Abstract
The South American tomato pinworm, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), is an invasive pest difficult to control. Insecticide application is quite common and remains the prevalent control method particularly in open-field cultivation systems. As a result, insecticide resistance to many chemical classes of insecticides has been described both in South America and in Europe. The development of insecticide resistance is relatively fast in this species, and the main mechanisms involved are altered target-site sensitivity and/or enhanced detoxification, depending on the chemical class. However, insecticide resistance mechanisms do not differ between South America and Europe and are mainly due to simple genotype variations leading to high levels of resistance. The presence of resistance alleles at low frequency, especially against newer chemistry, is of major concern, as they tend to spread with the invasions making tomato pinworm particularly difficult to control. The monitoring methods and management programmes developed for the species benefited from the pro-activity of the Insecticide Resistance Action Committee and its country groups, particularly in Brazil and Spain. Bioassay methods were developed, resistance monitoring activities initiated and resistance management guidance was provided. The implementation of integrated control programmes and appropriate resistance management strategies as part of such programs is of utmost importance to keep tomato pinworm infestations under economic damage thresholds, thus guaranteeing sustainable yields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
Insecticide use is the main management tactic employed against the tomato pinworm;
-
Insecticide resistance is common and globally spread, based on target-site insensitivity and/or enhanced detoxification;
-
Resistance management tactics were developed and are key for sustainable control, but global spread of resistance genotypes is a concern;
-
Risks of insecticide failure and other consequences of insecticide resistance are further issues of concern.
Early spread and control
Spread in South America and control constrains
Invasion of pest species is an ongoing and major concern in an increasingly globalized world where international trade and travel favour the introduction, establishment and spread outside their native ranges (Banks et al. 2015). Such invasions can have a strong impact and elicit profound environmental and economic effects in a broad range of ecosystems (Soliman et al. 2015; Bradshaw et al. 2016; Hill et al. 2016). While inspection and quarantine measures are the main practices to minimize the arrival of invasive pest species, relying on pesticides remains the major practice to control such pests once established on a broader scale (Lockwood et al. 2013; Liebhold et al. 2016). However, the presence of resistance alleles and the lack of effective insecticides due to missing registrations at the site of introduction could facilitate the fast spread of invasive pests. The South American tomato pinworm, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), is one of the most recent examples of such an invasive pest and of major concern among tomato growers all over the world.
The tomato pinworm, whose initial description dates back to 1917, apparently has the Peruvian central highlands in western South America as its native range (Guedes and Picanço 2012; Biondi et al. 2018). Historically, T. absoluta was exclusively reported from South America and Easter Island (Ripa et al. 1995). A succession of taxonomic revisions since the mid-1960s until the mid-1990s finally resulted in its current species name T. absoluta. This period coincided with the species’ spread through the South American continent reaching Brazil, the main tomato producer in the region, in the early 1980s (Guedes and Picanço 2012). Between the 1980s and late 2000s, the pinworm became the main tomato pest species in the region; a species notoriously difficult to control (Desneux et al. 2004; Guedes and Picanço 2012; Biondi et al. 2018).
The tomato pinworm infests young plants by larval penetration into the buds of young plant stems, and once foliage increases, the leaf-mining larvae attack the leaves leading to loss in photosynthesis capacity (Guedes and Picanço 2012; Biondi et al. 2018). Larvae are also known to attack the tomato fruits leading to serious yield losses and compromising crop production (Desneux et al. 2004; Guedes and Picanço 2012). The larval feeding habits and plant architecture make T. absoluta a difficult target for insecticide sprays (Guedes and Siqueira 2012; Biondi et al. 2018). Nonetheless, insecticide use was the only effective control method to prevent outbreaks. Thus, heavy reliance on insecticide use increased selection pressure, affecting the performance of important chemical classes of insecticides.
Patterns of insecticide use
The immediate threat to tomato production in Neotropical America led to intensive insecticide use against this pest in the invaded areas. When the species was first introduced in Brazil, farmers were applying insecticides 10–12 times per cultivation cycle. After a few years, this was increased to more than 30 applications per cropping cycle, i.e. 4–6 weekly sprays (Guedes and Siqueira 2012). However, early colonization of tomato fields, insect attack to multiple plant parts and protection by the plant canopy cause difficulty to control this pest species with insecticides. As a consequence, the level of insecticide efficacy achieved against the tomato pinworm is often low, what also favours additional spraying and insecticide overuse (Biondi et al. 2018). Formulation adjuvants and improved spraying technology do play major roles mitigating some of these problems, but the lack of control alternatives particularly for open-field tomatoes retains the need of multiple applications throughout the cropping cycle (Guedes and Siqueira 2012; Guedes and Picanço 2012; Biondi et al. 2018).
The scenario described above led to a dynamic succession of changes in the compounds used against the tomato pinworm in South America since the early spread of this species in the continent. Organophosphates and pyrethroids were among the few insecticides available for early T. absoluta control in tomatoes. These two classes of insecticides were used against the tomato pinworm starting from the 1960s and 1980s, respectively (Salazar and Araya 1997, 2001; Siqueira et al. 2000a; Lietti et al. 2005). The use of organophosphates soon declined, and cartap (a nereistoxin analogue) and abamectin (an avermectin) became available and were used in combination with pyrethroids (Siqueira et al. 2000b, 2001; Guedes and Siqueira 2012). By the late 1990s and early 2000s, the oxadiazine indoxacarb and chitin biosynthesis inhibitors (e.g. diflubenzuron, teflubenzuron and triflumuron) became available, and particularly the latter group was quite popular (Silva et al. 2011; Guedes and Picanço 2012).
Other chemical classes introduced for tomato pinworm control included the pyrroles (e.g. chlorfenapyr), spinosyns (e.g. spinosad) and the diamides (e.g. chlorantraniliprole and flubendiamide) (Silva et al. 2011; Gontijo et al. 2013; Silva et al. 2016a, b). Organic tomato production systems mostly rely on spinosad, azadirachtin, and Bacillus thuringiensis toxins (Bt toxins) (Silva et al. 2011; Biondi et al. 2018).
