Abstract
Semiochemicals such as herbivore-induced plant volatiles (HIPVs) and host chemicals serve as communication signals for parasitoids searching for oviposition sites. The braconid koinobiont endoparasitoid Dolichogenidea gelechiidivoris (Hymenoptera: Braconidae) efficiently parasitises larvae of Tuta absoluta (Lepidoptera: Gelechiidae), a major pest of tomato (Solanum lycopersicum). However, the attractive compounds used by the parasitoid to locate T. absoluta on host plants are not known. We therefore performed behavioural assays and chemical analyses to investigate the chemical basis of interactions between the parasitoid, the tomato plant and T. absoluta. Y-tube olfactometer bioassays revealed that D. gelechiidivoris was attracted to T. absoluta larvae-infested tomato plant volatiles and preferred volatiles of plants with a high infestation level than those with a low infestation level. The parasitoid was also attracted to volatiles of larval frass and to the sex pheromone of T. absoluta. Coupled gas chromatography–mass spectrometric analyses were performed on plant and frass volatiles. We found both qualitative and quantitative differences in volatile emission between healthy and T. absoluta larvae-infested tomato plants, where volatile emission rate increased with increasing infestation level. The most characteristic volatile compounds which distinguished T. absoluta larvae-infested plants from healthy plants were α-pinene, sabinene, β-myrcene, 2-carene, α-phellandrene, 3-carene, α-terpinene, β-phellandrene, (Z)-β-ocimene, (E)-β-ocimene, allo-ocimene, (E)-β-caryophyllene and methyl salicylate. With the exception of caryophyllene oxide, all larval frass volatile compounds were also found in tomato plant headspace volatiles. Olfactometer bioassays using synthetic compounds revealed that D. gelechiidivoris was attracted to α-pinene, β-myrcene, α-phellandrene, α-terpinene, β-ocimene, methyl salicylate and (E)-β-caryophyllene, and the 7-component blend of these attractants elicited the greatest attraction in the parasitoid. These findings open new avenues for exploiting these attractants as kairomone-based lures to recruit and retain the parasitoid in tomato fields for the biological control of T. absoluta.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Key message
-
We investigated semiochemicals mediating interactions between the braconid parasitoid Dolichogenidea gelechiidivoris, Tuta absoluta, and tomato plants.
-
Dolichogenidea gelechiidivoris was attracted to volatiles of T. absoluta larvae-infested tomato plants, larval frass, and sex pheromone of T. absoluta.
-
The blend of α-pinene, β-myrcene, α-phellandrene, α-terpinene, β-ocimene, methyl salicylate and (E)-β-caryophyllene was highly attractive to D. gelechiidivoris.
-
The attractive volatile blend and the sex pheromone could be deployed as kairomone-based lures to recruit and retain the parasitoid for biological control of T. absoluta.
Introduction
Kairomones are semiochemical molecules that are emitted by an organism to mediate interspecific interaction beneficial to the receiver organism (Dicke and Sabelis 1988; Kost 2008). Kairomones exploited by natural enemies are either plant volatiles or herbivore-associated chemicals such as pheromones (Afsheen et al. 2008; Turlings and Erb 2018; Ayelo et al. 2021). Herbivore-induced plant volatiles (HIPVs) are released by plants in response to herbivore feeding, and serve as long-range kairomones for parasitoids searching for hosts (Kessler and Baldwin 2001; Kaplan 2012; Turlings and Erb 2018; Ayelo et al. 2021). The HIPVs are a blend of compounds belonging to different chemical groups, while parasitoid attracting-compounds mainly belong to green leaf volatile and terpenoid groups (Mumm and Dicke 2010; Turlings and Erb 2018). The herbivore feeding behaviour, i.e. chewing or sap sucking, determines which plant defence pathway (jasmonic acid or salicylic acid) will be activated, and this influences the emission of HIPVs (Danner et al. 2018). Other biotic factors including the herbivore-infesting species, the level of infestation, the infesting instar, and the host plant species and genotype shape the compositions of HIPVs (Mumm and Dicke 2010). Quantitative and qualitative variations in the HIPV blends occur between healthy and herbivore-infested plants (Mumm and Dicke 2010; Danner et al. 2018), and these differences are known to induce different behavioural responses by parasitoid species to the HIPVs of host-infested plants (De Moraes et al. 1998; McCormick et al. 2012; Takabayashi and Shiojiri 2019).
Parasitoids also locate their hosts through kairomones emanating from the hosts or from the host by-products such as larval frass, larval secretions and adult sex pheromones (Vet and Dicke 1992; Afsheen et al. 2008). Herbivore larval frass volatiles mainly contain relatively small quantities of volatile compounds emitted by the host plants fed upon and other volatiles resulting from digestion and oxidation processes (Cordero et al. 2012), and may therefore only be active at short range, or act synergistically with HIPVs to enhance the long-range location of host-infested plants by natural enemies (Reddy et al. 2002; Dalen et al. 2015). Synergy between HIPVs and insect pheromones in enhancing mate and host finding behaviour by parasitoids has been documented (Reddy and Guerrero 2004; Xu et al. 2017). Herbivore pheromones are highly species-specific chemicals released for short and long-range communication with conspecific individuals, but which in turn can also be exploited by their natural enemies to locate them (Aukema and Raffa 2005); as seen in the braconid parasitoid Chelonus insularis Cresson, which is attracted to the sex pheromone of its host, Spodoptera frugiperda (Smith) (Roque-Romero et al. 2020).
The invasive South American tomato pinworm, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), is a serious constraint to tomato production both in open fields and in greenhouses (Desneux et al. 2010). Following its transatlantic invasion, T. absoluta was first detected in Spain in 2006 (Urbaneja et al. 2007). Soon after, the pest spread to and established in several countries in Europe, Asia, Haiti and almost the entire African continent with a devastating impact on tomato production (Desneux et al. 2010; Mansour et al. 2018; Verheggen & Fontus 2019; Han et al. 2019). Damage to crops by T. absoluta is mainly caused by the larvae which feed on leaves, fruits and stems of tomato plants, causing between 80 and 100% yield losses when no control measures are used (Desneux et al. 2010; Mansour et al. 2018 and references therein). Synthetic broad-spectrum chemical insecticides are often applied for T. absoluta control worldwide (Desneux et al. 2010; Biondi et al. 2018). These chemicals are, however, hazardous for human health and for the environment. The indiscriminate and frequent applications of these chemicals coupled with the multivoltine nature of T. absoluta have led to development of resistance in field populations of the pest to different classes of synthetic chemical insecticides, which inevitably makes their use unsustainable (reviewed in Guedes et al. 2019). Biological control using natural enemies offers an alternative and potentially sustainable way to control T. absoluta on tomato crops in the field (Desneux et al. 2010; Zappalà et al. 2013; Salas Gervassio et al. 2019).
The koinobiont endoparasitoid Dolichogenidea gelechiidivoris March (Syn. Apanteles gelechiidivoris March) (Hymenoptera: Braconidae) was imported into Kenya from Peru by the International Centre of Insect Physiology and Ecology (icipe) jointly with the International Potato Center (CIP) (Lima, Peru) for classical biological control of the pest in Africa. Dolichogenidea gelechiidivoris preferentially parasitises first and second instars of T. absoluta larvae (Aigbedion-Atalor et al. 2020), and it is known to be efficient in controlling T. absoluta in its native home range (Valencia and Peñaloza 1990; Palacios and Cisneros 1995; Vallejo 1999). However, when released for classical biological control, natural enemies are likely to emigrate or disperse from cropping field sites, thereby reducing their efficiency in controlling insect pests in the target crops (Heimpel and Asplen 2011). Interestingly, semiochemical-based lure applications have been pointed out as a sound solution to limit the dispersal of natural enemies and to recruit and retain them in the vicinity of target crops, thus enhancing biological control strategies (Kelly et al. 2014; Peri et al. 2018; Ayelo et al. 2021). A recent study reported that the egg parasitoid Trichogramma achaeae Nagaraja & Nagarkatti (Hymenoptera: Trichogrammatidae) was attracted to T. absoluta-infested tomato plant volatiles and the host sex pheromone (Gontijo et al. 2019). We hypothesised that the larval parasitoid D. gelechiidivoris also exploits plant and host semiochemicals to locate T. absoluta in the field. We, therefore, assessed the attractiveness of the parasitoid to healthy and T. absoluta-infested tomato plant volatiles and host kairomones in relation to host densities, analysed the plant and host frass volatiles, and then determined the compounds attractive to the parasitoid. We discuss our findings in the light of developing a kairomone attractant-based lure to recruit and retain D. gelechiidivoris in tomato crop fields to enhance the biological control of T. absoluta.
Materials and methods
Plants
Tomato (Solanum lycopersicum L. (Solanaceae)) plants (cv. Kilele F1 hybrid, Syngenta) were grown and maintained in a screen house at 28 ± 5 °C, 60 ± 10% RH at the International Centre of Insect Physiology and Ecology (icipe), Nairobi, Kenya. Nurseries were established by sowing seeds on a mixture of 1:1 soil: manure (i.e. goat dung) in a seed raising plastic tray. Three weeks later, the seedlings were transplanted on a 3:1 soil: manure mixture in plastic pots (15 × 10 cm) at a density of one plant per pot. The plants were watered as needed, and provided weekly with water-dipped fertiliser containing 18% N, 20% P2O5, and 21% K2O (Easygrow, Osho Chemical Industries Ltd). No chemical insecticides were applied on the plants.
