Abstract
Herbivore-induced plant volatiles (HIPVs) and host sex pheromones are important semiochemicals used by natural enemies to locate prey or hosts. The egg parasitoid Trichogramma achaeae Nagaraja & Nagarkatti has recently shown potential for use as a biological control agent of Tuta absoluta (Meyrick), a key pest of tomato crops worldwide. In this study, we used olfactometer tests to examine the behavioral response of T. achaeae females to T. absoluta sex pheromone or to HIPVs produced by tomato plants infested with T. absoluta eggs or larvae. Our results showed that T. achaeae was attracted to T. absoluta sex pheromone. Parasitoids were also innately attracted to volatiles produced by tomato plants, whether uninfested or infested. However, parasitoids could not distinguish between volatiles from uninfested or T. absoluta-infested tomato plants. We characterized the headspace volatiles of tomato plants used in the olfactometer tests and found out that oviposition and larval feeding by T. absoluta significantly enhanced HIPV emission. This study suggests that the sex pheromone of T. absoluta is a potential tool to manipulate the behavior of T. achaeae and improve its attraction to the tomato crop. The analysis of volatiles released by tomato plants, either infested or uninfested, coupled with the response of T. achaeae in the olfactometer tests was consistent with what was expected in terms of the foraging behavior of a generalist parasitoid. The results and implications are further discussed in the context of sustainable T. absoluta management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
The parasitoid Trichogramma achaeae is considered a suitable candidate for managing the invasive pest Tuta absoluta
-
We investigated the response of female parasitoids to T. absoluta sex pheromone, uninfested tomato plants, and plants infested with eggs or larvae of T. absoluta in an olfactometer
-
The results indicated that T. absoluta sex pheromone may be deployed by growers to attract these parasitoids to tomato crops
-
The parasitoids might depend on associative learning to respond appropriately to infested plants
Introduction
Plant- and host-derived semiochemicals play a key role in the foraging behavior of natural enemies of insect pests. Host-derived volatile compounds, such as kairomones, are the most reliable cues for orienting natural enemies to locate their prey/host at short distance; however, their exploitation is often hampered by the evolution of hosts that emit undetectable quantities of kairomone (Vet and Dicke 1992). On the other hand, host-derived volatile compounds, such as sex pheromones, are often produced in substantial amounts by females for attracting mates at short and long distances, which in turn can also be exploited secondarily by parasitoid females to locate insect hosts (Reddy et al. 2002; Noldus et al. 1991). For example, some egg parasitoids are known to rely on the host’s female sex pheromone to attach onto and travel on the female’s body, and then subsequently parasitize her newly oviposited eggs (Arakaki et al. 1996; Huigens et al. 2010).
Likewise, when fed upon by herbivorous arthropods, various plant species will emit specific volatile blends that could attract natural enemies (predators or parasitoids), repel conspecifics, and/or induce an increased defense of its own and of neighboring plants (Unsicker et al. 2009; Dicke and Baldwin 2010; Backer et al. 2015; Coppola et al. 2017). In general, herbivore-induced plant volatiles (HIPVs) consist of several groups of compounds including terpenoids, green leaf volatiles, and benzenoids (Mumm and Dicke 2010; Fatouros et al. 2012). HIPVs are produced through various metabolic pathways, but most are derived from the terpenoid or isoprenoid pathways (Sacchettini and Poulter 1997; Degenhardt et al. 2009). The qualitative and quantitative characteristics of HIPVs vary according to the herbivore involved and to the plant species, genotype, or age (Turlings et al. 1993; Takabayashi et al. 1994). Additionally, insect oviposition has also been reported to affect the volatile blend released by the host plant (Mumm et al. 2003; Kopke et al. 2008; Fatouros et al. 2012), which is often distinct from that induced by larval feeding (Hilker and Meiners 2011). All these aforementioned factors and their combination determine the ultimate response of herbivores and natural enemies to the HIPVs emitted, including responses of repellence or attraction.
The tomato pinworm Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) is a major pest of tomato crops in South and Central America, and more recently in Europe (Biondi et al., 2018; Giorgini et al. 2019). Tuta absoluta was first reported in eastern Spain in 2006 and since then has invaded other European countries (Desneux et al. 2010), the Middle East, Africa (Mansour et al. 2018), and parts of Asia (Campos et al. 2017; Han et al. 2019). The main damage caused by T. absoluta on tomato plants is due to larval feeding on leaves, stems, or fruits, leading to losses of as much as 100% in the absence of control measures (López 1991; Picanço et al. 1998; Tropea Garzia et al. 2012). Although T. absoluta is a well-studied horticultural pest, most of its control strategies are still based on insecticide use (Lietti et al. 2005; Guedes and Picanço 2012), the effectiveness of which is often undermined by insecticide resistance (Siqueira et al. 2000; Lietti et al. 2005; Silva et al. 2011; Roditakis et al. 2018; Silva et al. 2019) and/or reduced contact with the larvae hidden inside plant stems or fruits (Cocco et al. 2013).