Early monitoring: evolving methods and patterns of resistance
The frequent use of insecticides facilitated resistance development in T. absoluta as shown in resistance monitoring campaigns using different types of bioassays. Early studies used topical application, followed by filter paper impregnation assays with dried insecticide residue (Salazar and Araya 1997; Siqueira et al. 2000a, b, 2001; Guedes and Siqueira 2012). Later, a more applied leaf-dip method suitable for both fast- and slow-acting insecticides was developed (Galdino et al. 2011; Silva et al. 2011). This method was subsequently validated by the Insecticide Resistance Action Committee (IRAC) as IRAC Method No. 022 (Roditakis et al. 2013a, b) and became a widely accepted reference.
Resistance monitoring in the tomato pinworm revealed the dynamic nature of resistance in South America, shifting with the prevailing pattern of insecticide use. The early use of organophosphate and pyrethroid insecticides led to initial detection of resistance to these compounds first in Chile, later in Brazil and Argentina (Salazar and Araya 1997, 2001; Siqueira et al. 2000a; Lietti et al. 2005). Pyrethroid resistance became widespread and was most likely introduced into Europe at the onset of the tomato pinworm invasion from South America (Salazar and Araya 1997; Siqueira et al. 2000a; Silva et al. 2011; Haddi et al. 2012; Silva et al. 2015). Low-to-moderate levels of abamectin and cartap resistance were soon reported and increased subsequently (Siqueira et al. 2000b, 2001; Silva et al. 2016b), while pyrethroid resistance decreased to levels lower than tenfold (Silva et al. 2011). Low-to-moderate levels of indoxacarb resistance were later detected (Silva et al. 2011, 2016b), as well as high resistance ratios to chitin biosynthesis inhibitors resulting in field failure at the peak of their use in the mid-2000s (Silva et al. 2011).
Increased resistance to chitin biosynthesis inhibitors favoured the use of spinosad and an increase in resistance of the latter from low to high levels (Reyes et al. 2012; Campos et al. 2014, 2015). The latest chemical class of insecticides launched for tomato pinworm control is the diamides (Nauen 2006). However, after a few years of extensive use, resistance to these compounds was detected both in Brazil and in Europe (Campos et al. 2015; Roditakis et al. 2015,2017b; Silva et al. 2016a, 2019). Interestingly, resistance to chlorfenapyr was detected, but remained at low levels so far, most likely due to its limited use (Silva et al. 2016b); something similar is also observed in the case of Bt toxins (Silva et al. 2011).
Associated risk of control failure
Insecticide resistance detected in laboratory bioassays does not necessarily result in control failures under applied conditions. Unlike insecticide resistance, the risk or likelihood of control failure is rarely surveyed since it requires realistic exposure scenarios in respective bioassays, in addition to standard endpoints obtained in insecticide bioassays designed for this purpose (Guedes 2017). Nonetheless, the risk of control failure has received an increased attention and was recently surveyed and preliminarily mapped for tomato pinworm in Brazil. The findings discussed the effect of landscape topography on the spread of tomato pinworm resistance alleles (Silva et al. 2011; Gontijo et al. 2013; Silva et al. 2015), and it was suggested that the flat landscape of the Brazilian savannah seems to favour the spread of insecticide resistance. The concern of potential control failures and resistance spread has also been discussed for European hotspots for tomato pinworm (Roditakis et al. 2013b).
European invasion, subsequent spread and control
Invasion and associated patterns of insecticide use
Outside Neotropical America, this pest was first reported in Spain in late 2006 (Urbaneja et al. 2007) from where it further spread to coastal European and North African countries (Desneux et al. 2011; Campos et al. 2017; Biondi et al. 2018). Mapping of potential source of invasion into Europe suggested central Chile as the likely origin (Guillemaud et al. 2015). Subsequently, T. absoluta invaded Middle East countries and more recently moved southwards reaching several eastern and western sub-Saharan regions, and subsequently reaching South Africa by 2016 (Pfeiffer et al. 2013; Brévault et al. 2014; Tonnang et al. 2015; Visser et al. 2017; Sylla et al. 2017; Biondi et al. 2018; Mansour et al. 2018; Santana et al. 2019). Eastward, T. absoluta extended its range of distribution to India and the Himalayan region by 2017 (Sankarganesh et al. 2017; Sharma and Gavkare 2017; Han et al. 2018, 2019; Santana et al. 2019), and its presence, although unconfirmed, was reported from Pakistan and Tajikistan (Campos et al. 2017). The presence of T. absoluta was not yet reported from some major tomato-producing countries, including China, New Zealand, the USA and Australia (Biondi et al. 2018).
The introduction of T. absoluta into the Mediterranean region was accompanied by an extensive use of insecticides to keep it under control (Desneux et al. 2011), resulting in a significant increase in both average number of applications and pest control-related costs (Potting et al. 2013). Initially, and due to the lack of specifically registered compounds for tomato pinworm control, growers relied on broad-spectrum insecticides such as pyrethroids (Balzan and Moonen 2012). However, such a strategy proved ineffective in providing suitable control levels and highlighted the need of introducing new chemicals specifically targeting T. absoluta combined with field monitoring of insecticide susceptibility (Roditakis et al. 2013a, b). Since 2009, a wave of insecticide registrations for use against T. absoluta allowed a wider choice of products. Between 2009 and 2011, the number of active insecticide molecules specifically introduced to target T. absoluta reached 15 and 18 in Spain and Tunisia, respectively, encompassing some 13 distinct modes of action (Desneux et al. 2011; Abbes et al. 2012).