Insects rearing
Tuta absoluta colony was established using eggs, larvae and adults obtained from infested tomato samples collected from fields at the Kenyan Agricultural and Livestock Research Organisation (KALRO) (0°37′11.3″S 37°22′08.0″E) in Kimbimbi, Mwea Sub-county, Kirinyaga County. The insects were kept in Plexiglass cages (60 × 60 × 80 cm) in the laboratory under conditions set at 25 ± 2 °C, 65 ± 5% RH and 12L:12 D photoperiod regime. To maintain the colony, 6–8-week-old tomato plants were exposed for 3 days to T. absoluta adults for oviposition. Thereafter, the infested plants were removed and placed in another Plexiglass cage and kept for 2 weeks albeit, with an addition of tomato plants to ensure better development of larvae. Emerging adults were transferred into another Plexiglass cage for plant infestation. Tuta absoluta adults were fed on 80% honey solution smeared on the top inner side of the Plexiglass cages.
Dolichogenidea gelechiidivoris colony was initiated with parasitoid cocoons received from the International Potato Center (CIP), Peru and maintained at the icipe quarantine facility until the parasitoid adults emerged, as described in Aigbedion-Atalor et al. (2020). The rearing was done in laboratory Plexiglas cages (40 × 40 × 50 cm) at the quarantine facility at 25 ± 2 °C, 65 ± 5% RH and photoperiod regime of 12L:12D. The insects were kept on T. absoluta-infested tomato plants obtained by exposure of 6–8-week-old tomato plants to T. absoluta adults for 3 days. Thereafter, at three to 5 days post-infestation, the infested plants with batches of first and second larval instars were offered to the parasitoids for 48 h and renewed on 2-day intervals. Parasitoid-exposed plants were cut off and their petioles inserted into floral foams previously soaked into water, and then placed in other Plexiglass cages (30 × 30 × 30 cm). Healthy tomato plants were added to ensure satisfactory larval development. Emerging D. gelechiidivoris adults were aspirated and kept in small cylindrical plastic cups (2.5 × 7 cm) for 48 h to increase the chances of their mating before they were used in bioassay experiments. Parasitoids were fed on 80% honey solution smeared on the inner side top of the Plexiglass cages.
Y-tube olfactometer bioassays
Dolichogenidea gelechiidivoris responses to volatiles from different odour sources were tested in dual-choice experiments using a Y-tube olfactometer. The Y-tube glass devise (2.5 cm i.d.) consisted of a 12-cm long stem and two 6-cm arms which formed an angle of 60º. The Y-tube glass device was placed in an observation chamber made of a cardboard box (35 × 35 × 55 cm), which was illuminated with a 220–240 V cool fluorescent tube to provide a uniform lighting. Each arm of the olfactometer was connected to a 10-L glass jar serving as an odour source container. A vacuum pump (KNF lab LABOPORT N86KT.18, France) was used to generate a unidirectional airflow which was filtered by an active carbon filter, and then passed through the odour source containers at a constant wind speed of 150 mL min−1 set using a flow meter (AALBORG, Orangeburg, NY, USA). Although preliminary bioassays showed that the proportion of responsive parasitoids was quite similar when the Y-tube glass was oriented vertically (96.7%) or horizontally (90%), in subsequent bioassays, the Y-tube glass was oriented vertically in our tests, because the parasitoids move upwards in rearing cages. The plant pot was tightly wrapped with aluminium foil to prevent headspace volatile contamination from the soil. The test plants were then placed in the glass jars and left to acclimatise under the airflow for one hour before the start of the bioassays. A single parasitoid was introduced at the base of the stem of the Y-tube, and the insect’s first choice was recorded over a 10 min observation period. A choice was scored when the insect climbed and entered a given arm up to a distance of 3 cm and remained in the arm for 30 s. In total, fifty insects were tested per choice test over 10 days (five insects per day per choice test). Insects that had not made a choice after 5 min were considered as non-responsive (which accounted for between 0 and 6% of the insects tested), and hence were not included in the data analyses. One plant was used for five insects, and after three insects the Y-tube glass was cleaned with dichloromethane (solvent) and the volatile sources switched between arms to avoid positional bias. At the end of experiments on each day, the Y-tube glass was cleaned with Teepol odourless detergent and hot water, and rinsed with acetone and distilled water, then dried in the oven at 150 °C overnight. Naïve 2–5-day-old D. gelechiidivoris females were used in the bioassays as the parasitoid’s mature egg load peaked within this age interval (Aigbedion-Atalor et al. 2020). All bioassays were performed at the quarantine facility at 25 ± 2 °C, 65 ± 5% RH and 12L:12D photoperiod regime.
Responses of Dolichogenidea gelechiidivoris to plant volatiles
The olfactory responses of D. gelechiidivoris to volatiles from healthy and T. absoluta-infested tomato plants were investigated in dual choices, comparing: (i) air vs. air (control); (ii) air vs. healthy plant; (iii) air vs. T. absoluta-infested plant; (iv) healthy plant vs. T. absoluta-infested plant. Four-week-old plants were individually infested with 5, 10 or 20 T. absoluta larvae of first and second instars (1:1 ratio) which were left to feed on the plant for 4 days.
Responses of Dolichogenidea gelechiidivoris to host volatiles
The parasitoid olfactory responses to volatiles from T. absoluta larvae, larval frass and sex pheromone were tested; here the 10-L glass jar containers were replaced by 250-mL quick fit glass jars (Sigma Scientific, Gainesville, FL, USA). For the bioassays, the clean air (control) was tested against: (i) clean air; (ii) 10, 20 or 40 T. absoluta larvae; (iii) 15, 30 or 60 mg of T. absoluta larval frass; and (iv) T. absoluta female commercial sex-pheromone lure. The pheromones lures contain 90:10 ratio of (E,Z,Z)-3,8,11-tetradecatrien-1-yl acetate to (E,Z)-3,8-tetradecadien-1-yl acetate (both 98.5% purity) released by a rubber septum dispenser and were purchased from KOPPERT (Biological Systems, Ltd.). Tuta absoluta larval frass was collected from mined tomato plant leaves using a camel hairbrush, and thereafter immediately used in the bioassays. A sample of 60 mg of frass corresponded to the amount collected from approximately 40 third instar larvae feeding for 4 days. The collection of larvae was done by placing T. absoluta larvae-infested leaves in a plastic box, then paper towel was added and the container was closed with a ventilated-mesh lid. After 24 h, the larvae easily moved out of the mined leaves, which made easy the collection of the larvae without damaging them. Tuta absoluta first and second larval instars were used in the bioassays as the parasitoid D. gelechiidivoris preferentially parasitises these stages (Aigbedion-Atalor et al. 2020).
Collection of volatiles
Tomato plant headspace volatiles
Headspace volatiles were trapped onto prepacked 30-mg Super-Q adsorbents (Analytical Research Systems, Gainesville, FL, USA) using a dynamic push–pull system. A vacuum pump (KNF lab LABOPORT N86KT.18, France) was used to suck air which passed through a charcoal-filter and then entered the 10-L glass jar containers used in the behavioural experiments, at a rate of 200 mL min−1. We collected volatile compounds from healthy and T. absoluta-infested tomato plants, as well as from empty volatile collection chamber, and from pots containing soil with no plants (controls), with five replicates. The pots of the plant and the control were tightly wrapped in aluminium foil to prevent contamination from potting soil, and were then placed separately in the containers. The age of tomato plants and the infestation procedure were the same as described above. Volatiles were collected for 24 h and eluted with 150 μL of dichloromethane, then stored at − 80 °C. Just before analyses by gas chromatography-mass spectrometry (GC–MS), the headspace extract volume was concentrated to 50 μL under a gentle nitrogen air stream, then 5 μL of biphenyl (99% purity) solution (20 ng/μL) was added as internal standard.
Frass headspace volatiles
A Solid Phase Micro-Extraction (SPME) holding 65-μm fibre (PDMS-DVB StableFlex; Supelco Bellefonte, PA, USA) was used to collect T. absoluta larval frass volatiles. The SPME fibre was cleaned by conditioning in GC at 250 °C for 15 min before use. A 60 mg sample of larval frass was placed in a 2-mL storage vial with a rubber septum lid. The SPME fibre was deployed through the lid and held at 1–2 cm above the sample for 24 h, thereafter the volatiles were analysed by GC–MS, in five replicates.
Chemical analysis
Volatile analyses were performed using a 7890A Gas Chromatograph (Agilent Technologies) coupled with an HP-5MSI low bleed non-polar capillary column (5% phenyl and 95% methylpolysiloxane, 30 cm × 0.25 mm × 0.25 μm film thickness). An aliquot (1 μL) of headspace tomato volatiles was analysed in splitless mode. Whereas the SPME fibre loaded with the frass volatiles was injected into the GC injection port immediately after volatile trapping. Volatiles were analysed using helium as carrier gas at a flow rate of 1.2 mL min−1. For both the plant and frass volatile analyses, the oven temperature was set at 35 °C for 5 min, then increased at a rate of 10 °C min−1 to reach 280 °C which was held for 10.5 min. The ion source temperature was set at 250 °C with an interface temperature of 270 °C, and spectra were recorded at 70 eV. The compounds were identified using retention time, library mass spectra (NIST11, Wiley9, Adams and Chemecol), and Kovats retention indices (RIs). The RIs calculated using retention times of n-alkane (C8–C30) standards which were run as a mixture in a separate injection. The retention index (RI) was computed using the following formula: (RI) = [RT(X) − RT(n)]/[RT (n + 1) − RT(n)]*100 + (100*n), where RT(X) is the retention time of the studied compound X, RT(n) is the retention time of the alkane with n carbons that eluted before X, and RT(n + 1) is the retention time of the alkane with n + 1 carbons that eluted after X. Comparison with published mass spectra and Kovats retention indices from online NIST library was done, and synthetic standards, when available, were run to confirm the identification of some compounds by comparison of the expected retention time and the MS spectra. The peak area and concentration of the internal standard were used for quantification of the volatile organic compounds (VOCs), i.e. internal calibration which is a recommended method for quantification of relative amounts of volatile compounds using GC–MS (IOFI 2011; Ruiz-Hernández et al. 2018). In our study, the formula used for the quantification of the compounds were adapted from Wang et al. (2019), as follows:
and
where Ca is the concentration (ng⁄µL) of the analyte in the volatile eluent; PAa is the peak area of the identified analyte; PAis is the peak area of the internal standard; Cis is the concentration of internal standard (i.e. 20 ng/µL of biphenyl) and V is the volume of the volatile eluent (i.e. 50 µL) in which the aliquot (5µL) of internal standard has been applied; Rr is the release rate (ng/plant/h) which equals to the concentration (ng⁄µL) multiplied by the volume of the eluent (µL) and divided by the volatile collection period (24 h).