Several indigenous Afro-Eurasian natural enemies have been reported as potential biological control agents of T. absoluta, from which eulophid and braconid parasitoid wasps, and especially mirid predators, stand out as potentially more effective (Zappalà et al. 2013; Giorgini et al. 2019). Nevertheless, among the various natural enemies of T. absoluta studied to date, the egg parasitoids of the genus Trichogramma have received more substantial attention as potential biological control agents (Parra and Zucchi 2004; Pratissoli et al. 2005; Cagnotti et al. 2016; Cherif et al. 2018). For example, the generalist egg parasitoid Trichogramma achaeae Nagaraja & Nagarkatti (Hymenoptera: Trichogrammatidae) has been recently considered as a valid candidate to help manage T. absoluta, which infests greenhouse tomato crops in Mediterranean countries (Cabello et al. 2012; Cascone et al. 2015; Giorgini et al. 2019 and references therein). Trichogramma achaeae is a generalist parasitoid known to parasitize the eggs of lepidopteran species in at least ten different families and is considered native to Asia (China, India, and Russia). However, following accidental or deliberate introductions for biological control of lepidopteran pests, T. achaeae is now distributed worldwide, being recorded in Europe, Africa, and the Americas (Cabello et al. 2009; Wright and Stouthamer 2011; Polaszek et al. 2012). This parasitoid is also commercially available in various countries (Cabello et al. 2009; van Lenteren et al. 2018) following the consistent increase of the egg parasitism rate and pest control after inundative releases (Cabello et al. 2012; Oliveira et al. 2017).
To further improve this parasitoid efficiency, it is important to understand the chemical ecology of trophic interactions among tomato plants, T. absoluta, and T. achaeae. While it is known that T. absoluta eggs and larvae can elicit the production of HIPVs by tomato plants (Backer et al. 2015; Anastasaki et al. 2018), the responses of T. achaeae to those HIPVs remain unexplored. Likewise, it is unknown whether T. achaeae females could be attracted to T. absoluta sex pheromone. The pursuit of such knowledge is crucial to improve the effective use of T. achaeae in the augmentative biological control of T. absoluta in tomato greenhouses. The aim of our study was to use a series of olfactometer tests to assess the response (attraction) of T. achaeae female parasitoids to: (i) HIPVs induced by T. absoluta egg deposition on tomato plants, (ii) HIPVs induced by T. absoluta larvae feeding on tomato plants, or (iii) to synthetic sex pheromone of T. absoluta. Additionally, we characterized the HIPV chemical profile of tomato plants infested with T. absoluta eggs or larvae.
Materials and methods
Plants and insects
The tomato plants (cultivar ‘San Marzano nano’) used in the olfactometer tests were grown in a glasshouse under the following conditions: temperature of 24 ± 2 °C, relative humidity (RH) of 65 ± 5%, and photoperiod of 16L:8D. Plants used in the olfactometer tests were 5 weeks old, with 4–6 completely expanded leaves and a height of 18 cm.
Tuta absoluta was continuously reared inside bug dorms at the Istituto per la Protezione Sostenibile delle Piante (IPSP) under the same conditions described above. The original strain of T. absoluta was collected in 2015 in tomato greenhouses located in Battipaglia (Salerno, Italy). Trichogramma achaeae parasitoids used in the olfactometer tests were purchased biweekly from a commercial supplier (Agrobio, Almeria, Spain) in the form of pupae developed in the eggs of Ephestia kuehniella Zeller. Upon their arrival, the parasitoids were transferred to a climatic chamber at the same conditions described above until their use. The identity of the Trichogramma species was confirmed by COI and ITS2 gene amplification and sequencing as described by Cascone et al. (2015). Adult parasitoids were placed individually into micro-glass vials and sexed under a stereomicroscope 1–2 h prior to olfactometer tests. All female parasitoids used in olfactometer tests were 48–72 h old, mated, fed (1:1 water/honey solution), and naïve, i.e., with no previous oviposition experience or contact with plants.
Olfactometer tests
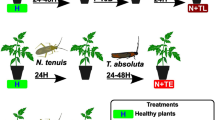
The behavioral response of T. achaeae females toward T. absoluta sex pheromone and HIPVs from infested tomato plants was assessed in a Y-tube olfactometer system (described in detail by Fatouros et al. 2012). Commercial pheromone dispensers or tomato plants were placed individually inside a 20-L glass jar, which was subsequently closed tightly for 15–20 min prior to the beginning of each choice test to allow the consistent diffusion of odor plumes and to reach a pressure balance inside the system. Each of the two glass jars was independently connected to an arm of the Y-tube, and the air flow was adjusted to 100 ml/minute for each arm. Female parasitoids were allowed 30 min to make a choice between the two odor sources. All tests were conducted between 10:00 and 14:00 h in a laboratory evenly illuminated by fluorescent lights and with an ambient temperature of 24 ± 2 °C.
During each run, we subjected 48–72-h-old female parasitoids to the following choice tests: (i) tomato plant infested with T. absoluta eggs versus uninfested tomato plant, (ii) tomato plant infested with T. absoluta eggs versus clean air, (iii) tomato plant infested with T. absoluta larvae versus uninfested tomato plant, (iv) clean air versus T. absoluta sex pheromone, (v) uninfested tomato plant versus clean air, and (vi) tomato plant infested with T. absoluta larvae versus clean air. The first–second instar larvae of T. absoluta used in tests (iii and vi) were engendered from eggs in tests (i and ii), and the plants were also maintained the same for these two tests (iii and vi). The reason for the larvae-infested treatment is that eggs and larvae of T. absoluta can co-occur on the same plants, and by associative learning, the adult parasitoids could increase their chance of encountering its specific host (the egg) on plants infested with multiple stages of the pest. In treatments involving egg infestation (i and ii), the tomato plants were subjected to T. absoluta oviposition 48–72 h prior to olfactometer tests. This exposure time resulted in plants with approximately 28.60 ± 2.00 (SE) T. absoluta eggs each (min. 20, max. 58 eggs). We allowed the eggs about 7–10 days after the last oviposition exposure date to hatch into larvae and then carried out the olfactometer test (iii and vi). Thereafter, each tomato plant had an average of 12.76 ± 1.10 (SE) T. absoluta larvae (min. 7, max. 24 larvae). During test iv, the T. absoluta sex pheromone was released by a rubber septum dispenser (Biogard, CBC Europe S.r.l) containing (E,Z,Z)-3,8,11-tetradecatrien-1-yl acetate and (E,Z)-3,8-tetradecadien-1-yl acetate.