Currently, a large number of insecticides representing several chemical classes are registered and used against T. absoluta, depending on the country (Table 1). These insecticide classes include organophosphates (chlorpyrifos, methamidophos), pyrethroids (deltamethrin, lambda-cyhalothrin, bifenthrin, permethrin), oxadiazines (indoxacarb), spinosyns (spinosad, spinetoram), avermectins (abamectin, emamectin benzoate), pyrroles (chlorfenapyr), benzoylureas (diflubenzuron, lufenuron, novaluron), diamides (chlorantraniliprole, flubendiamide), diacylhydrazines (chromafenozide, methoxyfenozide, tebufenozide), semicarbazones (metaflumizone), tetranortriterpenoids (azadirachtin) and nereistoxin analogues (cartap) (IRAC 2018). Moreover, commercial formulations of some bio-insecticides based on B. thuringiensis and Beauveria bassiana have been also widely used on tomato crops, as they are often more compatible with the tomato pinworm natural enemies (Biondi et al. 2012, 2013; Klieber and Reineke 2016). Other bio-insecticides are also available, like limonene and borax, but exhibiting more limited use (Soares et al. 2019).
Invasive (resistant) genotypes
From the status of a key tomato pest only in South American countries, T. absoluta largely and rapidly expanded its geographical distribution during the last 13 years. Since resistance to a range of insecticides was earlier reported in Brazil, Chile and Argentina before the pinworm invasion into Europe (Souza et al. 1992; Siqueira et al. 2000a, 2001; Salazar and Araya 2001; Lietti et al. 2005; Guedes and Siqueira 2012), it is not surprising that resistance alleles migrated outside the native geographical range of the pest into Europe.
Guillemaud et al. (2015) showed that the origin of the invading populations around the Mediterranean was most likely from Chile. Alleles conferring resistance to insecticides were initially present at low frequency but increased upon selection with insecticides. Molecular analysis of the voltage-gated sodium channel—targeted by pyrethroids—in T. absoluta, revealed three kdr/super-kdr-type mutations (M918T, T929I and L1014F) present at high frequencies within different field strains from both South America and Europe (Haddi et al. 2012). Similarly, Haddi et al. (2017) also detected at high frequency an A201S mutation linked with organophosphate resistance at the gene encoding the organophosphate target site, acetylcholinesterase (AChE), in different populations of T. absoluta.
Nonetheless, the presence of these mutations in the invading populations likely contributed to the fitness and spread of T. absoluta under the intensive and ongoing insecticide use against this species. Therefore, the development and use of diagnostic tests to detect the resistant genotypes in the invading populations are very important to properly design suitable management tactics as they will allow the selection and use of insecticides still effective against the prevailing resistant mechanisms (Guedes and Siqueira 2012; Haddi et al. 2012).
Method validation, monitoring and patterns of insecticide resistance
As mentioned above, initial attempts to control the pest using typical insecticides for lepidopteran pests such as pyrethroids and OPs resulted in control failures and major crop losses. A more concerted approach was followed by three research groups from Greece, Italy and Spain, who worked with a leaf-dip bioassay to monitor baseline susceptibility against a number of useful insecticides (Roditakis et al. 2018). Initial baselines were determined on tomato pinworm populations collected in Greece to key insecticides of different modes of action (Roditakis et al. 2013b), such as diamides, spinosad, emamectin benzoate and indoxacarb.
The susceptibility levels of T. absoluta to the tested insecticides were stable for several years. However, significant chlorantraniliprole resistance levels (> 700-fold) were detected in 2014 in Italian populations. The affected greenhouses suffered from major tomato crop losses due to pest control failure (Roditakis et al. 2015). At that time, low resistance levels to diamides were also reported in Greece already indicating development of diamide resistance. Despite the proactive instructional actions performed by many Greek farmers and agronomists, high resistance levels (resistance ratio (RR) > 600) were detected in Greece in 2015 (Roditakis et al. 2018) and subsequently in Israel (2016), UK (2015–2016; C. Bass, personal communication) and Spain (2018) (Roditakis et al. 2018; Zimmer 2018). Additional notable cases of insecticide resistance include indoxacarb (RR up to 91-fold in 2016/Greece), albeit not widespread and without associated control failure, and spinosad (RR over 480-fold in 2015–2016/UK; C. Bass, personal communication).
The development of diamide resistance led to an increased reliance on alternative modes of action for tomato pinworm management such as indoxacarb and spinosad. Very recently, an increasing trend in resistance levels to registered insecticides has been noticed, indicating a shift towards multiple resistance in field populations of T. absoluta (i.e. simultaneous resistance to different insecticides based on distinct mechanisms) (Roditakis 2018). This trend reinforces the need for resistance monitoring efforts using the established leaf-dip method in different countries and regions (Konuş 2014; Ugurlu Karaağaç 2015; Yalçin et al. 2015; Cherif et al. 2018; Zibaee et al. 2018). Although the number of populations tested so far and the range of insecticides evaluated is relatively limited—considering the present tomato pinworm spread—substantial knowledge on the insecticide resistance status among populations of T. absoluta was gained at a global scale (see below).
Molecular mechanisms of insecticide resistance
Early cases
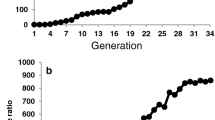
With the invasion of T. absoluta in Europe, the scientific community has seen a considerable increase in publications dealing with insecticide resistance, its mechanisms and management in this pest species (Fig. 1). In general, the most frequent mechanisms of resistance to insecticides involve: (a) increased detoxification by metabolic enzymes such as cytochrome P450s (CYP450), glutathione S-transferases and esterases; (b) target-site mutations by amino acid substitutions/deletions resulting in reduced sensitivity; and of lesser importance (c) altered behavioural responses and (d) reduced penetration (Li et al. 2007; Feyereisen et al. 2015). However, enhanced levels of detoxification enzymes and altered target sites are those mechanisms most commonly found also in T. absoluta against a range of chemical classes of insecticides (summarized in Table 1).