Chemicals
All synthetic standards were purchased from Merck, France. Chemical purities of standards were as follows: α-pinene (98%), sabinene (75%), β-myrcene (90%), 2-carene (97%), α-phellandrene (85%), 3-carene (90%), α-terpinene (95%), β-phellandrene (95%), β-ocimene (90%), linalool (97%), allo-ocimene (98%), methyl salicylate (97%), (E)-β-caryophyllene (98%), α-humulene (96%), p-cymene (99%), p-xylene (99%), α-terpineol (96%), terpinolene (90%), γ-terpinene (97%), γ-elemene (98%), β-elemene (96%), (Z)-3-hexen-1-ol (98%), 6-methyl-5-hepten-2-one (98%), geranyl acetone (97%), β-pinene (99%) and β-ionone (96%). The synthetic of β-ocimene contained the mixture of the isomers E and Z in a ratio of 2.5E: 1Z. Dichloromethane (99.9% purity) was purchased from Merck, Germany.
Bioassays with synthetic compounds
The VOCs α-pinene, sabinene, β-pinene, β-myrcene, 2-carene, α-phellandrene, 3-carene, α-terpinene, β-phellandrene, (Z)-β-ocimene, (E)-β-ocimene, linalool, allo-ocimene, methyl salicylate, (E)-β-caryophyllene and α-humulene were selected to test the attractiveness of synthetic compounds to the parasitoid. The synthetic of β-ocimene containing the mixture of E and Z isomers in a ratio of 2.5E: 1Z, was tested instead of the two isomers separately. The natural release rate (ng/plant/h) (Table 1) was considered as the reference dose, and each compound was tested at three doses corresponding to release rates of 1, 10 and 100 equivalent plants. Based on the results, seven compounds (i.e. α-pinene, β-myrcene, α-phellandrene, α-terpinene, β-ocimene, methyl salicylate and (E)-β-caryophyllene) were found to be attractive to the parasitoid, and therefore a 7-component blend of these compounds mixed at their most attractive doses (blend B1) was tested. Since this dose of blend B1 did not attract the parasitoid, four other blends were made up from blend B1 dose by dilutions to one-half (blend B2), one-fourth (blend B3), one-tenth (blend B4), and one-hundredth (blend B5), with which the attractiveness to the parasitoid was tested. The compounds were diluted in dichloromethane (solvent), and a 10 μL aliquot of the test solution was loaded on a 2 × 2 cm filter paper and tested against the control (filter paper loaded with 10 μL-dichloromethane). The solvent was left to evaporate for 30 s, then the impregnated filter papers were placed at the edge of the olfactometer arms, and were renewed for every insect. Fifty insects were tested individually per choice test as described above.
Statistical analyses
The proportions of odours chosen by the parasitoid D. gelechiidivoris were compared using a chi-squared test. The variable VOC concentrations were tested for normality using Shapiro–Wilk’s test, and homogeneity of variance using Bartlett’s test. Since the data were not normally distributed and their variance not homogenous, a nonparametric Kruskal–Wallis ANOVA was performed to compare amounts of VOCs between healthy and T. absoluta-infested tomato plants, using the post hoc Dunn’s test with Bonferroni’s adjustment for mean separation (Dinno 2015). The random forest (RF) analysis (Breiman 2001) was performed to select the VOCs that best distinguished T. absoluta-infested tomato plants from healthy plants based on the concentrations of VOCs. The mean decrease in accuracy (MDA) generated using the RF “importance” function enabled the selection of VOCs that significantly contributed to the discrimination analyses, and a higher MDA indicates a higher importance of the compound to the discrimination (Liaw and Wiener 2002; Ranganathan and Borges 2010). A classification error, commonly known as out of bag (OOB) error, allowed appreciating the classification accuracy (100%—OOB error) of the RF analysis output (Breiman 2001). Using concentrations of the most discriminating VOCs, a sparse partial least square discriminant analysis (sPLS-DA) biplot was performed in mixOmics package to illustrate the correlation between volatile compounds and healthy and T. absoluta-infested plants (Lê Cao et al. 2011; Hervé et al. 2018). The validation of the sPLS-DA model was assessed using the “perf” function and the “leave-one-group-out” cross-validation method in the mixOmics package, as well as the sPLS-DA parameters (R2X, R2Y, Q2) (Rohart et al. 2017). Heatmap clustering was also performed to illustrate variations in the discriminating VOCs across replicates of healthy and T. absoluta-infested tomato plants, using the “cim” function in the mixOmics package (Rohart et al. 2017). For bioassays with synthetic compounds, we used compounds that appeared at least thrice among the top discriminating VOCs (compounds indicated in dark black colour) responsible for differences between plants offered in dual-choice bioassays in the five modalities in which the parasitoid attraction was significant. All statistical analyses were performed using R statistical software, version 4.0.2 (R Core Team 2020).
Results
Responses of Dolichogenidea gelechiidivoris to plant volatiles
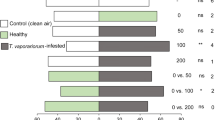
The parasitoid D. gelechiidivoris was not attracted to volatiles of healthy tomato plants when compared to clean air (χ2 = 0, df = 1, P = 1) (Fig. 1). In contrast, relative to clean air, the parasitoid was highly attracted to volatiles of tomato plants infested by T. absoluta at all the densities of host infestation tested (χ2 = 6.61, df = 1, P = 0.01; χ2 = 17.52, df = 1, P < 0.0001; χ2 = 15.19, df = 1, P < 0.0001, for 5, 10 and 20 T. absoluta larvae/plant). Similarly, D. gelechiidivoris preferred the volatiles of tomato plants infested with 5 (χ2 = 4.69, df = 1, P = 0.03), 10 (χ2 = 10.58, df = 1, P = 0.0006) or 20 T. absoluta larvae (χ2 = 14.38, df = 1, P = 0.0001) over those of healthy tomato plants (Fig. 1). Moreover, the parasitoid was more attracted to volatiles of tomato plants infested with a high density of 20 T. absoluta larvae than to the volatiles of plants with low infestation levels, i.e. 5 (χ2 = 8.16, df = 1, P = 0.004) or 10 T. absoluta larvae (χ2 = 6.61, df = 1, P = 0.01) (Fig. 1).
Behavioural responses of Dolichogenidea gelechiidivoris to volatiles of Tuta absoluta larvae-infested and healthy tomato plants in Y-tube olfactometer choice tests, as a percentage of the parasitoids which responded. Fifty insects were tested per choice test. nr = number of non-responsive parasitoids (i.e. insects which made no choice). P stands for levels of significance with ns = no significant difference (P > 0.05); *, **, *** = significant differences, respectively, at P < 0.05, P < 0.01 and P < 0.001 from χ2 test at α = 0.05
Responses of Dolichogenidea gelechiidivoris to host volatiles
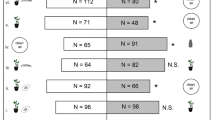
Volatiles of Tuta absoluta larvae did not attract the parasitoid, D. gelechiidivoris, at any of the tested densities of 10 (χ2 = 0.02, df = 1, P = 0.887), 20 (χ2 = 0.73, df = 1, P = 0.391) or 40 larvae (χ2 = 3.38, df = 1, P = 0.065) compared to clean air (Fig. 2). Similarly, the parasitoid was not attracted to volatiles from T. absoluta larval frass at the doses of 15 mg (χ2 = 0.98, df = 1, P = 0.322) or 30 mg (χ2 = 2.04, df = 1, P = 0.153) when compared to clean air. On the other hand, volatiles of T. absoluta larval frass at a dose of 60 mg did attract the parasitoid compared to clean air (χ2 = 8.16, df = 1, P = 0.004) (Fig. 2). Moreover, D. gelechiidivoris was more attracted to the commercial sex pheromone of T. absoluta than to clean air (χ2 = 6.02, df = 1, P = 0.014) (Fig. 2).