Each of the six choice tests was replicated 20–26 times, where a replicate was represented by a group of ten female parasitoids placed inside a glass tube that was inserted at the end of the Y-tube and allowed 30 min to make a choice. Groups of replicates (2–4) were conducted on 6–8 different dates, where a new set of pair of odors was always provided. A choice was scored only when a female parasitoid touched the very end of one of the Y-tube arms, or when it was collected in the trapping bulb connected to the Y-tube near the end of each arm. Females that did not make a choice were referred to as ‘unresponsive’ and were excluded from the statistical analysis (see below). In order to avoid any spatial bias, the position of the Y-tube arms was also reversed after running each choice test replicate.
Chemical analysis and HIPV profiling
After each olfactometer choice test, the tomato plants (uninfested, egg- and larva-infested) were individually placed inside a 20-L glass jar for a 3-h volatile extraction from the headspace of those plants. There were 6–8 replicates for the chemical analysis/HIPV profiling of infested plants (with eggs or larvae) and 14 for uninfested plants (each plant considered as one replicate). The volatiles were collected in Tenax traps and stored at − 20 °C for later volatile profiling by means of gas chromatography. Tomato plants infested with T. absoluta eggs from test (ii) were subjected to volatile extraction only after larvae emergence and being used in the olfactometer test (iii), whereas tomato plants infested with eggs from test (i) and uninfested plants were subjected to volatile extraction immediately after being used in the olfactometer. Aboveground parts of all plants (stem and leaves) were weighed out using a precision scale (Mettler Toledo) after volatile collection (destructive sample).
After trapping on Tenax (30 mg) and carboxen (30 mg) packed tubes, the samples were analyzed by CIS4–TDU–GC/MS. Gerstel TDU was heated at 300 °C for 7 min under a helium stripping flow of 30 ml min−1. The TDU unit was directly assembled over the PTV injector (CIS4 Gerstel, Germany) with a liner-in-liner coupling, which eliminates the carryover effect and analyte loss. During this stage, the CIS4 was cooled to − 20 °C by computer-controlled liquid CO2 pulsed flow. After cryo-trapping on a Tenax packing liner, the PTV was quickly ramped to 260 °C for desorption and the analyte was transferred to CIS4. An Agilent 7890 GC equipped with a 5975 MSD was used for the analysis, all from Agilent Tech. (Palo Alto, CA, USA). Helium was used as the carrier gas, and the flow was kept constant at 1.2 ml/min. The chromatographic settings were as follows: injector in splitless mode set at 260 °C, J&W Innowax column (50 m, 0.20 mm i.d., 0.4 um df); oven temperature program: initial temperature 40 °C for 1 min, then 10 °C min−1 increase until 130 °C, then 5 °C min−1 increase until 210 °C, then 20 °C min−1 increase until 260 °C, hold time 3 min. The mass spectrometer was operating with an electron ionization of 70 eV, in scan mode in the m/z range 29–330, at three scans sec−1.
The deconvoluted peak spectra obtained by Agilent MassHunter software were matched against the NIST 11 spectral library for tentative identification. Kovats’ retention indices were calculated for further compound confirmation and compared with those reported in the literature for the chromatographic column used. Authentic standards were also injected to confirm compound identity.
Data analysis
A two-sided binomial proportion test was carried out on the olfactometer data to test for differences in odor choice made by T. achaeae females. Only data on female parasitoids that made a choice between odor sources within 30 min were included in the analysis. The unresponsive females were excluded from the statistical analysis. Therefore, a total of 194, 158, 146, 156, 119, and 192 female parasitoids were included in the analyses for choice tests (i), (ii), (iii), (iv), (v), and (vi), respectively. Differences in the fresh weight of tomato plants (uninfested, egg- or larva-infested) after volatile extraction were also tested using ANOVA. The analysis was run using R software (R Core Team 2018).
A partial least squares-discriminant analysis (PLS-DA) was used to determine whether HIPV profiles classify the treatments assessed (uninfested plant, and plant infested by eggs or larvae) in different groups. PLS-DA was performed using the R packages mixOmics (Rohart et al. 2017) and RVAideMemoire (Hervé 2017). Additionally, MANOVA and ANOVA were run to test for differences in HIPV concentration among uninfested or egg- and larva-infested tomato plants, which was followed by a pairwise comparison using the post hoc Tukey test at P < 0.05 (R Core Team 2018).
Results
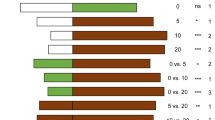
In the olfactometer tests, T. achaeae females preferred the odor from synthetic Tuta absoluta sex pheromone as opposed to clean air (χ2 = 4.00, P = 0.04) (Fig. 1). Likewise, a greater proportion of female parasitoids preferred the odor of uninfested tomato plants (χ2 = 4.07, P = 0.04) and of tomato plants infested with T. absoluta eggs (χ2 = 3.97, P = 0.04) or larvae (χ2 = 5.05, P = 0.02), when contrasted with clean air (Fig. 1). However, there was no significant difference regarding parasitoid choice when contrasting either larvae-infested (χ2 = 1.98, P = 0.15) or egg-infested tomato plants (χ2 = 0.05, P = 0.94) against uninfested tomato plants. There was no significant difference in the fresh weight of the tomato plants used among the different infestation treatments (uninfested, egg- or larva-infested) (F = 2.83, P = 0.07).