The first cases of resistance and control failure with pyrethroids were observed in South America before the species was introduced into Europe (Salazar and Araya 1997; Siqueira et al. 2000b; Lietti et al. 2005; Silva et al. 2011). Meaning many years before Haddi et al. (2012) could unravel the mechanisms of pyrethroid resistance in this species by showing the presence of target-site mutations in the voltage-gated sodium channel, similarly to those described in other pest species (Table 1; Rinkevich et al. 2013). Cloning of the para-type sodium channel IIS4-IIS6 region from resistant strains revealed three mutations commonly associated with resistance to pyrethroids across a range of insect species (M918T, T929I and L1014). Genotyping of various populations of T. absoluta from countries in South America and Europe revealed the presence of a L1014F mutation at maximum frequency in almost all the populations (Haddi et al. 2012; Silva et al. 2015), suggesting its fixation in most of them. Associated with this mutation, two others greatly enhancing pyrethroid resistance were also present (called super-kdr), and leading to observed field failures of pyrethroid applications.
Pyrethroids may also be metabolized by detoxification enzymes, such as esterases and CYP450s, but such mechanisms appear to be of limited importance in T. absoluta. However, over-expressed CYP450s in combination with target-site mutations can have strong implications for pyrethroid resistance as recently shown in Anopheles gambiae (Vontas et al. 2018). Nonetheless, elevated levels of CYP450s have been suggested to be involved in tomato pinworm resistance against cartap, because their suppression by piperonyl butoxide increased the susceptibility of cartap-resistant strains (Siqueira et al. 2000a). In contrast, only a minor role was suggested for esterases and glutathione S-transferases. CYP450s potentially mediate the demethylation and sulfoxidation of cartap as detoxification mechanism rather than its activation (Lee et al. 2004), which may explain the suppression of cartap resistance by piperonyl butoxide in T. absoluta. A major involvement of CYP450 in abamectin resistance was also suggested earlier, aided by enhanced esterase activity (Siqueira et al. 2001). More recent studies on insecticide resistance in T. absoluta did not yet result in the identification of individual CYP450s driving high levels of metabolic resistance to any chemical class of insecticides.
Organophosphates have been among those insecticides failing to control T. absoluta in South America many years ago, but the mechanisms conferring resistance remained elusive until the invasion of this pest into Europe. Two recent studies on resistance of T. absoluta to organophosphates showed the presence of the mutation A201S in the acetylcholinesterase (ace1) gene (Haddi et al. 2017; Zibaee et al. 2018). The authors concluded that this mutation was already present in the invading population(s) of the tomato pinworm, which is consistent with the organophosphate use and resistance history in South America (Salazar and Araya 1997; Siqueira et al. 2000a; Lietti et al. 2005).
Recent cases
A number of insecticides recently introduced for Lepidoptera control also worked well against leaf-mining species including T. absoluta. Among them, spinosyns (macrocyclic lactones) comprise insecticides derived from the soil bacterium Saccharopolyspora spinosa, represented by spinosad (a natural compound) and the semi-synthetic derivative spinetoram (Crossthwaite et al. 2017). They act by binding to an allosteric site at nicotinic acetylcholine receptors (nAChR), and since the first field-relevant case of spinosad resistance in the beet armyworm, Spodoptera exigua (Hübner) (Moulton et al. 2000), various other failures in different pest species were reported (Sparks et al. 2012).
Resistance to spinosad was first reported in T. absoluta populations from Chile with a potential involvement of CYP450 and esterases as the main mechanisms (Reyes et al. 2012). Two years later, Campos et al. (2014) reported autosomal, recessive and monogenic resistance to spinosad in a population of T. absoluta from Brazil with high cross-resistance to spinetoram. This population did not show resistance to other insecticides tested, and the lack of elevated detoxification enzyme levels and synergism suggested target-site insensitivity as the potential mechanism of spinosad resistance. Ultimately, resistance of T. absoluta to spinosyns was associated with a single mutation G275E in the α6 subunit of the nAChR (Silva et al. 2016c), as described earlier in the western flower thrips (Puinean et al. 2013). However, because the frequency of this mutation in the selected strain was as low as in the parental strain, Silva et al. (2016c) speculated that other mechanisms may contribute to the observed resistance level, thus adding complexity to the initial scenario of monogenic resistance (Campos et al. 2014). Indeed, an exon-skipping event that resulted in the expression of a non-functional α6 subunit of the nAChR in spinosad-resistant strains was later reported (Berger et al. 2016a, b). The authors provided functional electrophysiological evidence that spinosad no longer affects nAChR receptors devoid of the exon sequence, albeit they have not provided direct evidence since they expressed a homo-pentameric nAChR consisting of α7 subunits which are considered the closest vertebrate homologs of insect α6 subunits. Additional target-site mutations described and conferring spinosad resistance in other pests include indels, resulting in premature stop codons leading to loss of function in truncated α6 subunits (Scott 2008).
Diamides were the latest chemical class of insecticides launched to the market with initial registrations for the diamondback moth (Plutella xylostella (L.)) control in the Philippines and Thailand (Nauen 2006). Diamide insecticides bind to insect ryanodine receptors, which are large homotetrameric calcium channels mediating upon activation calcium release from intracellular stores in neuromuscular tissue, leading to muscle contraction (Lümmen 2013). Indeed, the first case of insect resistance to diamide insecticides was reported in P. xylostella from Philippines and Thailand (Troczka et al. 2012), followed by China (Wang and Wu 2012) and several other countries, including India, Japan, Korea, Vietnam and USA (Steinbach et al. 2015). However, soon after diamondback moth, Roditakis et al. (2015) and Silva et al. (2016a) reported the first cases of diamide resistance in greenhouse and field populations of T. absoluta, respectively, and Silva et al. (2019) confirmed altered target-site resistance reported earlier by Roditakis et al. (2017a, b).
