations. Finally, the search for genes responsible for resistance may offer a potential tool in the IPM of these mites.
References
Amrine JW, Manson DCM (1996) Preparation, mounting and descriptive study of eriophyoid mites. In: Lindquist EE, Sabelis M, Bruin J (eds) Eriophyoid mites - Their Biology, Natural Enemies and Control. Elsevier Science Publishing, Amsterdam (World Crop Pests, vol 6, pp 383–396)
Amrine JW Jr, Duncan GH, Jones AT, Gordon SC, Roberts IM (1994) Cecidophyopsis mites (Acari: Eriophyidae) on Ribes spp. (Grossulariaceae). Int J Acarol 20(3):139–168
Avgin S, Bahadiroğlu G (2004) The effect of Colomerus vitis (Pgst.) (Acarina: Eriophyidae) on the yield and quality of grapes in Islahiye, Gaziantep. J Agric Sci 14(2):73–78
Balasubramanian M, Purushothamam D (1972) Phenols in healthy and galled leaves of Pongamia glabra Vent. caused by an eriophyid mite, Eriophyes cherianii Massee (Eriophyidae: Acarina). Ind J Exp Biol 10:394–395
Beck SD, Maxwell FG (1976) Use of plant resistance. In: Huffaker CB, Messenger PS (eds) Theory and practice of biological control. Academic press, New York, pp 615–631
Bergh JC, Weiss CR (1993) Pear rust mite, Epitrimerus pyri (Acari: Eriophyidae) oviposition and nymphal development on Pyrus and non–Pyrus hosts. Exp Appl Acarol 17:215–224
Bernard MB, Horne PA, Hoffmann AA (2005) Eriophyid mite damage in Vitis vinifera (grapevine) in Australia: Calepitrimerus vitis and Colomerus vitis (Acari: Eriophyidae) as the common cause of the widespread “Restricted Spring Growth” syndrome. Exp Appl Acarol 35:83–109
Bernays EA, Chapman RF (1994) Host-plant selection by phytophagous Insects. Chapman & Hall, New York 312 pp
Boczek J, Zawadzki W, Davis R (1984) Studies on the morphological and biological differences in Aculus fockeui (Nalepa et Trouessart) (Acari: Eriophyidae) on various host plants. Int J Acarol 10:81–87
Bronner R, Westphal E, Dreger F (1991) Pathogenesis-related proteins in Solanum dulcamara L. resistant to the gall mite Aceria cladophthirus Nalepa (syn. Eriophyes cladophthirus Nal.). Physiol Mol Plant Pathol 38(2):93–104
Carew ME, Goodisman MAD, Hoffmann AA (2004) Species status and population structure of grapevine eriophyoid mites (Acari: Eriophyoidae). Entomol Exp Appl 111:87–96
Casado CG, Heredia A (1999) Structure and dynamics of reconstituted cuticular waxes of grape berry cuticle (Vitis vinifera L.). J Exp Bot 50(331):175–182
Castagnoli M, Liguori M, Nannelli R (1997) Le popolazioni degli Acari nei vigneti inerbiti del Chianti: confronto tra cultivar. Redia 80:15–31
Cetin H, Arslan D, Ozcan A (2011) Influence of Eriophyid mites (Aculus olearius Castagnoli and Aceria oleae (Nalepa) (Acarina: Eriophyidae)) on some physical and chemical characteristics of Ayvalık variety olive fruit. J Sci Food Agric 91:498–504
Commenil P, Brunet L, Audran JC (1997) The development of grape berry cuticle in relation to susceptibility to bunch rots disease. J Exp Bot 48:1599–1607
Craemer C, Saccaggi DL (2013) Frequent quarantine interception in South Africa of grapevine Colomerus species (Trombidiformes: Prostigmata: Eriophyidae): taxonomic and distributional ambiguities. Int J Acarol 39(3):239–243
de Lillo E (2001) A modified method for eriophyid mite extraction (Acari: Eriophyoidea). Int J Acarol 27(1):67–70