Proportion (%) of Trichogramma achaeae adult females choosing tomato plant volatiles induced by eggs or larvae of Tuta absoluta, or the synthetic sex pheromone of this pest. Female parasitoids had a choice between: (i) tomato plant infested with T. absoluta eggs versus uninfested tomato plant, (ii) tomato plant infested with T. absoluta eggs versus clean air, (iii) tomato plant infested with T. absoluta larvae versus uninfested tomato plant, (iv) clean air versus synthetic sex pheromone of T. absoluta, (v) uninfested tomato plant versus clean air, and (vi) tomato plant infested with T. absoluta larvae versus clean air. N = number of female parasitoids that chose one of the ‘odor’ options within 30 min (‘responsive females’). *Statistical difference between the proportions of parasitoids choosing one of the odor choices (two-sided Z-test, P < 0.05). N.S. no significant statistical difference
Tuta absoluta oviposition and larval feeding enhanced significantly the emission of HIPVs by the tomato plants (MANOVA, Pillai’s trace = 1.68, F20,30 = 3.563, P = 0.002) (Table 1). In total, 15 plant-related volatile compounds were identified from the headspace of uninfested, egg- and larvae-infested tomato plants (Table 1). Although the uninfested and larvae-infested tomato plants emitted similar HIPV amounts for the majority of identified compounds, a significant difference in the amounts of sabinene and limonene was recorded (Table 1). Additionally, tomato plants infested with T. absoluta eggs released a significantly higher quantity of α-terpinene and p-cymene than that released by uninfested plants (Table 1). Lastly, the most abundant compounds found in the HIPV blends of all treatments were methyl salicylate, β-phellandrene, limonene, α-pinene, 2-carene, and α-phellandrene (Table 1).
Projection to latent structures discriminant analysis (PLS-DA) of all treatments together presented three major clusters of samples, where the two T. absoluta treatments (plants infested with eggs or larvae) separated from the control (uninfested plants) and from each other (Fig. 2). The first two significant PLS components explained 44% and 13% of the total variance, respectively. Among all VOCs emitted by tomato plants, sabinene, p-symene, 3-carene, methyl salicylate, and limonene most contributed to the separation between the experimental treatments, with variable importance for the projection (VIP) values > 1.
Discussion
The deployment of semiochemicals that could enhance the performance of biological control agents represents a profitable and modern strategy for plant protection. This is particularly important for invasive pests whose control still relies mostly on the repeated application of synthetic insecticides (Giorgini et al. 2019). Our results show that both host- and plant-derived cues influence the foraging behavior of T. achaeae. Specifically, olfactometer tests showed that female parasitoids were significantly attracted toward the sex pheromone of T. absoluta. In fact, the exploitation of sex pheromone to locate host eggs is relatively common among egg parasitoids, including Trichogramma species. For example, Reddy et al. (2002) demonstrated that the sex pheromone components of Plutella xylostella (L.), individually or as synthetic blends, attracted both the egg parasitoid Trichogramma chilonis Ishii and the larval parasitoid Cotesia plutellae (Kurdjumov). Likewise, Lewis et al. (1982) also documented that a synthetic blend of Heliothis zea (Boddie) sex pheromone increased significantly egg parasitism by Trichogramma pretiosum Riley in greenhouse and in cotton fields. A similar result could be expected for T. achaeae in European greenhouses, or in tomato fields where mating disruption is deployed to manage T. absoluta (Cocco et al. 2013; Perez-Hedo et al. 2017; Biondi et al., 2018; Giorgini et al. 2019). However, field and greenhouse experiments are necessary to confirm this hypothesis regarding T. achaeae. For example, there have been some discrepancies between laboratory and greenhouse results regarding the attraction of other Trichogramma species to T. absoluta sex pheromone (Ahamdi and Poorjavad 2018), which worked better under laboratory conditions. Additionally, applying synthetic pheromone at the right timing and dose is crucial to guarantee the attraction of specific parasitoids, and thus these parameters should be also investigated.
Although the infestation by T. absoluta, especially oviposition, increased the emission of HIPVs (VOCs) by tomato plants (Table 1), T. achaeae females did not show a preference for egg-infested or larva-infested plants over uninfested tomato plants (Fig. 1). This pattern has also been observed for other natural enemies of T. absoluta such as the mirid predator Nesidiocoris tenuis (Heuter) (Naselli et al. 2017). In contrast, in our study, female parasitoids preferred uninfested, egg-infested, or larvae-infested tomato plants when they were tested against clean air. These results indicate that T. achaeae females are innately attracted to volatiles produced by tomato plants, regardless of the infestation status. In fact, it has been documented that certain plant species (e.g., tomato) naturally release specific compounds, such as sesquiterpenes, that significantly influence the attraction of Trichogramma species (Nordlund et al. 1985). The absence of T. achaeae’s preference for T. absoluta-infested plants, despite the higher release of volatiles in respect to control plants, suggests that naïve parasitoids may not be able to distinguish between infested and uninfested plants. If that is the case, the associative learning ability of T. achaeae females could become crucial for exploiting chemical cues released by infested tomato plants. In fact, some Trichogramma species have been reported to be able to learn the different components of host plant volatiles and to adapt their behavior accordingly (Pashalidou et al. 2010; Wilson and Woods 2016). Moreover, Vet and Dicke (1992) also theorized that generalist parasitoids, compared to specialists, should rely more strongly on learning infested-plant cues to adjust their foraging behavior since they have not coevolved with a specific host.