Troczka et al. (2012) elucidated the mechanism of resistance to diamides in the diamondback moth and detected a G4946E mutation in the C-terminal transmembrane domain of the ryanodine receptor. This mutation was associated with diamide resistance and evolved independently in Philippine and Thailand populations of P. xylostella. Later on, Steinbach et al. (2015) provided functional evidence by radioligand binding studies that the mutation is indeed conferring resistance to diamides. Other mutations such as an I4790 M described in Chinese strains of diamondback moth (Guo et al. 2014) were close to G4946E as shown by ryanodine receptor homology modelling (Steinbach et al. 2015). Diamide resistance levels found in European and Brazilian strains of T. absoluta were as high as those reported for diamondback moth, thus encouraging the investigation of the possible role of target-site mutations (Table 1). Roditakis et al. (2017b) sequenced respective domains of the ryanodine receptor gene of diamide-resistant T. absoluta and indeed detected two mutations, G4903E and I4746 M, corresponding to the positions already described for P. xylostella. The authors also detected two novel mutations, G4903 V and I4746T, in some of the resistant T. absoluta strains, and radioligand binding studies with thoracic membrane preparations provided functional evidence that these mutations alter the affinity of the Tuta ryanodine receptor to diamides. Nevertheless, the amino acid substitution G4903E/V is considered the most important to define the sensitivity of lepidopteran ryanodine receptors to diamides (Nauen and Steinbach 2016). This perception is supported by recent cellular studies on functionally expressed ryanodine receptor constructs carrying the G4946E mutation (Troczka et al. 2015), and by CRISPR/Cas9 genome-edited fruit flies carrying the G4903 V mutation (Douris et al. 2017).
Indoxacarb and metaflumizone are the major sodium channel blockers in the market and used against T. absoluta. No resistance to metaflumizone has been reported up to date in T. absoluta (Karaagaç 2015; Silva et al. 2016b; Table 1). In contrast, resistance to indoxacarb was reported in T. absoluta (Silva et al. 2011; Roditakis et al. 2013b). Roditakis et al. (2017a) reported two mutations (F1845Y and V1848I) in the voltage-gated sodium channel of T. absoluta associated with resistance to indoxacarb. These mutations were previously reported in P. xylostella (Wang et al. 2016) and impaired both indoxacarb and metaflumizone efficacy. Therefore, these mutations found in T. absoluta may cause cross-resistance to both sodium channel blockers, what still needs to be confirmed.
Insecticide resistance management
Basis
The discovery and development of new insecticides these days are more difficult and costly than ever before. Therefore, strategies delaying the fast development of resistance to new and existing insecticides need to be implemented in all agri- and horticultural settings (Sparks and Nauen 2015). Any insecticide resistance management (IRM) strategy must be proactive, as resistance is likely to develop if no actions are taken to prevent it. The basis of an IRM strategy is composed of two components, one more general aimed to reduce selection pressure, and another one more specifically aimed at avoiding selection of resistance mechanisms (Bielza 2008).
Insecticide resistance is selected by the repeated use of the same compounds of the same modes of action over many generations. Therefore, the first component of an IRM strategy seeks to lower the selection pressure by reducing pest populations and optimizing insecticide use (Bielza 2008). An Integrated Pest Management (IPM) programme calls for alternative management strategies, including cultural control (proper watering and fertilization, sanitation, weed removal, crop rotation and anti-insect nets), behaviourally mediated control (use of pheromone or colour traps), biological control (use of predators, parasitoids and pathogens), and genetic control (host plant resistance) (Desneux et al. 2004; Guedes and Picanço 2012; Biondi et al. 2018). The use of one or more of these alternative strategies may reduce the need for insecticides, thus decreasing the selection pressure on pest population. Moreover, the use of alternate biological control and/or chemical control in different crop periods may reduce insecticide selection pressure. Nonetheless, despite these alternative tools, chemical control remains a primary tool in many situations, and its use must be optimized.
Another more specific component of an IRM strategy focuses on avoiding selection of resistance mechanisms. This component is based on the rotation/alternation of insecticides without cross-resistance. Here, the key is knowing which resistance mechanisms prevail in compromising insecticide efficacy to avoid inadvertent selection for such particular resistance mechanisms. Functionally, the tactic involves avoiding the tank mix or the repeated use of the same insecticide, mode of action or insecticides affected by the same resistance mechanism (cross-resistance) (Bielza 2008). The rotation scheme must consider the length of a pest generation, because it is essential to ensure that successive generations of the pest are not exposed to the same insecticide mode of action or insecticides showing cross-resistance. Within each generation, a repeated application is acceptable (block application), but never among generations. If the resistance mechanisms and cross-resistance patterns are not fully identified, then rotation schemes should use insecticides with different modes of action (MoA). To this end, the MoA Classification Scheme developed by IRAC is a useful tool to select compounds for a rotation scheme (Sparks and Nauen 2015).
Current efforts
Soon after the tomato pinworm introduction to Spain, a proactive IRM strategy was adopted. Stakeholders involved in controlling T. absoluta collaborated with IRAC Spain to develop an IRM strategy based on both components mentioned above: (a) lower selection pressure by reducing pest population and optimizing insecticide use and (b) rotation of insecticides without cross-resistance (IRAC Spain 2009). Firstly, an IPM approach was promoted adopting non-chemical control methods, such as traps, insect-proof netting and biological control (Bielza et al. 2016). In addition, the proper use of insecticides was encouraged (rates, timing, coverage, intervals, thresholds). Secondly, a MoA rotation scheme was designed in Spain, using a window of 30 days based on the pest’s generation time to ensure that consecutive generations are not exposed to the same MoA (Fig. 2). Very importantly, this IRM strategy was disseminated by massive circulation among growers, technicians, cooperatives, distributers, officials, industry, etc., similar to outreach activities in Brazil and Greece (i.e. https://goo.gl/3flIui, https://goo.gl/jXqMCs). Summarizing these experiences, IRAC international provides effective recommendations for sustainable and effective resistance management of T. absoluta (IRAC 2014) (Table 2).