Behavioural responses of Dolichogenidea gelechiidivoris to volatiles of larvae, larval frass, and commercial sex pheromone of Tuta absoluta in a Y-tube olfactometer choice tests, as a percentage of respondent parasitoids. Fifty insects were tested per choice test. nr = number of non-responsive parasitoids (i.e. insects which made no choice). cph = one rubber septum loaded with commercial sex pheromone of T. absoluta. P stands for levels of significance with ns = no significant difference (P > 0.05); *, ** = significant differences, respectively, at P < 0.05 and P < 0.01 from χ2 test at α = 0.05
Analysis of tomato volatiles
Forty VOCs were identified in the headspace volatile profiles of healthy and T. absoluta larvae-infested tomato plants (Table1; Fig. 3). These VOCs belong to eight chemical classes: monoterpenes (22), sesquiterpenes (7), ketones (4), aldehydes (2), benzenoids (2), an ester (1), a homoterpene (1) and an alcohol (1) (Table 1). Quantitative and qualitative differences were observed in the composition of constitutive and T. absoluta larvae-induced tomato plant volatiles which were dominated by monoterpenes and sesquiterpenes (Table 1; Fig. 3). Monoterpenes increased by twofold to fourfold in T. absoluta larvae-infested tomato plants relative to healthy plants. Relative to the total volatile emission per plant, monoterpenes accounted for 93.8, 92.8, 88.8 and 86.1%, respectively, for healthy plants and plants infested with 5, 10 and 20 T. absoluta larvae; 2-carene and β-phellandrene being the most abundant monoterpenes (Table 1). Sesquiterpenes increased by twofold to tenfold in T. absoluta larvae-infested plants compared to healthy plants, and represented 2.4, 2.4, 4.7 and 5.8% of the total released volatiles, respectively, in healthy plants, and 5, 10 and 20 T. absoluta larvae-infested plants, with (E)-β-caryophyllene, α-humulene and δ-elemene as the most abundant sesquiterpenes (Table 1). Specifically, VOC emission rates increased with the host infestation density, as seen in the emission of α-pinene, β-myrcene, 3,7,7-trimethyl-1,3,5-cycloheptatriene, 2-carene, α-phellandrene, α-terpinene, β-phellandrene, (E)-β-ocimene, (E)-β-caryophyllene, δ-elemene and α-humulene which increased in T. absoluta larvae-infested plants compared to healthy plants. Fifteen VOCs were specific to T. absoluta-infested tomato plant volatiles, and not detected among the volatiles of healthy plants. These VOCs included the alcohol (Z)-3-hexenol; the aldehydes benzaldehyde and benzene acetaldehyde; the monoterpenes sabinene, linalool, allo-ocimene, neo-allo ocimene, α-terpineol, β-cyclocitral; the sesquiterpene β-elemene; the ester (Z)-3-hexenyl butanoate; the benzenoid ester methyl salicylate; the ketones 6-methyl-5-hepten-2-one, (Z)-jasmone and β-ionone (Table 1). On the other hand, emission rates of some VOCs including γ-terpinene, guaiadiene-6,9 and an unidentified monoterpene did not significantly vary between healthy and T. absoluta larvae-infested tomato plants (Table 1).
GC/MS profiles of headspace volatiles from healthy plants and plants infested with 5, 10 and 20 Tuta absoluta (Ta) larvae. The numbers correspond to the names of the volatile compounds listed in Table 1. IS = internal standard (biphenyl)
Analysis of Tuta absoluta larval frass volatiles
Fifteen VOCs were detected in the headspace of T. absoluta larval frass volatiles, of which 7 were monoterpenes, 6 sesquiterpenes, a ketone and a benzenoid ester (Table 2, Fig. 4). With the exception of caryophyllene oxide, all VOCs detected in the larval frass were found in the tomato plant headspace volatiles listed in Table 1.
GC–MS profile of Tuta absoluta larval frass volatiles. Numbers correspond to names of the volatile compounds listed in Table 2
Determination of discriminating volatile organic compounds
The mean decrease in accuracy (MDA) in the random forest (RF) analysis revealed 19 VOCs as the most discriminating compounds (MDA ≥ 60) between healthy plants and plants infested with 5, 10 and 20 T. absoluta larvae (Fig. 5a). The sparse partial least square discriminant analysis (sPLS-DA) showed the distribution of the different plant treatments in four clusters, where plants infested by 5, 10 and 20 T. absoluta larvae were separated from each other, and from the control, i.e. healthy plants (Fig. 5b). The sPLS-DA biplot showed that all the discriminating VOCs were closely associated with 20 T. absoluta larvae-infested tomato plants, the volatiles of which elicited the greatest attraction in the parasitoid (Fig. 5c). A proportion of 83.3% of the total variation in volatile emission was explained by the two first dimensions of the sPLS-DA. Dimension 1 accounted for 76.7% of the total variation and was highly correlated mainly with methyl salicylate, (E)-β-ocimene, β-myrcene, β-phellandrene, α-humulene, (E)-β-caryophyllene, 2-carene and α-pinene. Whereas dimension 2 explained 6.6% of the total variation, and was closely associated mainly with linalool, 3-carene, α-phellandrene, β-pinene and allo-ocimene. Clustering heatmap also showed these VOCs to be abundant in tomato plants infested with 20 T. absoluta larvae (Fig. 5d). Moreover, the heatmap clustered the samples in two main categories, i.e. one composed of the plants infested with 20 T. absoluta larvae, and another group containing all the remaining samples (i.e. combination of healthy plants and others infested with 5 and 10 T. absoluta larvae) which, in turn, were also separated among themselves.
Determination of the most discriminating volatiles and their correlation with healthy tomato plants and tomato plants infested with 5, 10 and 20 Tuta absoluta larvae (abbreviated 5Ta-inf, 10Ta-inf and 20Ta-inf, respectively, with five replicates). a The 25 most discriminating VOCs between healthy and infested tomato plants are listed in decreasing importance based on the mean decrease in accuracy in the random forest analysis with a classification accuracy of 100%. b A sparse partial least square discriminant analysis (sPLS-DA) plot displaying the distribution of healthy and infested tomato plants (R2X = 0.789, R2Y = 0.594, Q2 = 0.532), and c sPLS-DA biplot showing the correlation of VOCs with healthy and infested tomato plants using the 19 top discriminating VOCs (mean decrease in accuracy, MDA ≥ 60) (R2X = 0.789, R2Y = 0.594, Q2 = 0.532). (d) Clustering heatmap showing the abundance (in decreasing colour intensity) of the top discriminating VOCs across replicates of healthy and T. absoluta-infested tomato plants
Using the RF analysis, we went further in details by highlighting the VOCs that mostly discriminated plants offered in dual choices where the parasitoid displayed attraction responses. The findings revealed that the top VOCs discriminating T. absoluta larvae-infested plants from healthy plants resulted mainly from quantitative variations, and included α-pinene, sabinene, β-pinene, 2-carene, α-phellandrene, 3-carene, α-terpinene, β-phellandrene, (Z)-β-ocimene, (E)-β-ocimene, (E)-β-caryophyllene, α-humulene, β-myrcene, allo-ocimene and geranyl acetone, with the exception of methyl salicylate which was not detected in healthy plants (Fig. 6a–c). Similarly, α-pinene, β-myrcene, 2-carene, β-phellandrene, (Z)-β-ocimene, (E)-β-ocimene, (E)-β-caryophyllene and linalool appeared to be the VOCs that contributed most to distinguishing between plants with high infestation density, i.e. 20 T. absoluta larvae and those with low infestation density, i.e. 5 and 10 T. absoluta larvae (Fig. 6d–e). The multidimensional scaling (MSD) ordination plot also showed that healthy and infested plants differed in the abundance of their VOCs (Fig. 6f).
The 25 most predictive volatiles are listed in order of decreasing importance (difference in colour intensity) based on mean decrease in accuracy in the random forest analysis using VOC concentrations. Histogram showing volatile compounds that distinguish: a 5 Tuta absoluta (Ta) larvae-infested plants versus healthy plants; b 10 T. absoluta larvae-infested plants versus healthy plants; c 20 T. absoluta larvae-infested plant versus healthy plants; d 20 T. absoluta-infested plant versus 5 T. absoluta-infested plants; e 20 T. absoluta larvae-infested plants versus 10 T. absoluta larvae-infested plants (for each analysis, the classification accuracy was ≥ 95%). f Multidimensional Scaling (MDS) ordination plot associated with RF analysis, showing the distribution of healthy plants and plants infested with varying densities of T. absoluta larvae, based on the concentrations of their VOCs
The compounds that appeared at least three times among the top discriminating VOCs (compounds indicated in dark black colour, Fig. 6) were α-pinene, sabinene, β-pinene, β-myrcene, 2-carene, α-phellandrene, 3-carene, α-terpinene, β-phellandrene, (Z)-β-ocimene, (E)-β-ocimene, linalool, allo-ocimene, methyl salicylate, (E)-β-caryophyllene, and α-humulene (Fig. 6). Eight of the selected compounds were detected in the larval frass volatiles (Table 2, Fig. 4), which also attracted the parasitoid (Fig. 2). We therefore focused on these discriminating VOCs to test the parasitoid responses to synthetic compounds.
Bioassays with synthetic compounds
The parasitoid D. gelechiidivoris was attracted to α-pinene at doses of 123 ng (χ2 = 5.22, df = 1, P = 0.022) and 1230 ng (χ2 = 8.16, df = 1, P = 0.004), but not at a dose of 12.3 ng (χ2 = 0.73, df = 1, P = 0.391) compared to the control (DCM) (Fig. 7). Similarly, α-phellandrene did not attract the parasitoid at a dose of 15.4 ng, while higher doses of 154 ng (χ2 = 5.78, df = 1, P = 0.016) and 1.54 μg (χ2 = 8.16, df = 1, P = 0.004) attracted the parasitoid compared to the control. Attraction of D. gelechiidivoris to β-ocimene occurred only at a dose of 320 ng (χ2 = 8.82, df = 1, P = 0.003), but no attraction was observed for lower doses of 32 ng (χ2 = 0.567, df = 1, P = 0.33) and 3.2 ng (χ2 = 0.5, df = 1, P = 0.479) compared to the control. Likewise, β-caryophyllene at 1000 ng dose (χ2 = 7.22, df = 1, P = 0.007) attracted the parasitoid, whereas a dose of 100 ng (χ2 = 2.52, df = 1, P = 0.112) or 10 ng (χ2 = 0.5, df = 1, P = 0.479) compared to the control did not. Dolichogenidea gelechiidivoris was also attracted to 260 ng methyl salicylate (χ2 = 5.78, df = 1, P = 0.016), 580 ng β-myrcene (χ2 = 6.61, df = 1, P = 0.01) and 850 ng α-terpinene (χ2 = 4.17, df = 1, P = 0.041), but it was not attracted to lower doses of one-tenth and one-hundredth compared to the control (Fig. 7). On the other hand, sabinene, β-pinene, 2-carene, 3-carene, β-phellandrene, linalool, allo-ocimene and α-humulene were not attractive to the parasitoid at doses tested in our experiments (Fig. 7).