Alternatively, the amount of volatiles produced by T. absoluta (eggs or larvae)-infested plants in our experiment may not have been sufficient to allow T. achaeae females to distinguish between infested and uninfested tomato plants. For example, while limonene and sabinene have been shown to attract egg parasitoids (e.g., Trichogrammatidae) (Rani and Sandhyarani 2012; Dicke 1994), their ‘higher’ concentrations (limonene and sabinene) on T. absoluta-infested plants in our experiment (Table 1) were not enough to allow T. achaeae to clearly distinguish between uninfested and infested tomato plants. Following the same line of reasoning, other studies have documented that a higher density of T. absoluta infestation will result in greater amounts of HIPVs (Silva et al. 2017), which attract more Trichogramma parasitoids (Alsaedi et al. 2016). Additionally, it appears that egg parasitoids such as T. pretiosum released in greenhouses are also more attracted to tomato plants containing a higher number of T. absoluta eggs (Faria et al. 2008). Thus, testing the response of T. achaeae parasitoids to HIPVs from tomato plants sustaining higher densities of T. absoluta eggs and larvae should be considered in future studies.
The most abundant compounds found in the headspace of T. absoluta egg- and larva-infested and uninfested tomato plants were methyl salicylate, β-phellandrene, limonene, α-pinene, 2-carene, and α-phellandrene, which is in line with previous studies (Silva et al. 2017). Nonetheless, it is not uncommon to find some variation in the results of current and previous studies regarding the amount of headspace compounds found in tomato plants (Farag and Paré 2002; Degenhardt et al. 2010; Proffit et al. 2011; Backer et al. 2015). Such differences in the emitted blends may be explained by variations in the size and structure of the volatile collection chambers, plant age and cultivar, herbivore density, and the environmental conditions (Dudareva et al. 2006). In our study, volatile extraction and olfactometer tests were conducted on plants of similar age and morphological structure (5.5 ± 1.0 leaves per plant) as well as similar egg (28.60 ± 2.00) and larvae (12.76 ± 1.10) densities per plant. In any case, the PLS-DA analysis showed that uninfested and infested tomato plants had significantly different volatile profiles, with these differences appearing 72 h after T. absoluta oviposition.
In Mediterranean countries, T. achaeae has been considered a potential candidate for the biological control of T. absoluta by inundative releases in tomato greenhouses (Urbaneja et al. 2012; El-Arnaouty et al. 2014; Kortam et al. 2017). Nevertheless, the necessity of multiple parasitoid releases in each season has been considered a drawback by several researchers (Cabello et al. 2009; Chailleux et al. 2012, 2013). Regardless, T. achaeae has been a key component of T. absoluta IPM programs in some Mediterranean greenhouse districts by complementing the activity of mirid predators, which may not be very effective if used alone. In this context, the specific timing that biocontrol agent is released results in better control of T. absoluta. For example, T. achaeae and mirid predators could work in unison, especially if released at different times: the former targeting early egg infestation and the latter subsequently targeting remaining eggs and larvae of T. absoluta (Urbaneja et al., 2012; Chailleux et al. 2013; Zappalà et al. 2013). This approach is also potentially favorable to reduce the damage linked to the phytophagous behavior of the mirid predators, which occurs frequently when they are released at the beginning of the season (Giorgini et al. 2019). Field tests in different conditions (protected, open field) are needed to investigate this assertion.
Semiochemicals are of paramount importance to help natural enemies locate and recognize their hosts or prey. Understanding the nature of these chemicals and their ecological role in trophic interactions is essential to design more sustainable strategies for pest management, including biological control. Our data indicate that female parasitoids of T. achaeae respond to both host sex pheromone and volatiles released by tomato plants, regardless of their infestation status. Nonetheless, further studies will be necessary to investigate how the host sex pheromone could be deployed to manipulate T. achaeae and thus improve the parasitism of T. absoluta in greenhouses and open fields. Additionally, it is crucial to assess the optimal releasing rate of these semiochemicals to enhance the performance of T. achaeae. Lastly, further studies should investigate the potential for T. achaeae to learn the cues from T. absoluta-infested tomato plants to adjust their foraging behavior accordingly, as well as the influence of host density on the T. achaeae response to HIPVs. The information obtained through such studies would complement our current results and would generate mechanistic knowledge that will promote a more efficient and sustainable management of T. absoluta.
Author contribution
PC, MG, and EG conceived and designed the experiments. MM worked on HIPV profiling and data analysis. LG, PC, HR, GS, and LI worked on olfactometer bioassays. LG analyzed olfactometer data. LG, PC, MG, and EG wrote and edited the manuscript. All authors reviewed and approved the final version of the manuscript.