Insecticide treatment windows established based on the respective modes of action (MoA) aiming the management of Tuta absoluta and using the minimum duration of a single generation (30 days). Each colour represents a different mode of action. Multiple applications of the same MoA are possible within a treatment window. When a treatment window is completed, a different MoA should be selected for use in the next 30 days, and if possible, a different MoA should even be applied in a third MoA treatment window. The example shown is based on a suitable situation with four different MoAs available and working equally good against T. absoluta. (Color figure online)
Beyond pinworm resistance
Insecticide resistance refers to individuals and populations of a species, but the consequences of this phenomenon go beyond these hierarchical levels of organization (Guedes et al. 2016, 2017), a fact frequently neglected. Insecticide application, although targeting a single (or few) pest species or populations, necessarily reaches other non-targeted populations and species leading to various consequences. These consequences are discussed below. Another concern is the expression of insecticide-induced hormesis, and induction/cross-induction of detoxification enzymes in the tomato pinworm. However, as neither phenomenon has yet been reported in the tomato pinworm, they will be just briefly addressed since they may favour inadvertent selection for insecticide resistance.
Hormesis, induction and inadvertent selection
Hormesis, or more precisely insecticide-induced hormesis, is a biphasic dose–response phenomenon characterized by a stimulatory effect associated with the exposure to low (sublethal) doses of compounds that are toxic at higher doses (Cutler 2013; Guedes and Cutler 2014; Cutler and Guedes 2017). The concern here is that insecticide-resistant populations also express hormesis, but at higher doses than susceptible populations (Guedes et al. 2010, 2017). Therefore, the sublethal doses inducing hormesis for a resistant population might be as high as the field label rates. This is particularly important when heterozygotes express an almost completely resistant phenotype as described for diamide insecticides in T. absoluta populations carrying a ryanodine receptor target-site mutation (Roditakis et al. 2017b). In such a case, the field rate used of an insecticide not only leads to control failure of the targeted pest species, but will actually favour the population growth of the resistant population.
The (epigenetic) induction and cross-induction of detoxification enzymes is another issue relevant within the context of insecticide resistance. Detoxification enzymes are broadly recognized as important insecticide resistance mechanisms when up-regulated (and over-expressed) in resistant populations (Sparks and Nauen 2015). However, the fact that these detoxification enzymes are inducible should not be neglected, nor the fact that induction may also take place among insecticide-resistant populations exposed to sublethal concentrations of insecticides. This exposure may basically prime the insects against further exposure to the same or other compounds (Bielza et al. 2007). This phenomenon is sometimes referred to as hormetic priming or conditioning and has been detected for esterases and cytochrome P450 monooxygenases (Rix et al. 2004; Cutler and Guedes 2017).
Hormesis and induction of detoxification enzymes may both contribute to decreased insecticide efficacy and potential control failure, albeit not yet described in T. absoluta. However, both phenomena may also contribute to or even shape inadvertent selection for insecticide resistance in targeted and non-targeted species, and tomato crops represent a risk in this respect. A reason for that is the wide coexistence of two important pest species, the tomato pinworm and whiteflies, which usually require frequent insecticide applications for their control. A recent report of whitefly resistance to cartap and chlorantraniliprole in Neotropical America, two insecticides used against the tomato pinworm but not against whiteflies, reinforce this concern (Dângelo et al. 2018). Past use of cartap and the current frequent use of the diamide chlorantraniliprole against the pinworm in the region apparently led to the development of resistance to these compounds in whiteflies, what may compromise the future use of related compounds, like the diamed cyantraniliprole, against the latter (Siqueira et al. 2000a, b; Silva et al. 2016a, b, c; Roditakis et al. 2017b).
Community stress
Insecticide use, and insecticide resistance as one of its main consequences, is important in shaping community context and patterns of community structure (Guedes et al. 2016, 2017). Insect outbreaks, and particularly secondary pest outbreaks, are one of the potential consequences of community stress due to insecticide use and resistance. Curiously, the subject remains largely neglected with focus on natural enemy assemblages, when considered, neglecting even their associated host complex (Martin et al. 2013; Arias-Martín et al. 2016; Guedes et al. 2017). This is unfortunate, because when tomato and the tomato pinworm are considered, the interplay between them and whiteflies, besides the tomato borer Neoleucinodes elegantalis (Guenée) and their natural enemies, do offer a complex scenario whose potential relevance remains unrecognized.
Knowledge gaps and future outlook
The research on insecticide resistance in the tomato pinworm increased considerably after its invasion in Europe (Fig. 2). This fact provides new insights for the management of the species and concerns about the spread of insecticide-resistant genotypes to other regions (Guedes and Siqueira 2012; Haddi et al. 2012; Guillemaud et al. 2015; Biondi et al. 2018). Nonetheless, the knowledge remains patchy and the information available covers a limited geography considering the ongoing global expansion of this species. Furthermore, the temporal and spatial scales of development and spread of insecticide resistance in the tomato pinworm are speculative and would aid in predicting future concerns on newly invaded or non-invaded areas. Last, prime information obtained in the species genetics, population structure, and patterns and mechanisms of insecticide resistance allowed initial establishment of resistance management programs, which require regional adaptation by local experts. However, the consequences of the implementation of such control strategies on other species are largely neglected and remain a knowledge gap relevant to the tomato cropping system, which are worth pursuing. There is good reason to appreciate the level of information produced so far regarding insecticide resistance in the tomato pinworm, but there is even more reason to focus ahead attempting to address several of the largely unexplored points discussed in this review. The tomato pinworm spread and its ongoing increase in importance reinforce the need to address major challenges regarding the spread and control of any invasive pest by a concerted approach among all stakeholders.
Author contributions
All authors participated in the designing and drafting of the manuscript, each focusing on the respective area(s) of expertise. The authors are responsible for the content.