de Lillo E, Aldini P (2001) Functional morphology of some leg sense organs in Pediculaster mesembrinae (Acari: Siteroptidae) and Phytoptus avellanae (Acari: Phytoptidae). In: Halliday RB, Walter DE, Proctor HC, Norton RA, Colloff M (eds) Acarology: Proc X Int Congr. CSIRO Publishing, Australia, pp 217–225
de Lillo E, Monfreda R (2004) “Salivary secretions” of eriophyoids (Acari: Eriophyoidea): first results of an experimental model. Exp Appl Acarol 34(3–4):291–306
de Lillo E, Di Palma A, Nuzzaci G (2002) Morphological adaptations of mite chelicerae to different trophic activities (Acari). Entomologica, Bari 35(2001):125–180
de Lillo E, Nuzzaci G, Di Palma A (2005) Sensorial structures in mites and perspectives of research. Phytophaga XIV(2004):59–81
Dellei A, Szendrey L (1991) The mite infestation of different grapevine varieties in the vineyards of Heves County. Növén 27(2):55–61
Dhaliwal GS, Dilawari VK (1993) Advances in Host Resistance to Insects. Kalyani Publishers, India p 443
Dres M, Mallet J (2002) Host races in plant-feeding insects and their importance in sympatric speciation. Philos Trans R Soc Lond B 357:471–492
Duso C, de Lillo E (1996) Damage and control of Eriophyoid mites in crops: 3.2.5 Grape. In: Lindquist EE, Sabelis MW, Bruin J (eds) Eriophyoid mites - Their Biology, Natural Enemies and Control. Elsevier Science Publishing, Amsterdam (World Crop Pests, vol 6, pp 571–582)
Duso C, Castagnoli M, Simoni S, Angeli G (2010) The impact of eriophyoids on crops: recent issues on Aculus schlechtendali, Calepitrimerus vitis and Aculops lycopersici. Exp Appl Acarol 51:151–168
Gholami M, Khanjani M, Mirab-balou M (2005a) Study on resistance of different cultivares of grape to Colomerus vitis in west of Iran. In: Proceeding of the 4th Horticultural Science of Iran, Mashhad, pp 183–184
Gholami M, Khanjani M, Mirab-balou M (2005b) Study on effective agents in population dynamic of Colomerus vitis in west of Iran. In: Proceeding of the 4th Horticultural Science of Iran, Mashhad, pp 190–191
Gibson RW, Valencia L (1978) A survey of potato species for resistance to the mite Polyphagotarsonemus latus, with particular reference to the protection of Solanum berthaultii and S. tarijense by glandular hairs. Potato Res 21:217–223
Gonçalves MIF, Maluf W, Gomes LAA, Barbosa LV (1998) Variation of 2-Tridecanone level in tomato plant leaflets and resistance to two mite species (Tetranychus sp.). Euphytica 104:33–38
Herr R (1991) Untersuschungen zur Resistenz der Gattung Ribes gegen die Johannisbeergallmilbe, Cecidophyopsis ribis (Westw.) (Acari, Eriophyidae). J Appl Entomol 112(2):181–193
Hochwender CG, Janson EM, Cha DH, Fritz RS (2005) Community structure of insect herbivores in a hybrid system: Examining the effects of browsing damage and plant genetic variation. Ecol Entomol 30:170–175
Howe GA, Schaller A (2008) Direct defenses in plants and their induction by wounding and insect herbivores. In: Schaller A (ed) Induced plant resistance to herbivory, vol vol 7C. Springer Science, Berlin, pp 7–30
Hoy MA (2011) Agricultural acarology. introduction to integrated mite management. CRC Press, Boca Raton 410
Irigoyen JJ, Emerich DW, Sanchez-Diaz M (1992) Alfalfa leaf senescence include by drought stress: photosynthesis, hydrogen, peroxid, metabolism, lipid peroxidation and ethylene evolution. Physiol Plant 84:67–72
Ishaaya I, Sternlicht M (1969) Growth accelerators and inhibitors in lemon buds infested by Aceria sheldoni (Ewing) (Acarina: Eriophyidae). J Exp Bot 20(65):796–804