Behavioural responses of Dolichogenidea gelechiidivoris to synthetic compounds tested at three doses corresponding to release rates by 1, 10 and 100 equivalent plants in one hour. Fifty insects were tested per choice test. nr = number of non-responsive insects (i.e. insects that made no choice). DCM = dichloromethane. P = statistical significance level with ns = no significant difference (P > 0.05); *, ** = significant differences at P < 0.05 and P < 0.01 from χ2 test at α = 0.05
Surprisingly, the parasitoid D. gelechiidivoris was not attracted to the blend of the seven attractive compounds (α-pinene, β-myrcene, α-phellandrene, α-terpinene, β-ocimene, methyl salicylate and (E)-β-caryophyllene) when mixed at their most attractive doses (blend B1) compared to the control (DCM) (χ2 = 1.02, df = 1, P = 0.213) (Fig. 8). However, subsequent dilutions of B1 elicited varying degrees of attraction in the parasitoid. The blend B2 composed of half of the B1 dose was relatively attractive to the parasitoid (χ2 = 6.02, df = 1, P = 0.014), whereas the blend B3 composed of one-fourth of the B1 dose was the most attractive to the parasitoid (χ2 = 11.76, df = 1, P = 0.0006) compared to the control. The parasitoid was also attracted to the blend B4 composed of one-tenth of B1 dose (χ2 = 9.19, df = 1, P = 0.002), but not to blend B5, i.e. one-hundredth of B1 dose (χ2 = 0.5, df = 1, P = 0.48) when compared to the control (Fig. 8).
Behavioural responses of Dolichogenidea gelechiidivoris to a 7-component blend of the attractive compounds mixed at their most attractive doses (B1) which is subsequently diluted to one-half (B2), one-fourth (B3), one-tenth (B4), and one-hundredth (B5). Fifty insects were tested per choice test. nr = number of non-responsive insects (i.e. insects that made no choice). DCM = dichloromethane. P = statistical significance level with ns = no significant difference (P > 0.05); *, **, *** = significant differences, respectively, at P < 0.05, P < 0.01 and P < 0.001 from χ2 test at α = 0.05
Discussion
We report the attractiveness of the braconid parasitoid D. gelechiidivoris to tomato volatiles-induced by feeding of T. absoluta larvae, and to the host kairomones, and identify the attractive compounds using a Y-tube olfactometer. Y-tube olfactometer and wind tunnel are both suited for the study of behavioural responses of braconid parasitoids to semiochemicals, as seen in the parasitoids Aphidius ervi Haliday, Agathis bishopi (Nixon) and Cotesia glomerata (L.) (Hymenoptera: Braconidae) which were found to be active both in wind tunnel and Y-tube olfactometer, orienting more towards volatiles of host-infested plants than to those of healthy plants (Du et al. 1996; Steinberg et al. 1992; Zimba et al. 2015). In our study, we only observed the choice of odours by D. gelechiidivoris, and no other foraging behaviours like landing on sources, flight capacity, use of visual cues, take off ability, etc. for which the use of wind tunnel is mandatory.
Tuta absoluta larvae-infested tomato plant volatiles attracted the parasitoid D. gelechiidivoris because of the quantitative and qualitative differences in the volatile composition of infested tomato plants relative to healthy plants. We found that D. gelechiidivoris was more attracted to volatiles of tomato plants infested with T. absoluta larvae than to volatiles of healthy tomato plants. This is in line with findings obtained with closely related species. Suckling et al. (2012) reported that, compared to volatiles of healthy apple (Malus domestica) seedlings, the parasitoid Dolichogenidea tasmanica (Cameron) (Hymenoptera: Braconidae) was more attracted to volatiles of apple seedlings infested with the light brown apple moth, Epiphyas postvittana (Walker) (Lepidoptera: Tortricidae). Our findings also showed that D. gelechiidivoris olfactory attraction was greater towards plants with high host infestation density, i.e. 20 T. absoluta larvae than to those with low (5 or 10) host infestation density. Such positive density-dependent olfactory responses for volatiles of host-infested plants have also been reported in other plant-host-parasitoid tritrophic interactions. For example, Cotesia vestalis (Haliday) (Hymenoptera: Braconidae) was reported to prefer volatiles of cabbage plants infested with a high density (15–30 larvae) of its host, Plutella xylostella (L.) (Lepidoptera: Plutellidae) when compared to volatiles of plants with a low (5) infestation density (Shiojiri et al. 2010; Girling et al. 2011). With no surprise, we found that D. gelechiidivoris was not attracted to volatiles of healthy tomato plants when compared to clean air. The same pattern has been reported in other natural enemies searching for T. absoluta in tomato plants, as for the parasitoid Trichogramma achaeae which was not attracted to volatiles of healthy tomato plants compared to clean air (Gontijo et al. 2019).
Responses of natural enemies to specific host-infested plants are a result of quantitative and qualitative differences in the compositions of plant volatiles (De Moraes et al. 1998; McCormick et al. 2012). The behavioural responses of D. gelechiidivoris and the tomato plant volatile emission rates were found to be positively correlated with the infestation density of T. absoluta larvae. We found that the density of infesting T. absoluta larvae led to quantitative variations in the volatile composition between healthy and infested tomato plants, as previously reported by De Backer et al. (2015). Specifically, in our study, increasing the level of T. absoluta larval infestation positively correlated with increased emission of volatiles, namely the monoterpenes α-pinene, β-myrcene, α-phellandrene, α-terpinene and (E)-β-ocimene, and the sesquiterpene (E)-β-caryophyllene. The increase in the rate of volatile emissions upon leaf mining by T. absoluta larvae could be explained by the increase in feeding intensity and the plant defence pathway activated by the insects. Indeed, leaf mining larvae are known to activate both jasmonic acid (JA) and salicylic acid (SA) pathways which may act synergistically to induce volatile emission in host plants (Yang et al. 2020). Some authors reported that activation of JA pathway in tomato plants led to the upregulation of defence genes and enhanced the production of secondary metabolites and herbivore-induced volatiles in the plants (Chen et al. 2006; Degenhardt et al. 2010). The induced JA level and the volatile emission were reported to be positively correlated with the duration and intensity of herbivore feeding, as seen in the armyworm Spodoptera exigua (Hübner) and S. littoralis (Boisd.) (Lepidoptera: Noctuidae) larvae of which feeding on maize plants resulted in the increase in both JA level and emission of terpene volatiles, as the infestation density and feeding duration increased (Schmelz et al. 2003; Turlings et al. 2004). Qualitative differences have also been observed in plant volatiles whereby herbivory on plants likely results in the emission of herbivore-specific plant volatiles (De Moraes et al. 1998; McCormick et al. 2012). We found that T. absoluta larvae feeding at a high density on tomato plants led to the emission of novel compounds that were not detected in the headspace volatiles of healthy tomato plants. These VOCs included the alcohol (Z)-3-hexenol; the ester (Z)-3-hexenyl butanoate; the benzenoid ester methyl salicylate; the monoterpenes sabinene, linalool and allo-ocimene; and the sesquiterpene β-elemene. Silva et al. (2017) reported that the volatile compounds specific to T. absoluta larvae-infested tomato plants were mainly alcohols: 3-methylbutan-1-ol and (Z)-2-penten-1-ol; and esters such as (Z)-2-penten-1-yl acetate ester, (Z)-2-penten-1-yl butyrate and (Z)-3-hexen-1-yl crotonate, but emissions of novel monoterpenes and sesquiterpenes were not observed. The differences in the specific T. absoluta larvae-induced tomato plants volatiles between our study and that of Silva et al. (2017) could be because of differences in the tomato cultivars used (Kilele in this study vs. Moneymaker in the study by Silva et al. (2017)), consistent with Proffit et al. (2011) who reported qualitative differences in the volatile compounds from tomato plant cultivars.