References
Abbes K, Harbi A, Chermiti B (2012) The tomato leafminer Tuta absoluta (Meyrick) in Tunisia: current status and management strategies. EPPO Bull 42:226–233
Arias-Martín M, García M, José M et al (2016) Effects of three-year cultivation of Cry1Ab-expressing Bt maize on soil microarthropod communities. Agric Ecosyst Environ 220:125–134
Balzan MV, Moonen AC (2012) Management strategies for the control of Tuta absoluta (Lepidoptera: Gelechiidae) damage in open-field cultivations of processing tomato in Tuscany (Italy). EPPO Bull 42:217–225
Banks NC, Paini DR, Baylists KL, Hodda M (2015) The role of global trade and transport network topology in the human-mediated dispersal of alien species. Ecol Lett 18:188–199. https://doi.org/10.1111/ele.12397
Barati R, Hejazi MJ, Mohammadi SA (2018) Insecticide susceptibility in Tuta absoluta (Lepidoptera: Gelechiidae) and metabolic characterization of resistance to diazinon. J Econ Entomol 111:1555–1557
Berger M, Piunean AM, Randall E et al (2016a) Insecticide resistance mediated by an exon skipping event. Mol Ecol 25:5692–5704
Berger M, Puinean AM, Bielza P, Jacobson R, Field LM, Bass C, Williamson M (2016) Mechanisms of spinosad resistance in European populations of the tomato leafminer. In: XXV International Congress of Entomology, 25–30 September, Orlando, FL, USA
Bielza P (2008) Insecticide resistance management strategies against the western flower thrips, Frankliniella occidentalis. Pest Manag Sci 64:1131–1138
Bielza P, Espinosa PJ, Quinto V, Abellán J, Contreras J (2007) Synergism studies with binary mixtures of pyrethroid, carbamate and organophosphate insecticides on Frankliniella occidentalis (Pergande). Pest Manag Sci 63:84–89
Bielza P, Garcíaa-Vidal L, Martínez-Aguirre MR (2016) Tuta absoluta—insecticide resistance management of this invasive species. In: XXV international congress of entomology, 25–30 September, Orlando, FL, USA
Biondi A, Desneux N, Siscaro G, Zappala L (2012) Using organic certified rather than synthetic pesticides may not be safer for biological control agents: selectivity and side effects of 14 pesticides on the predator Orius laevigatus. Chemosphere 87:803–812
Biondi A, Zappalà L, Stark JD, Desneux N (2013) Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PLoS ONE 8:e76548
Biondi A, Guedes RNC, Wan F-H, Desneux N (2018) Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: past, present, and future. Annu Rev Entomol 63:239–258
Bradshaw CJA, Leroy B, Bellard C et al (2016) Massive yet grossly underestimated global costs of invasive insects. Nat Commun 7:12986
Brévault T, Sylla S, Diatte M, Bernadas G, Diarra K (2014) Tuta absoluta Meyrick (Lepidoptera: Gelechiidae): a new threat to tomato production in Sub-Saharan Africa. Afr Entomol 22:441–444
Campos MR, Rodrigues ARS, Silva WM et al (2014) Spinosad and the tomato borer Tuta absoluta: a bioinsecticide, an invasive pest threat, and high insecticide resistance. PLoS ONE 9:e103235
Campos MR, Silva TB, Silva WM et al (2015) Susceptibility of Tuta absoluta (Lepidoptera: Gelechiidae) Brazilian populations to ryanodine receptor modulators. Pest Manag Sci 71:537–544
Campos MR, Biondi A, Adiga A, Guedes RN, Desneux N (2017) From the Western Palaearctic region to beyond: Tuta absoluta 10 years after invading Europe. J Pest Sci 90:787–796
Cherif A, Harbaoui K, Zappalà L, Grissa-Lebdi K (2018) Efficacy of mass trapping and insecticides to control T. absoluta in Tunisia. J Plant Dis Prot 125:51–61
Crossthwaite AJ, Bigot A, Camblin P, Goodchild J, Lind RJ, Slater R, Maienfisch P (2017) The invertebrate pharmacology of insecticides acting at nicotinic acetylcholine receptors. J Pestic Sci 42:67–83
Cutler GC (2013) Insects, insecticides and hormesis: evidence and considerations for study. Dose-Response 11:154–177
Cutler GC, Guedes RNC (2017) Occurrence and significance of insecticide-induced hormesis in insects. In: Duke SO, Kudsk P, Solomon K (eds) Pesticide dose: effects on the environment and target and non-target organisms. American Chemical Society, Washington, pp 101–119
Dângelo RAC, Campos MR, Silva PS, Guedes RNC (2018) Insecticide resistance and control failure likelihood of the whitefly Bemisia tabaci (MEAM1; B biotype): a Neotropical. Ann Appl Biol 172:88–99
Desneux N, Wajnberg E, Wyckhuys KAG et al (2010) Biological invasion of European tomato crops by Tuta absoluta: ecology, geographic expansion and prospects for biological control. J Pest Sci 83:197–215
Desneux N, Luna MG, Guillemaud T, Urbaneja A et al (2011) The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: the new threat to tomato world production. J Pest Sci 84:403–408
Douris V, Papapostolou KM, Ilias A, Roditakis E, Kounadi S, Riga M, Nauen E, Vontas J (2017) Investigation of the contribution of RyR target-site mutations in diamide resistance by CRISPR/Cas9 genome modification in Drosophila. Insect Biochem Mol Biol 87:127–135
Feyereisen R, Dermauw W, van Leeuwen T (2015) Genotype to phenotype, the molecular and physiological dimensions of resistance in arthropods. Pest Biochem Physiol 121:61–67
Galdino TVS, Picanço MC, de Morais EGF et al (2011) Metodologia de bioensaio para estudos de toxicidade de formulações comerciais de inseticidas a Tuta absoluta (Meyrick, 1917). Cienc Agrotecnol 35:869–877
Gontijo PC, Picanço MC, Pereira EJG et al (2013) Spatial and temporal variation in the control failure likelihood of the tomato leaf miner, Tuta absoluta. Ann Appl Biol 162:50–59
Guedes RNC (2017) Insecticide resistance, control failure likelihood and the First Law of Geography. Pest Manag Sci 73:479–484