Johnson HB (1975) Plant pubescence: an ecological perspective. Bot Rev 41:233–258
Khanjani M, Hadad Irani-Nejad K (2009) Injurious Mites of Agricultural Crops in Iran, 2nd edn. Bu-Ali Sina Univ Press Center, Iran p 526
Khanjani M, Khalghani J (2008) Principles of pests control (Insect & mites). Pub Ministry of jihad-e-agriculture, Agricultural extension, Education and Research Organization press center, Iran p 360
Kielkiewicz M, Soika G, Olszewska-Kaczynska I (2011) A comparative evaluation of the consequences of Phytoptus tetratrichus Nalepa (Acari: Eriophyoidea) feeding on the content and tissue distribution of polyphenolic compounds in leaves of different linden taxa. Acarologia 51(2):237–250
Kozlowski J (1995) Fecundity, oviposition rate and survival of Aculus schlechtendali (Nal.) on the leaves of selected apple varieties. Pr Nauk Inst Ochrony Rosl 36(1–2):128–131
Kozlowski J (1998) Czynniki warunkujace wrazliwosc odmian jabloni i reakcja odmian na pordzewiacza jabloniowego–Aculus schlechtendali (Nalepa). Rozprawy Naukowe Instytutu Ochrony Roslin w Poznaniu, Poland 2:119
Kozlowski J, Kozlowska M (1996) The influence of feeding of the apple rust mite Aculus schlechtendali (Nal) (Acarina: Eriophyidae) on biochemical changes in leaves of different apple cultivars. J Plant Prot Res 37(1–2):67–71
Leite GLD, Picanҫo M, Guedes RNC, Zanuncio JC (1999) Influence of canopy height and fertilization levels on the resistance of Lycopersicon hirsutum to Aculops lycopersici (Acari: Eriophyidae). Exp Appl Acarol 23(8):633–642
Li HJ, Conner RL, Liu ZY, Li YW, Chen Y, Zhou YL, Duan XY, Shen TM, Chen Q, Graf RJ, Jia X (2007) Characterization of wheat-triticale lines resistant to powdery mildew, stem rust, stripe rust, wheat curl mite, and limitation on spread of WSMV. Plant Dis 91(4):368–374
Linder C, Jermini M, Zufferey V (2006) Impact of the erineum mite Colomerus vitis on Muscat. IOBC/WPRS Bull 36:273–277
Loughner R, Goldman K, Loeb G, Nyrop J (2008) Influence of leaf trichomes on predatory mite (Typhlodromus pyri) abundance in grape varieties. Exp Appl Acarol 45:111–122
Luchian V, Săvulescu E, Tudose M (2008) Behavior of several varieties of Vitis vinifera L. to the attack caused by Colomerus vitis Pagst. and Tetranychus urticae Koch. Lucrări Ştiinţifice Seria B 52:123–127
Mariau D (1977) Aceria (Eriophyes) guerreronis: un important ravageur des cocoteraies africaines et américaines. Oléagineux 32(3):101–111
Martin-Tanguy J (1997) Conjugated polyamines and reproductive development: biochemical, molecular and physiological approaches. Physiol Plant 100:675–688
Michalska K (2003) Climbing of leaf trichomes by Eriophyid Mites impedes their location by predators. J Insect Behav 16(6):833–844
Michalska K, Skoracka A, Navia D, Amrine JW, Ueckermann EA (2010) Behavioural studies on Eriophyoid mites: an overview. Exp Appl Acarol 51(1/3):31–59
Mohiseni AA, Golmohammadi M, Kooshki MH (2011) Investigations on the resistance of 25 olive genotypes to Aceria oleae and Oxycenus niloticus (Acari: Eriophyidae) under greenhouse condition. Plant Prot 33(2):39–48
Monfreda R, de Lillo E (2006) Attuali conoscenze sulle secrezioni salivari negli Acari Eriophyoidea. Atti Accad Naz It Entomol Rend 53(2005):379–388
Monfreda R, Spagnuolo M (2004) Enzyme activity of an eriophyoid “salivary” secretion: preliminary report on polygalacturonase. In: Proceedings of the V symposium of the european association of acarologist, Berlin, Germany, 26–30 July. Phytophaga, XIV:611–614