Herbivory-induced plant volatile compounds, either individually or as a blend, mediate the attraction of natural enemies to host plants (Turlings and Erb 2018; Ayelo et al. 2021). We found that the parasitoid D. gelechiidivoris was attracted to α-pinene, β-myrcene, α-phellandrene, α-terpinene, β-ocimene, (E)-β-caryophyllene, of which the emission rate increased with an increase in the intensity of T. absoluta herbivory, and to methyl salicylate, a volatile specifically induced by herbivory. These attractants have also been reported to act as kairomone for other natural enemy species. For example, the braconid parasitoid A. ervi was attracted to methyl salicylate and (E)-β-caryophyllene identified in the volatiles of tomato plants infested by Macrosiphum euphorbiae (Sasso et al. 2009), and to α-phellandrene and (E)-β-ocimene detected in Acyrthosiphon pisum-infested bean plant volatiles (Takemoto and Takabayashi 2015). Moreover, the parasitoid Microplitis croceipes (Cresson) (Hymenoptera: Braconidae) was attracted to α-pinene identified in Heliothis virescens (Fab.) (Lepidoptera: Noctuidae) larvae-infested cotton plant volatiles (Morawo and Fadamiro 2014). The attractive compounds were active at different equivalent plant doses. While the responses of D. gelechiidivoris to (E)-β-caryophyllene, α-phellandrene and α-pinene slightly increased between doses corresponding to the release rates (i.e. dose/h) of 10 and 100 equivalent plants, the other attractants were active only at release rates of 100 equivalent plants. The increase in doses to the release rates of 10 and 100 equivalent plants is guided by the expectation that in a tomato field, thousands of plants older than those used in our bioassays continuously release volatiles to attract natural enemies. Volatile emission by tomato plants has been reported to increase with increasing plant age (Jansen et al. 2008), and with increased temperature (Copolovici et al. 2012). We thus speculate that volatile release rate in a tomato field could be higher than that of plants in the laboratory. Furthermore, as odour plume concentration is diluted by the wind over distances in the field, it is likely that kairomone doses to be applied to attract natural enemies in the field would be higher than doses that elicited attraction when tested in laboratory assays (Takemoto and Takabayashi 2015). In this regard, the increase in volatile concentrations in the dose–response assays could help get insights into the threshold concentrations detectable by, and attractive to, the parasitoid D. gelechiidivoris for future field application purposes. We observed that the parasitoid did not display an avoidance or repellent behaviour to the doses tested, indicating that these doses were in the relevant range for the parasitoid attraction. We found that the highest attraction for D. gelechiidivoris was obtained with the 7-component blend (containing 308 ng α-pinene, 145 ng β-myrcene, 385 ng α-phellandrene, 213 ng α-terpinene, 80 ng β-ocimene, 250 ng (E)-β-caryophyllene and 65 ng methyl salicylate) that elicited a similar attraction level as the volatiles of 20-T. absoluta larvae-infested tomato plant which was the most attractive to the parasitoid among all the infested plants. Similarly, the parasitoid Lytopylus rufipes Nees (Hymenoptera: Braconidae) showed the strongest attraction to a blend of five compounds, i.e. (Z)-3-hexenyl acetate, linalool, (E)-β-ocimene, (E)-3,8-dimethyl-1,4,7-nonatriene and (E,E)-α-farnesene (Liu et al. 2019). Insects use blend of odourants when searching for hosts in nature (Thomas-Danguin et al. 2014; Conchou et al. 2019), and odourant mixtures elicit faster olfactory processing responses in insects and are more reliable for insect olfaction than single odourants (Chan et al. 2018). However, the attractiveness of an odourant blend depends on its composition, and the concentration and ratio of the individual compounds (Beyaert et al. 2010; Cha et al. 2011). The non-attraction of the parasitoid to the blend of the seven attractants when mixed at their most attractive doses (blend B1) could be explained by the composition and the high concentration of the blend B1. High concentrations of an attractant can turn into eliciting a neutral or even repellent behaviour from natural enemies (Ren et al. 2017 and references therein). Goelen et al. (2021) reported that a blend of benzaldehyde and styrene (mixed at their most attractive doses) was attractive to the parasitoid, whereas a 5-component blend containing benzaldehyde and styrene at their most attractive doses did not attract the parasitoid at any of the doses tested. In a blend of attractants, some molecules can exert an inhibitory effect on the activity of another molecule depending on their concentrations and ratios, thereby reducing the overall detection and the excitatory activity of the blend on the insect’s olfactory neurons (Hatano et al. 2015).
Natural enemies also locate their hosts and prey using kairomones emanating from them, and known to be released from diverse sources including larval frass, glandular defensive larval secretions, and pheromones (Afsheen et al. 2008). The detection of host-associated volatile components by D. gelechiidivoris may enhance the parasitoid foraging behaviour in finding T. absoluta on host plants in the field. Indeed, in this study, we demonstrated that volatiles of the larval frass of T. absoluta attracted D. gelechiidivoris. Similarly, Chuche et al. (2006) reported that the parasitoid Dibrachys cavus (Walker) (Hymenoptera: Pteromalidae) was attracted to its host, Lobesia botrana (Denis & Schiffermüller) (Lepidoptera: Tortricidae) larval frass volatiles. In our study, with the exception of caryophyllene oxide which could have resulted from oxidation processes, all T. absoluta larval frass volatile compounds were identified in the headspace volatiles of the host plant. These findings corroborate those in previous studies reporting that volatiles from frass of leaf-chewing herbivores, like Chrysolina herbacea contained compounds of the digested materials of the host plant, Mentha spp., with oxidation of 1,8 cineole into hydroxy-1,8-cineoles, occurring in the insect’s frass (Cordero et al. 2012; Pizzolante et al. 2017). Among the volatiles we identified in the T. absoluta larval frass, the monoterpenes β-myrcene and α-terpinene, the sesquiterpene (E)-β-caryophyllene, and the benzenoid ester methyl salicylate attracted D. gelechiidivoris.
Our findings also revealed that the parasitoid D. gelechiidivoris was attracted to the commercial sex pheromone of T. absoluta. The results are in agreement with those of previous studies reporting that the sex pheromone of T. absoluta attracted Trichogramma egg parasitoids (Ahmadi and Poorjavad 2018; Gontijo et al. 2019). Tuta absoluta sex pheromones are used in attract and kill, mass trapping and mating disruption control strategies for the management of the pest in the field (Megido et al. 2013). This control strategy could benefit the parasitoid D. gelechiidivoris which eavesdrops upon the pheromone, or harm the success of parasitisation if the parasitoid individuals are trapped by the pheromone-baited sticky traps used in tomato crop fields. However, greenhouse and field experiments are needed to confirm the attraction of D. gelechidiivoris to T. absoluta sex pheromone. This is particularly important because discrepancies were observed between laboratory and greenhouse or field results regarding the attraction of Trichogramma species to T. absoluta sex pheromones (Ahmadi and Poorjavad 2018). Still, positive results were found for the parasitoid Telenomus euproctidis Wilcox (Hymenoptera: Scelionidae) which was attracted to solvent extract of pheromone glands and synthetic sex pheromone of its host, Orgyia postica (Walker) (Lepidoptera: Lymantriidae) in both laboratory and field (Arakaki et al. 2011).
In summary, we report that T. absoluta larvae-infested tomato plants and the host larval frass release volatiles that are attractive to the braconid parasitoid D. gelechiidivoris. The attractive volatile compounds could be involved in the long-range kairomones exploited by the parasitoid in locating tomato plants where to find T. absoluta larvae for oviposition. Specifically, we show that D. gelechiidivoris is attracted to the terpenoids α-pinene, β-myrcene, α-phellandrene, α-terpinene, β-ocimene, (E)-β-caryophyllene, and to the benzenoid ester methyl salicylate, when tested individually or in a 7-component blend. Moreover, we demonstrate that T. absoluta sex pheromone elicits attraction behaviour in the parasitoid and may therefore also serve as a kairomone for the parasitoid in finding T. absoluta on host plants in the field. The findings of our study thus elucidate the role of semiochemicals that could be exploited to recruit and retain the parasitoid D. gelechiidivoris in the field. In combination with other integrated pest management (IPM) methods, semiochemicals are being applied in crop fields to enhance biological control of insect pests. In this context, our findings open new avenues for development of kairomone-based lure to enhance the biological control of T. absoluta for the suppression of this pest within a holistic IPM programme.
References s
Afsheen S, Wang X, Li R, Zhu C-S, Lou Y-G (2008) Differential attraction of parasitoids in relation to specificity of kairomones from herbivores and their by-products. Insect Sci 15:381–397. https://doi.org/10.1111/j.1744-7917.2008.00225.x
Ahmadi S, Poorjavad N (2018) Behavioral and biological effects of exposure to Tuta absoluta (Lepidoptera: Gelechiidae) sex pheromone on several Trichogramma (Hymenoptera: Trichogrammatidae) populations. J Econ Entomol 111(6):1–9. https://doi.org/10.1093/jee/toy212
Aigbedion-Atalor PO, Mohamed SA, Hill MP, Zalucki MP, Azrag AGA, Srinivasan R, Ekesi S (2020) Host stage preference and performance of Dolichogenidea gelechiidivoris (Hymenoptera: Braconidae), a candidate for classical biological control of Tuta absoluta in Africa. Biol Control 144:104215. https://doi.org/10.1016/j.biocontrol.2020.104215