References
Ahamdi S, Poorjavad M (2018) Behavioral and biological effects of exposure to Tuta absoluta (lepidoptera: gelechiidae) sex pheromone on several Trichogramma (hymenoptera: trichogrammatidae) populations. J Econ Entomol. https://doi.org/10.1093/jee/toy212 (in press)
Alsaedi G, Ashouri A, Talaei-Hassanloui R (2016) Behavioral responses of the three Trichogramma species to different odor sources. J Entomol Zool Stud 4(4):1924
Anastasaki E, Drizou F, Milonas PG (2018) Electrophysiological and oviposition responses of Tuta absoluta females to herbivore-induced volatiles in tomato plants. J Chem Ecol. https://doi.org/10.1007/s10886-018-0929-1
Arakaki N, Wakamura S, Yasuda T (1996) Phoretic egg parasitoid, Telenomus euproctidis (Hymenoptera: Scelionidae), uses sex pheromone of tussock moth Euproctis taiwana (Lepidoptera: Lymantriidae) as a kairomone. J Chem Ecol 22:1079–1085
Backer LD, Megido RC, Fouconnier ML, Brostaux Y, Francis F, Verheggen F (2015) Tuta absoluta-induced plant volatiles: attractiveness towards the generalist predator Macrolophus pygmaeus. Arthropod-Plant Interact 9:465–476. https://doi.org/10.1007/s11829-015-9388-6
Biondi A, Guedes RNC, Wan FH, Desneux N (2018) Ecology, worldwide spread, and management of the invasive south american tomato pinworm, Tuta absoluta: past, present, and future. Annu Rev Entomol 63:239–258
Cabello T, Gallego JR, Vila E, Soler A, del Pino M, Carnero A et al (2009) Biological control of the South American tomato pinworm, Tuta absoluta (Lepidoptera: Gelechiidae), with releases of Trichogramma achaeae (Hym.: Trichogrammatidae) in tomato greenhouses of Spain. IOBC/WPRS Bull 49:225–230
Cabello T, Gallego JR, Fernandez FJ, Gamez M, Vila E, del Pino M, Hernandez-Suarez E (2012) Biological control strategies for the South American tomato moth (Lepidoptera: Gelechiidae) in greenhouse tomatoes. J Econ Entomol 105(6):2085–2096
Cagnotti CL, Hernández CM, Andormo AV, Viscarret M, Riquelme M, Botto EN, López SN (2016) Acceptability and suitability of Tuta absoluta eggs from irradiated parents to parasitism by Trichogramma nerudai and Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Agric For Entomol 18:198–205
Campos MR, Biondi A, Adiga A, Guedes RNC, Desneux N (2017) From the Western Palaearctic region to beyond: Tuta absoluta 10 years after invading Europe. J Pest Sci 90:787–796
Cascone P, Carpenito S, Slotsbo S, Iodice L, Sørensen GJ, Holmstrup M, Guerrieri E (2015) Improving the efficiency of Trichogramma achaeae to control Tuta absoluta. Biocontrol 60:761–771
Chailleux A, Desneux N, Seguret J, Do Thi Khanh H, Maignet P, Tabone E (2012) Assessing European egg parasitoids as a mean of controlling the invasive South American tomato pinworm Tuta absoluta. PLoS ONE 7:e48068
Chailleux A, Biondi A, Han P, Tabone E, Desneux N (2013) Suitability of the pest–plant system Tuta absoluta (Lepidoptera: Gelechiidae)—tomato for Trichogramma (Hymenoptera: Trichogrammatidae) parasitoids and insights for biological control. J Econ Entomol 106:2310–2321
Cherif A, Mansour R, Attia-Barhoumi S, Zappalà L, Grissa-Lebdi K (2018) Effectiveness of different release rates of Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae) against Tuta absoluta (Lepidoptera: Gelechiidae) in protected and open field tomato crops in Tunisia. Biocontrol Sci Technol. https://doi.org/10.1080/09583157.2018.1542485
Cocco A, Deliperi S, Delrio G (2013) Control of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in greenhouse tomato crops using the mating disruption technique. J Appl Entomol 137:16–28
Coppola M, Cascone P, Madonna V, Di Lelio I, Esposito F, Avitabile C, Romanelli A, Guerrieri E, Vitiello A, Pennacchio F, Rao R, Corrado G (2017) Plant-to-plant communication triggered by systemin primes anti-herbivore resistance in tomato. Sci Rep. https://doi.org/10.1038/s41598-017-15481-8
Degenhardt J, Kollner TG, Gershenzon J (2009) Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 70:1621–1637
Degenhardt DC, Refi-Hind S, Stratmann JW, Lincoln DE (2010) Systemin and jasmonic acid regulate constitutive and herbivore-induced systemic volatile emissions in tomato, Solanum lycopersicum. Phytochemistry 71:2024–2037
Desneux N, Wajnberg E, Wyckhuys KAG et al (2010) Biological invasion of European tomato crops by Tuta absoluta: ecology, geographic expansion and prospects for biological control. J Pest Sci 83:197–215
Dicke M (1994) Local and systemic production of volatile herbivore-induced terpenoids: their role in plant-carnivore mutualism. J Plant Physiol 143:465–472
Dicke M, Baldwin IT (2010) The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci 15:167–175
Dudareva N, Negre F, Nagegowda DA, Orlova I (2006) Plant volatiles: recent advances and future perspectives. Crit Rev Plant Sci 25:417–440
El-Arnaouty SA, Pizzol J, Galal HH, Kortam MN, Afifi AI et al (2014) Assessment of two Trichogramma species for the control of Tuta absoluta in North African tomato greenhouses. Afr Entomol 22:801–809