Monfreda R, Nuzzaci G, de Lillo E (2008) Detection, extraction, and collection of Eriophyoid mites. Zootaxa 1662:35–43
Navia D, de Moraes GJ, Querino RB (2006) Geographic variation in the coconut mite, Aceria guerreronis Keifer (Acari: Eriophyidae): a geometric morphometric analysis. Int J Acarol 32(3):301–314
Ning X, Xue FC, Hua CC, Zong MC (1996) Morphological and biochemical parameters of tea varieties resistant to pink mite (Acaphylla theae Watt). J Tea Sci 16(2):125–130
O’Dowd JD, Willson MF (1997) Leaf domatia and the distribution and abundance of foliar mites in broadleaf deciduous forest in Wisconsin. Am Midl Nat 137:337–348
Oldfield GN (1996) Toxemias and other non-distortive feeding effects. In: Lindquist EE, Sabelis M, Bruin J (eds) Eriophyoid mites—Their Biology, Natural Enemies and Control. Elsevier Science Publishing, Amsterdam (World Crop Pests, vol 6, pp 243–250)
Panda N, Khush GS (1995) Host plant resistance to insects. IRRI-CABI, p 431
Petanović R, Kielkiewicz M (2010) Plant-eriophyoid mite interactions: specific and unspecific morphological alterations. Part II. Exp Appl Acarol 51:81–91
Peterson RKD, Higley LG (eds) (2001) Biotic stress and yield loss. CRC Press, Boca Raton, pp 205–232
Reddy MV, Sheila VK, Murthy AK, Padma N (1995) Mechanism of resistance to Aceria cajani in pigeonpea. Int J Trop Plant Dis 13(1):51–57
Royalty RN, Perring TM (1996) Nature of damage and its assessment. In: Lindquist EE, Sabelis M, Bruin J (eds) Eriophyoid mites - Their Biology, Natural Enemies and Control. Elsevier Science Publishing, Amsterdam (World Crop Pests, vol 6, pp 493–512)
Sadasivam S, Macickam A, (1992) Biochemical methods (Second edition). New Age International (P) Limited Publishers New Delhi and TNAU Coimbatore, India 256 p
SAS Institute (2003) GLM: a guide to statistical and data analysis, version 9.1. SAS Institute Cary
Schmeits TGJ, Sassen MMA (1978) Suction marks in nutrition cells of a gall on leaves of Acer pseudoplatanus L., caused by Eriophyes macrorhynchus typicus Nal. Acta Bot Neerl 27:27–33
Shi A, Tomczyk A (2001) Impact of feeding of eriophyid mite Epitrimerus gibbosus (Nalepa) (Acari: Eriophyoidea) on some biochemical components of blackberry (Rubus spp.). Bull Polish Acad Sci Biol Sci 49(1):41–47
Simoni S, Sabelis MW (2010) Glandular trichome ‘forests’ on tomato leaves act as a predation refuge for tomato russet mites. In: Proceedings of XIII international congress of acarology, p 254
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Skoracka A (2009) Description of Abacarus lolii n. sp. (Prostigmata: Eriophyoidea: Eriophyidae), a cryptic species within a grass–feeding Abacarus complex. Int J Acarol 35(5):405–417
Skoracka A, Kuczyński L (2006) Infestation parameters and morphological variation of the wheat curl mite Aceria tosichella Keifer (Acari: Eriophyoidea). In: Gabryś G, Ignatowicz S (eds) Advances in polish acarology. SGGW, Warszawa, pp 330–339
Skoracka A, Kuczynski L, Magowski W (2002) Morphological variation in different host populations of Abacarus hystrix (Acari: Prostigmata: Eriophyoidea). Exp Appl Acarol 26:187–193
Smith CM (2005) Plant resistance to arthropods. Molecular and conventional approaches. Springer, Dordrecht The Netherlands, p 423
Smith LM, Stafford EM (1948) The bud mite and the erineum mite of grapes. Hilgardia 18(7):317–334