Arakaki N, Yamazawa H, Wakamura S (2011) The egg parasitoid Telenomus euproctidis (Hymenoptera: Scelionidae) uses sex pheromone released by immobile female tussock moth Orgyia postica (Lepidoptera: Lymantriidae) as kairomone. Appl Entomol Zool 46:195–200. https://doi.org/10.1007/s13355-011-0031-4
Aukema BH, Raffa KF (2005) Selective manipulation of predators using pheromones: responses to frontalin and ipsdienol pheromone components of bark beetles in the Great Lakes region. Agric for Entomol 7:193–200. https://doi.org/10.1111/j.1461-9555.2005.00250.x
Ayelo PM, Pirk CWW, Yusuf AA, Chailleux A, Mohamed SA, Deletre E (2021) Exploring the kairomone-based foraging behaviour of natural enemies to enhance biological control: A Review. Front Ecol Evol 9:641974. https://doi.org/10.3389/fevo.2021.641974
Beyaert I, Wäschke N, Scholz A, Varama M, Reinecke A, Hilker M (2010) Relevance of resource-indicating key volatiles and habitat odour for insect orientation. Anim Behav 79(5):1077–1086. https://doi.org/10.1016/j.anbehav.2010.02.001
Biondi A, Guedes RNC, Wan F-H, Desneux N (2018) Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: past, present, and future. Annu Rev Entomol 63:239–258. https://doi.org/10.1146/annurev-ento-031616-034933
Breiman L (2001) Random Forests. Mach Learn 45(1):5–32. https://doi.org/10.1023/A:1010933404324.Cha
Chan HK, Hersperger F, Marachlian E, Smith BH, Locatelli F, Szyszka P, Nowotny T (2018) Odorant mixtures elicit less variable and faster responses than pure odorants. PLoS Comp Biol 14(12):e1006536
Chen H, Jones AD, Howe GA (2006) Constitutive activation of the jasmonate signaling pathway enhances the production of secondary metabolites in tomato. FEBS Lett 580:2540–2546. https://doi.org/10.1016/j.febslet.2006.03.070
Chuche J, Xuéreb A, Thiéry D (2006) Attraction of Dibrachys cavus (Hymenoptera : Pteromalidae) to its host frass volatiles. J Chem Ecol 32:2721–2731. https://doi.org/10.1007/s10886-006-9195-8
Conchou L, Lucas P, Meslin C, Proffit M, Staudt M, Renou M (2019) Insect odorscapes: from plant volatiles to natural olfactory scenes. Front Physiol 10:972. https://doi.org/10.3389/fphys.2019.00972
Copolovici L, Kännaste A, Pazouki L, Niinemets Ü (2012) Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. J Plant Physiol 169(7):664–672. https://doi.org/10.1016/j.jplph.2011.12.019
Cordero C, Zebelo SA, Gnavi G, Griglione A, Bicchi C, Maffei ME, Rubiolo P (2012) HS-SPME-GC×GC-QMS volatile metabolite profiling of Chrysolina herbacea frass and Mentha spp. leaves. Anal Bioanal Chem 402(5):941–1952. https://doi.org/10.1007/s00216-011-5600-4
Dalen M, Knudsen GK, Norli HR, Thöming G (2015) Sources of volatiles mediating host location behaviour of Glypta haesitator, a larval parasitoid of Cydia nigricana. Biol Control 90:128–140. https://doi.org/10.1016/j.biocontrol.2015.05.019
Danner H, Desurmont GA, Cristescu SM, van Dam NM (2018) Herbivore-induced plant volatiles accurately predict history of coexistence, diet breadth, and feeding mode of herbivores. New Phytol 220:726–738. https://doi.org/10.1111/nph.14428
De-Backer L, Megido RC, Fauconnier ML, Brostaux Y, Francis F, Verheggen F (2015) Tuta absoluta-induced plant volatiles: attractiveness towards the generalist predator Macrolophus pygmaeus. Arthropod-Plant Interact 9(5):465–476. https://doi.org/10.1007/s11829-015-9388-6
De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nat 393(6685):570–573. https://doi.org/10.1038/31219
Degenhardt DC, Refi-Hind S, Stratmann JW, Lincoln DE (2010) Systemin and jasmonic acid regulate constitutive and herbivore-induced systemic volatile emissions in tomato, Solanum lycopersicum. Phytochem 71:2024–2037. https://doi.org/10.1016/j.phytochem.2010.09.010
Desneux N, Wajnberg E, Wyckhuys KAG, Burgio G, Arpaia S, Narváez-Vasquez CA, González-Cabrera J et al (2010) Biological invasion of European tomato crops by Tuta absoluta: ecology, geographic expansion and prospects for biological control. J Pest Sci 83(3):197–215. https://doi.org/10.1007/s10340-010-0321-6
DH Cha., Linn CE, Teal PEA, Zhang A, Roelofs WL, Loeb GM, (2011) Eavesdropping on plant volatiles by a specialist moth: significance of ratio and concentration. PLoS ONE 6(2):e17033. https://doi.org/10.1371/journal.pone.0017033
Dicke M, Sabelis MW (1988) Infochemical terminology: based on cost-benefit analysis rather than origin of compounds? Funct Ecol 2:131–139. https://doi.org/10.2307/2389687
Dinno A (2015) Nonparametric pairwise multiple comparisons in independent groups using Dunn’s Test. Stata J 15:292–300. https://doi.org/10.1177/1536867X1501500117
Du YJ, Poppy GM, Powell W (1996) Relative importance of semiochemicals from first and second trophic levels in host foraging behavior of Aphidius ervi. J Chem Ecol 22(9):1591–1605. https://doi.org/10.1007/BF02272400
Girling RD, Stewart-Jones A, Dherbecourt J, Staley JT, Wright DJ, Poppy GM (2011) Parasitoids select plants more heavily infested with their caterpillar hosts: a new approach to aid interpretation of plant headspace volatiles. Proc R Soc B Biol Sci 278(1718):2646–2653
Goelen T, Vuts J, Sobhy IS, Wäckers F, Caulfield JC, Birkett MA et al (2021) Identification and application of bacterial volatiles to attract a generalist aphid parasitoid: from laboratory to greenhouse assays. Pest Manag Sci 77(2):930–938. https://doi.org/10.1002/ps.6102
Gontijo L, Cascone P, Giorgini M, Michelozzi M, Rodrigues HR, Spiezia G, Iodice L, Guerrieri E (2019) Relative importance of host and plant semiochemicals in the foraging behavior of Trichogramma achaeae, an egg parasitoid of Tuta absoluta. J Pest Sci 92(4):1479–1488. https://doi.org/10.1007/s10340-019-01091-y
Guedes RNC, Roditakis E, Campos MR, Haddi K, Bielza P, Siqueira HAA et al (2019) Insecticide resistance in the tomato pinworm tuta absoluta: patterns, spread, mechanisms, management and outlook. J Pest Sci 92(4):1329–1342. https://doi.org/10.1007/s10340-019-01086-9
Han P, Bayram Y, Shaltiel-Harpaz L, Sohrabi F, Saji A, Esenali UT, Jalilov A et al (2019) Tuta absoluta continues to disperse in Asia: damage, ongoing management and future challenges. J Pest Sci 92(4):1317–1327. https://doi.org/10.1007/s10340-018-1062-1
Hatano E, Saveer AM, Borrero-Echeverry F, Strauch M, Zakir A, Bengtsson M et al (2015) A herbivore-induced plant volatile interferes with host plant and mate location in moths through suppression of olfactory signalling pathways. BMC Biol 13:75. https://doi.org/10.1186/s12915-015-0188-3
Heimpel GE, Asplen MK (2011) A ‘Goldilocks’ Hypothesis for dispersal of biological control agents. Biocontrol 56(4):441–450. https://doi.org/10.1007/s10526-011-9381-7
Hervé MR, Nicolè F, Lê Cao K-A (2018) Multivariate analysis of multiple datasets: a practical guide for chemical ecology. J Chem Ecol 44(3):215–234. https://doi.org/10.1007/s10886-018-0932-6
IOFI (2011) Guidelines for the quantitative gas chromatography of volatile flavouring substances, from the working group on methods of analysis of the International Organization of the Flavor Industry (IOFI). Flavour Fragr J 26(5):297–299. https://doi.org/10.1002/ffj.2061
Jansen R, Hofstee JW, Verstappen F, Bouwmeester H, Posthumus M, van Henten E (2008) A method to detect baseline emission and plant damage induced volatile emission in a greenhouse. Acta Hortic 801:1415–1422. https://doi.org/10.17660/ActaHortic.2008.801.174
Kaplan I (2012) Attracting carnivorous arthropods with plant volatiles : the future of biocontrol or playing with fire? Biol Control 60(2):77–89. https://doi.org/10.1016/j.biocontrol.2011.10.017
Kelly JL, Hagler JR, Kaplan I (2014) Semiochemical lures reduce emigration and enhance pest control services in open-field predator augmentation. Biol Control 71:70–77. https://doi.org/10.1016/j.biocontrol.2014.01.010
Khan M, Mousa AA, Syamasundar KV, Alkhathlan HZ (2012) Determination of chemical constituents of leaf and stem essential oils of Artemisia monosperma from Central Saudi Arabia. Nat Prod Commun 7(8):1079–1082. https://doi.org/10.1177/1934578x1200700829
Kost C (2008) Chemical Communication. In Jorgensen SE, Fath BD (eds) Encyclopedia of Ecology. Oxford: Elsevier, 557–575. http://hdl.handle.net/11858/00-001M-0000-0012-A137-1.
Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Sci 291(5511):2141–2144. https://doi.org/10.1126/science.291.5511.2141
Lê Cao K-A, Boitard S, Besse P (2011) Sparse PLS Discriminant Analysis: biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinform 12:253. https://doi.org/10.1186/1471-2105-12-253
Liaw A, Wiener M (2002) Classification and regression by Random Forest. R News 2(3):18–22
Liu CM, Matsuyama S, Kaionoh Y (2019) Synergistic effects of volatiles from host-infested plants on host-searching behavior in the parasitoid wasp Lytopylus rufipes (Hymenoptera: Braconidae). J Chem Ecol 45:684–692. https://doi.org/10.1007/s10886-019-01088-y
Mansour R, Brévault T, Chailleux A, Cherif A, Grissa-Lebdi K, Haddi K, Mohamed SA et al (2018) Occurrence, biology, natural enemies and management of Tuta absoluta in Africa. Entomol Gen 38:38–112. https://doi.org/10.1127/entomologia/2018/0749
McCormick AC, Unsicker SB, Gershenzon J (2012) The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci 17(5):303–310. https://doi.org/10.1016/j.tplants.2012.03.012
Megido RC, Haubruge E, Verheggen FJ (2013) Pheromone-Based management strategies to control the tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). A Review. Biotechnol. Agron Soc Environ 17(3):475–482. http://orbi.ulg.ac.be/handle/2268/154676.
Morawo T, Fadamiro H (2014) Attraction of two larval parasitoids with varying degree of host specificity to single components and a binary mixture of host-related plant volatiles. Chemoecol 24(4):127–135. https://doi.org/10.1007/s00049-014-0154-5
Mumm R, Dicke M (2010) Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Can J Zool 88(7):628–667. https://doi.org/10.1139/Z10-032
Palacios M, Cisneros F (1995) Management of the potato tuber moth. Program 4, Integrated Pest Management. International Potato Center, Program Report, Peru, 84–91.