Farag MA, Paré PW (2002) C6 green leaf volatiles trigger local and systemic VOC emissions in tomato. Phytochemistry 61:545–554
Faria CA, Torres JB, Fernandes AMV, Farias AMI (2008) Parasitism of Tuta absoluta in tomato plants by Trichogramma pretiosum Riley in response to host density and plant structures. Cienc Rural 38:1504–1509
Fatouros NE, Lucas-Barbosa D, Weldegergis BT, Pashalidou FG, van Loon JJA et al (2012) Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLoS ONE 7(8):e43607. https://doi.org/10.1371/journal.pone.0043607
Giorgini M, Guerrieri E, Cascone P, Gontijo L (2019) Current strategies and future outlook for managing the Neotropical tomato pest Tuta absoluta (Meyrick) in the Mediterranean Basin. Neotrop Entomol. https://doi.org/10.1007/s13744-018-0636-1
Guedes RNC, Picanço MC (2012) The tomato borer Tuta absoluta in South America: pest status, management and insecticide resistance. EPPO Bull 42:211–216
Han P, Bayram Y, Shaltiel-Harpaz L, Sohrabi F, Saji A, Esenali UT, Jalilov A, Ali A, Shashank PR, Ismoilov K, Lu ZZ, Wang S, Zhang GF, Wan FH, Biondi A, Desneux N (2019) Tuta absoluta continues to disperse in Asia: damage, ongoing management and future challenges. J Pest Sci. https://doi.org/10.1007/s10340-018-1062-1
Hervé M (2017) RVAideMemoire: testing and plotting procedures for biostatistics. https://CRAN.R-project.org/package=RVAideMemoire. Accessed 2 Aug 2018
Hilker M, Meiners T (2011) Plants and insect eggs: how do they affect each other? Phytochemistry 72:1612–1623
Huigens ME, Woelk JB, Pashalidou FG, Bukovinszky T, Smid HM, Fatouros NE (2010) Chemical espionage on species-specific butterfly anti-aphrodisiacs by hitchhiking Trichogramma wasps. Behav Ecol 21:470–478
Kopke D, Schröder R, Fischer HM, Gershenzon G, Hilker M, Schmidt A (2008) Does egg deposition by herbivorous pine sawflies affect transcription of sesquiterpene synthases in pine? Planta 22:427–438
Kortam MN, El Arnaouty SA, Fatnassi H, Afifi AI, Pizzol J, Suloma A, Poncet C (2017) The effect of microclimatic parameters on two Trichogramma species used to control Tuta absoluta. IOBC-WPRS Bull 124(131):137
Lewis WJ, Nordlund DA, Gueldne RC, Teal PEA, Tumlinson JH (1982) Kairomones and their use for management of entomophagous insects: XIII. Kairomonal activity for Trichogramma spp. of abdominal tips, excretion, and a synthetic sex pheromone blend of Heliothis zea (Boddie) moths. J Chem Ecol 8:1323–1331
Lietti MMM, Botto E, Alzogaray RA (2005) Insecticide resistance in argentine populations of Tuta absoluta. Neotrop Entomol 34:113–119
López E (1991) Polilla del tomate: problema crítico para la rentabilidad del cultivo de verano. Empresa y Avance Agrícola 1:6–7
Mansour R, Brévault T, Chailleux A, Cherif A, Grissa-Lebdi K, Haddi K, Mohamed SA, Nofemela RS, Oke A, Sylla S, Tonnang HEZ, Zappalà L, Kenis M, Desneux N, Biondi A (2018) Occurrence, biology, natural enemies and management of Tuta absoluta in Africa. Entomol Gen 38(2):83–112
Mumm R, Dicke M (2010) Variation in natural plant products and the attraction of bodyguards for indirect plant defense. Can J Zool 88:628–667
Mumm R, Schrank K, Wegener R, Schulz S, Hilker M (2003) Chemical analysis of volatiles emitted by Pinus sylvestris after induction by insect oviposition. J Chem Ecol 29:1235–1252
Naselli M, Zappalà L, Gugliuzzo A, Tropea Garzia G, Biondi A, Rapisarda C, Cincotta F, Condurso C, Verzera A, Siscaro G (2017) Olfactory response of the zoophytophagous mirid Nesidiocoris tenuis to tomato and alternative host plants. Arthropod–Plant Interact 11:121–131
Noldus LPJJ, van Lenteren JC, Lewis WJ (1991) How Trichogramma parasitoids use moth sex pheromones as kairomones: orientation behaviour in a wind tunnel. Physiol Entomol 16:313–327
Nordlund DA, Chalfant RB, Lewis WJ (1985) Response of Trichogramma pretiosum females to volatile synomones from tomato plants. J Entomol Sci 20(3):372–376
Oliveira L, Durão AC, Fontes J, Roja IS, Tavares J (2017) Potential of Trichogramma achaeae (Hymenoptera: Trichogrammatidae) in biological control of Tuta absoluta (Lepidoptera: Gelechiidae) in Azorean greenhouse tomato crops. J Econ Entomol 110:2010–2015
Parra JRP, Zucchi RA (2004) Trichogramma in Brazil: feasibility of use after twenty years of research. Neotrop Entomol 33:271–281
Pashalidou FG, Huigens ME, Dicke M, Fatouros NE (2010) The use of oviposition-induced plant cues by Trichogramma egg parasitoids. Ecol Entomol 35:748–753
Perez-Hedo M, Suay R, Alonso M, Ruocco M, Giorgini M, Poncet C, Urbaneja A (2017) Resilience and robustness of IPM in protected horticulture in the face of potential invasive pests. Crop Prot 97:119–127
Picanço MC, Leite GLD, Guedes RNC, Silva EA (1998) Yield loss in trellised tomato affected by insecticidal sprays and plant spacing. Crop Prot 17:447–452
Polaszek A, Rugman-Jones PF, Stouthamer R, Hernandez-Suarez E, Cabello T (2012) Molecular and morphological diagnoses of five species of Trichogramma: biological control agents of Chrysodeixis chalcites (Lepidoptera: Noctuidae) and Tuta absoluta (Lepidoptera: Gelechiidae) in the Canary Islands. Biocontrol 57:21–36