Smith L, Cristofaro M, de Lillo E, Monfreda R, Paolini A (2009) Field assessment of host plant specificity and potential effectiveness of a prospective biological control agent, Aceria salsolae, of Russian thistle, Salsola tragus. Biol Control 48(3):237–243
SPSS (2004) SPSS base 13.0 User’s guide. SPSS, Chicago
Stoeva A, Harizanova V, de Lillo E, Cristofaro M, Smith L (2012) Laboratory and field experimental evaluation of host plant specificity of Aceria solstitialis, a prospective biological control agent of yellow starthistle. Exp Appl Acarol 56(1):43–55
Systat Software (2008) Sigma plot statistics users guide, version 11.0. Systat Software, Inc, San Jose
Tafazoli E, Hkmati J, Firuz P (1992) Vines. Shiraz Univ Press, 1st Edition, 343 p [In Persian]
Tandon P (1984) Peroxidase-catalyzed IAA-oxidation in presence of cofactors and auxin protectors isolated from Eriophyes incited Zizyphus gall tissue. Cecid Int 6(1,2,3):69–81
Tandon P, Arya HC (1980) Presence of auxin protectors in Eriophyes induced Zizyphus stem galls. Experientia 36(8):958–959
Thomsen J (1988) Feeding behaviour of Eriophyes tiliae tiliae Pgst. and suction track in the nutritive cells of the galls caused by the mites. Entomol Meddr 56(2):73–78
Tomczyk A, Boczek J (2006) Physiological and biochemical changes in tree leaves infested by some Eriophyoid mites (Acari: Eriophyoidea). In: Gabrys G, Ignatowicz S (eds) Advances acarology. Warszaw, Poland, pp 413–419
Tomoioaga L, Comsa M (2010) Monitoring the population of Eriophyoid mites, the species Calepitrimerus vitis and Colomerus vitis, in the vineyards specific conditions of Central Transylvania. Bull Univ Agric Sci Vet Med Cluj-Napoca Hort 67(1):499
van Houten YM, Glas JJ, Hoogerbrugge H, Rothe J, Bolckmans KJF, Simoni S, van Arkel J, Alba JM, Kant MR, Sabelis MW (2013) Herbivory-associated degradation of tomato trichomes and its impact on biological control of Aculops lycopersici. Exp Appl Acarol 60(2):127–138
Vidović B, Stanisavljević L, Petanović R (2010) Phenotypic variability in five Aceria spp. (Acari: Prostigmata: Eriophyoidea) inhabiting Cirsium species (Asteraceae) in Serbia. Exp Appl Acarol 52:169–181
Walling LL (2000) The myriad plant responses to herbivores. J Pl Growth Regul 19:195–216
Walton VM, Dreves AJ, Gent DH, James DJ, Martin RR, Chambers U, Skinkis PA (2007) Relationship between rust mite Calepitrimerus vitis (Nalepa), bud mite Colomerus vitis (Pagenstecher) (Acari: Eriophyidae) and Short Shoot Syndrome in Oregon vineyards. Int J Acarol 33(4):307–318
Ward JH (1963) Hierarchical grouping to optimize an objective function. JASA 58:236–244
Acknowledgments
The authors are strongly in debt to Drs. V.M. Walton (Department of Horticulture, Oregon State University, Corvallis, USA) and S. Simoni (CRA-ABP Agricultural Research Council, Research Center for Agrobiology and Pedology, Florence, Italy) for their contribution in improving the paper by their critical review. The study was supported by Bu-Ali University (2010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khederi, S.J., de Lillo, E., Khanjani, M. et al. Resistance of grapevine to the erineum strain of Colomerus vitis (Acari: Eriophyidae) in western Iran and its correlation with plant features. Exp Appl Acarol 63, 15–35 (2014). https://doi.org/10.1007/s10493-014-9778-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-014-9778-y