Peri E, Moujahed R, Wajnberg E, Colazza S (2018) Applied chemical ecology to enhance insect parasitoid efficacy in the biological control of crop pests. In: Tabata J (ed) Chemical ecology of insects: applications and associations with plants and microbes, London. Taylor & Francis, New York, pp 234–267
Pizzolante G, Cordero C, Tredici SM, Vergara D, Pontieri P, Giudice LD, Capuzzo A et al (2017) Cultivable gut bacteria provide a pathway for adaptation of Chrysolina herbacea to Mentha aquatica volatiles. BMC Plant Biol 17(30):1–20. https://doi.org/10.1186/s12870-017-0986-6
Proffit M, Birgersson G, Bengtsson M, Reis R, Witzgall P, Lima E (2011) Attraction and oviposition of Tuta absoluta females in response to tomato leaf volatiles. J Chem Ecol 37(6):565–574. https://doi.org/10.1007/s10886-011-9961-0
R Core Team. (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org.
Ranganathan Y, Borges RM (2010) Reducing the babel in plant volatile communication: Using the forest to see the trees. Plant Biol 12(5):735–742. https://doi.org/10.1111/j.1438-8677.2009.00278.x
Reddy GVP, Holopainen JK, Guerrero A (2002) Olfactory responses of Plutella xylostella natural enemies to host pheromone, larval frass, and green leaf cabbage volatiles. J Chem Ecol 28(1):131–143. https://doi.org/10.1023/A:1013519003944
Reddy GVP, Guerrero A (2004) Interactions of insect pheromones and plant semiochemicals. Trends Plant Sci 9(5):253–261. https://doi.org/10.1016/j.tplants.2004.03.009
Ren LL, Balakrishnan K, Luo YQ, Schütz S (2017) EAG response and behavioral orientation of Dastarcus helophoroides (Fairmaire) (Coleoptera: Bothrideridae) to synthetic host-associated volatiles. PLoS ONE 12(12):e0190067. https://doi.org/10.1371/journal.pone.0190067
Rohart F, Gautier B, Singh A, Lê Cao K-A (2017) MixOmics: An R package for ‘Omics feature selection and multiple data integration. PLOS Comput Biol 13:e1005752. https://doi.org/10.1371/journal.pcbi.1005752
Roque-Romero L, Cisneros J, Rojas JC, Ortiz-Carreon FR, Malo EA (2020) Attraction of Chelonus insularis to host and host habitat volatiles during the search of Spodoptera frugiperda eggs. Biol Control 140:104100. https://doi.org/10.1016/j.biocontrol.2019.104100
Ruiz-Hernández V, Roca MJ, Egea-Cortines M, Weiss J (2018) A comparison of semi-quantitative methods suitable for establishing volatile profiles. Plant Methods 14:67. https://doi.org/10.1186/s13007-018-0335-2
Salas Gervassio NGS, Aquino D, Vallina C, Biondi A, Luna MG (2019) A re - examination of Tuta absoluta parasitoids in South America for optimized biological control. J Pest Sci 92(4):1343–1357. https://doi.org/10.1007/s10340-018-01078-1
Sasso R, Iodice L, Woodcock CM, Pickett JA, Guerrieri E (2009) Electrophysiological and behavioural responses of Aphidius ervi (Hymenoptera: Braconidae) to tomato plant volatiles. Chemoecol 19(4):195–201. https://doi.org/10.1007/s00049-009-0023-9
Schmelz EA, Alborn HT, Banchio E, Tumlinson JH (2003) Quantitative relationships between induced jasmonic acid levels and volatile emission in Zea mays during Spodoptera exigua herbivory. Planta 216:665–673. https://doi.org/10.1007/s00425-002-0898-y
Shiojiri K, Ozawa R, Kugimiya S, Uefune M, van Wijk M, Sabelis MW, Takabayashi J (2010) Herbivore-specific, density-dependent induction of plant volatiles: honest or ‘cry wolf’ signals? PLoS ONE 5:1–11. https://doi.org/10.1371/journal.pone.0012161
Silva DB, Weldegergis BT, van Loon JJA, Bueno VHP (2017) Qualitative and quantitative differences in herbivore-induced plant volatile blends from tomato plants infested by either Tuta absoluta or Bemisia tabaci. J Chem Ecol 43(1):53–65. https://doi.org/10.1007/s10886-016-0807-7
Steinberg S, Dicke M, Vet LEM, Wanningen R (1992) Response of the braconid parasitoid Cotesia (=Apanteles) glomerata to volatile infochemicals: effects of bioassay set-up, parasitoid age and experience and barometric flux. Entomol Exp Appl 63(2):163–175. https://doi.org/10.1111/j.1570-7458.1992.tb01571.x
Suckling DM, Twidle AM, Gibb AR, Manning LM, Mitchell VJ, Sullivan TES et al (2012) Volatiles from apple trees infested with light brown apple moth larvae attract the parasitoid Dolichogenidea tasmanica. J Agric Food Chem 60(38):9562–9566. https://doi.org/10.1021/jf302874g
Takabayashi J, Shiojiri K (2019) Multifunctionality of herbivory-induced plant volatiles in chemical communication in tritrophic interactions. Cur Opin Insect Sci 32:110–117. https://doi.org/10.1016/j.cois.2019.01.0032214-5745/ã
Takemoto H, Takabayashi J (2015) Parasitic wasps Aphidius ervi are more attracted to a blend of host-induced plant volatiles than to the independent compounds. J Chem Ecol 41:801–807. https://doi.org/10.1007/s10886-015-0615-5
Thomas-Danguin T, Sinding C, Romagny S, Mountassir FE, Atanasova B, Le Berre E et al (2014) The perception of odor objects in everyday life: a review on the processing of odor mixtures. Front Physiol 5:504. https://doi.org/10.3389/fpsyg.2014.00504
Turlings TCJ, Davison AC, Tamo C (2004) A six-arm olfactometer permitting simultaneous observation of insect attraction and odour trapping. Physiol Entomol 29:45–55. https://doi.org/10.1111/j.1365-3032.2004.0362.x
Turlings TCJ, Erb M (2018) Tritrophic interactions mediated by herbivore-induced plant volatiles : mechanisms, ecological relevance, application potential. Ann Rev Entomol 63:433–452. https://doi.org/10.1146/annurev-ento-020117-043507
Urbaneja A, Vercher R, Navarro V, Garcí-Marí F, Porcuna JL (2007) La Polilla Del Tomate, Tuta absoluta. Phytoma España 194:16–23
Valencia L, Peñaloza J (1990) Control biológico de las palomillas de la papa. Periódico Rural.–‘El Boyacense'. Tunja, Colombia. Ministerio de Agricultura, ICA, 4p.
Vallejo FA (1999) Mejoramiento Genético y Producción de Tomate En Colombia”. Universidad Nacional de Colombia, Sede Palmira, Cali (Colombia)
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Ann Rev Entomol 37:141–172. https://doi.org/10.1146/annurev.en.37.010192.001041
Verheggen F, Fontus RB (2019) First record of Tuta absoluta in Haiti. Entom Gen 38(4):349–353
Wang C, Zhang W, Li H, Mao J, Guo C, Ding R et al (2019) Analysis of volatile compounds in pears by HS-SPME-GC×GC-TOFMS. Mol 24(9):1775. https://doi.org/10.3390/molecules24091795
Xu H, Desurmont G, Degen T, Zhou G, Laplanche D, Henryk L, Turlings TCJ (2017) Combined use of herbivore-induced plant volatiles and sex pheromones for mate location in braconid parasitoids. Plant Cell Environ 40(3):330–339. https://doi.org/10.1111/pce.12818
Yang J-N, Wei J-N, Kang L (2020) Feeding of pea leafminer larvae simultaneously activates jasmonic and salicylic acid pathways in plants to release a terpenoid for indirect defense. Insect Sci. https://doi.org/10.1111/1744-7917.12820
Zappalà L, Biondi A, Alma A, Al-Jboory IJ, Arnò J, Bayram A, Chailleux A et al (2013) Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle East, and their potential use in pest control strategies”. J Pest Sci 86(4):635–647. https://doi.org/10.1007/s10340-013-0531-9
Zimba K, Hill MP, Moore SD, Heshula U (2015) Agathis bishopi (Hymenoptera: Braconidae) as a potential tool for detecting oranges infested with Thaumatotibia leucotreta (Lepidoptera: Tortricidae). J Ins Behav 28(5):618–633
Funding
This research was supported by the French National Research Agency (ANR) through CIRAD (Award no. ANR-16-CE32-0010-01); the Biovision Foundation Tuta IPM project (project ID: BV DPP-012/2019-2021); and the Norwegian Agency for Development Cooperation, the Section for research, innovation, and higher education (Grant No. RAF-3058 KEN-18/0005). Financial support was also granted by the University of Pretoria and the National Research Foundation through the NRF grants of AAY (Incentive Funding for Rated Researchers (IFRR) Grant No. 109380; Y-rated Researchers Grant No. RDYR180504326262) and CWWP (Grant No. CPRR160502163617). The authors also gratefully acknowledge financial supports provided by the following organisations and agencies: the UK’s Foreign, Commonwealth & Development Office (FCDO); the Swedish International Development Cooperation Agency (SIDA); the Swiss Agency for Development and Cooperation (SDC); the Federal Democratic Republic of Ethiopia; and the Kenyan Government. P.M.A. was supported by the University of Pretoria and the Deutscher Akademischer Austauschdienst (DAAD) In-Region Postgraduate Scholarship (Personal Grant No. 91672680). The views expressed herein do not necessarily reflect the official opinion of the donors.
Author information
Authors and Affiliations
Contributions
P.M.A., S.A.M, A.C., A.A.Y., C.W.W.P. and E.D. conceptualised and designed the research and provided intellectual inputs. P.M.A. conducted the experiments, analysed the data and drafted the manuscript. All authors proofread the manuscript and approved the final version for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Donald Weber.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ayelo, P.M., Mohamed, S.A., Chailleux, A. et al. The parasitoid Dolichogenidea gelechiidivoris eavesdrops on semiochemicals from its host Tuta absoluta and tomato. J Pest Sci 95, 633–652 (2022). https://doi.org/10.1007/s10340-021-01424-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-021-01424-w