Pratissoli D, Thuler RT, Andrade GS, Zanotti LCM, Silva AF (2005) Estimate of Trichogramma pretiosum to control Tuta absoluta in stalked tomato. Pesqui Agropecu Bras 40:715–718
Proffit M, Birgersson G, Bengtsson M, Reis R, Witzgall P, Lima E (2011) Attraction and oviposition of Tuta absoluta females in response to tomato leaf volatiles. J Chem Ecol 37:565–574
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. Accessed 2 Aug 2018
Rani PU, Sandhyarani K (2012) Specificity of systemically released rice stem volatiles on egg parasitoid, Trichogramma japonicum Ashmead behavior. J Appl Entomol 136:749–760
Reddy GVP, Holopainen JK, Guerrero A (2002) Olfactory responses of Plutella xylostella natural enemies to host pheromone, larval frass, and green leaf cabbage volatiles. J Chem Ecol 28:131–143
Roditakis E, Vasakis E, García-Vidal L, Martínez-Aguirre MDR, Rison JL, Haxaire-Lutun MO, Nauen R, Tsagkarakou A, Bielza P (2018) A four-year survey on insecticide resistance and likelihood of chemical control failure for tomato leaf miner Tuta absoluta in the European/Asian region. J Pest Sci 91:421–435. https://doi.org/10.1007/s10340-017-0900-x
Rohart F, Gautier B, Singh A, Lê Cao K-A (2017) mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput Biol 13(11):e1005752. https://doi.org/10.1371/journal.pcbi.1005752
Sacchettini JC, Poulter CD (1997) Biochemistry-creating isoprenoid diversity. Science 277:1788–1789
Silva GA, Picanço MC, Bacci L, Crespo ALB, Rosado JF, Guedes RNC (2011) Control failure likelihood and spatial dependence of insecticide resistance in the tomato pinworm, Tuta absoluta. Pest Manag Sci 67:913–920
Silva DB, Weldegergis BT, Van Loon JJA, Bueno VHP (2017) Qualitative and quantitative differences in herbivore-induced plant volatile blends from tomato plants infested by either Tuta absoluta or Bemisia tabaci. J Chem Ecol 43:53–65
Silva JE, Ribeiro LMS, Vinasco N, Guedes RNC, Siqueira HAA (2019) Field-evolved resistance to chlorantraniliprole in the tomato pinworm Tuta absoluta: inheritance, cross-resistance profile, and metabolism. J Pest Sci. https://doi.org/10.1007/s10340-018-1064-z
Siqueira HAA, Guedes RNC, Picanco MC (2000) Insecticide resistance in populations of Tuta absoluta (Lepidoptera:Gelechiidae). Agric For Entomol 2:147–153
Takabayashi J, Dicke M, Posthumus MA (1994) Volatile herbivore-induced terpenoids in plant-mite interactions: variation caused by biotic and abiotic factors. J Chem Ecol 20:1329–1354
Tropea Garzia G, Siscaro G, Biondi A, Zappalà L (2012) Tuta absoluta, a South American pest of tomato now in the EPPO region: biology, distribution and damage. EPPO Bull 42:205–210
Turlings TCJ, Wackers FI, Vet LEM, Lewis WJ, Tumlinson JH (1993) Learning of host-finding cues by hymenopterous parasitoids. In: Papaj DR, Lewis WJ (eds) Insect learning. Chapman and Hall, New York, pp 51–78
Unsicker SB, Kunert G, Gershenzon J (2009) Protective perfumes: the role of vegetative volatiles in plant defense against herbivores. Curr Opin Plant Biol 12:479–485
Urbaneja A, González-Cabrera J, Arnó J, Gabar R (2012) Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Manag Sci 68:1215–1222
van Lenteren JC, Bolckmans K, Köhl J, Ravensberg WJ, Urbaneja A (2018) Biological control using invertebrates and microorganisms: plenty of new opportunities. Biocontrol 63:39–59
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Ann Rev Entomol 37:141–172
Wilson JK, Woods HA (2016) Innate and learned olfactory responses in a wild population of the egg parasitoid Trichogramma (Hymenoptera: Trichogrammatidae). J Insect Sci 16(1):1–8
Wright MG, Stouthamer R (2011) First report of Trichogramma achaeae (Hymenoptera: Trichogrammatidae) from Hawaii. Proc Hawaii Entomol Soc 43:67
Zappalà L, Biondi A, Alma A, Al-Jboory IJ, Arnò J, Bayram A, Chailleux A, El-Arnaouty A, Gerling D, Guenaoui Y, Shaltiel-Harpaz L, Siscaro G, Stavrinides M, Tavella L, Aznar RV, Urbaneja A, Desneux N (2013) Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle East, and their potential use in pest control strategies. J Pest Sci 86(4):635–647
Acknowledgements
This work has been supported by the EU FP7 project ‘Ameliorating the Sustainable Control of Invasive Insects’ (PIRSES 318246). The work was also supported by ‘Fundação de Amparo a Pesquisa do Estado de Minas Gerais’—FAPEMIG (grant FORTIS-TCT-10254/2014). The authors would like to show their gratitude to Gabriele Cencetti (IBBR-CNR, ARCA Laboratory) for helping with GC–MS analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Biondi and N. Desneux.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gontijo, L., Cascone, P., Giorgini, M. et al. Relative importance of host and plant semiochemicals in the foraging behavior of Trichogramma achaeae, an egg parasitoid of Tuta absoluta. J Pest Sci 92, 1479–1488 (2019). https://doi.org/10.1007/s10340-019-01091-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-019-01091-y