Guedes RNC, Cutler GC (2014) Insecticide-induced hormesis and arthropod pest management. Pest Manag Sci 70:690–697
Guedes RNC, Picanço MC (2012) The tomato borer Tuta absoluta in South America: pest status, management and insecticide resistance. EPPO Bull 42:211–216
Guedes RNC, Siqueira HAA (2012) The tomato borer Tuta absoluta: insecticide resistance and control failure. CAB Rev Perspect Agric Vet Sci Nutr Nat Resour 7:1–7
Guedes NMP, Tolledo J, Corrêa AS, Guedes RNC (2010) Insecticide-induced hormesis in an insecticide-resistant strain of the maize weevil, Sitophilus zeamais. J Appl Entomol 134:142–148
Guedes RNC, Smagghe G, Stark JD, Desneux N (2016) Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu Rev Entomol 61:43–62
Guedes RNC, Walse SS, Throne JE (2017) Sublethal exposure, insecticide resistance, and community stress. Curr Opin Insect Sci 21:47–53
Guillemaud T, Blin A, Legoff I et al (2015) The tomato borer, Tuta absoluta, invading the Mediterranean Basin, originates from a single introduction from Central Chile. Sci Rep 5:8371
Guo L, Liang P, Zhou X, Gao X (2014) Novel mutations and mutation combinations of ryanodine receptor in a chlorantraniliprole resistant population of Plutella xylostella (L.). Sci Rep 4:6924
Haddi K, Berger M, Bielza P, Cifuentes D, Field LM, Gorman K, Rapisarda C, Williamson MS, Bass C (2012) Identification of mutations associated with pyrethroid resistance in the voltage-gated sodium channel of the tomato leaf miner (Tuta absoluta). Insect Biochem Mol Biol 42:506–513
Haddi K, Berger M, Bielza P et al (2017) Mutation in the ace-1 gene of the tomato leaf miner (Tuta absoluta) associated with organophosphates resistance. J Appl Entomol 141:612–619
Han P, Zhang Y-N, Lu Z-Z, Wang S, Ma D-Y, Biondi A et al (2018) Are we ready for the invasion of Tuta absoluta? Unanswered key questions for elaborating an Integrated Pest Management package in Xinjiang, China. Entomol Gen 38:113–125
Han P, Bayram Y, Shaltiel-Harpaz L, Sohrabi F, Saji A, Esenali UT et al (2019) Tuta absoluta continues to disperse in Asia: damage, ongoing management and future challenges. J Pest Sci. https://doi.org/10.1007/s10340-018-1062
Hill MP, Clusella-trullas S, Terblanche JS, Richardson DM (2016) Drivers, impacts, mechanisms and adaptation in insect invasions. Biol Invasions 18:883–891
IRAC (2014) Tuta absoluta—the tomato leafminer or tomato borer. Recommendations for Sustainable and Effective Resistance Management. http://www.irac-online.org/pests/tuta-absoluta/presentations/. Accessed 23 Oct 2018
IRAC Spain (2009) Prevención de resistencias en Tuta absoluta. http://www.irac-online.org/countries/spain/publications/. Accessed 23 Oct 2018
Karaagaç SU (2015) Enzyme activities and analysis of susceptibility levels in Turkish Tuta absoluta populations to chlorantraniliprole and metaflumizone insecticides. Phytoparasitica 43:693–700
Klieber J, Reineke A (2016) The entomopathogen Beauveria bassiana has epiphytic and endophytic activity against the tomato leaf miner Tuta absoluta. J Appl Entomol 140:580–589
Konuş M (2014) Analysing resistance of different T. absoluta (Meyrick) (Lepidoptera: Gelechiidae) strains to abamectin insecticide. Turkish J Biochem 39:291–297
Lee S-J, Caboni P, Tomizawa M, Casida JE (2004) Cartap hydrolysis relative to its action at the insect nicotinic channel. J Agric Food Chem 52:95–98
Li X, Schuler MA, Berenbaum MR (2007) Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Ann Rev Entomol 52:231–253
Liebhold AM, Berec L, Brockerhoff EG et al (2016) Eradication of invading insect populations: from concepts to applications. Annu Rev Entomol 61:335–352
Lietti MMM, Botto E, Alzogaray RA (2005) Insecticide resistance in argentine populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Neotrop Entomol 34:113–119
Lockwood JL, Hoopes MF, Marchetti MP (2013) Invasion ecology, 2nd edn. Wiley, New York
Lümmen P (2013) Calcium channels as molecular target sites of novel insecticides. Adv Insect Physiol 44:287–347
Mansour R, Brévault T, Chailleux A et al (2018) Occurrence, biology, natural enemies and management of Tuta absoluta in Africa. Entomol Gen 38:83–112
Martin EA, Reineking B, Seo B, Steffan-dewenter I (2013) Natural enemy interactions constrain pest control in complex agricultural landscapes. Proc Natl Acad Sci USA 110:5534–5539
Moulton JK, Pepper DA, Dennehy TJ (2000) Beet armyworm (Spodoptera exigua) resistance to spinosad. Pest Manag Sci 56:842–848
Nauen R (2006) Insecticide mode of action: return of the ryanodine receptor. Pest Manag Sci 62:690–692
Nauen R, Steinbach D (2016) Resistance to diamide insecticides in lepidopteran pests. In: Horowitz AR, Ishaaya I (eds) Advances in insect control and in resistance management. Springer, Dordrecht, pp 219–240
Pfeiffer DG, Muniappan R, Sall D et al (2013) First record of Tuta absoluta (Lepidoptera: Gelechiidae) in Senegal. Fla Entomol 96:661–662
Potting RP, Van Der Gaag DJ, Loomans A et al (2013) Pest risk analysis—Tuta absoluta, tomato leaf miner moth. Ministry of Agriculture, Nature and Food Quality. Plant Protection Service of the Netherlands, Utrecht
Puinean AM, Lansdell SJ, Collins T et al (2013) A nicotinic acetylcholine receptor transmembrane point mutation (G275E) associated with resistance to spinosad in Frankliniella occidentalis. J Neurochem 124:590–601
Reyes M, Rocha K, Alarcón L et al (2012) Metabolic mechanisms involved in the resistance of field populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) to spinosad. Pestic Biochem Physiol 102:45–50
Rinkevich FD, Du Y, Dong K (2013) Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids. Pestic Biochem Physiol 106:93–100
Ripa SR, Rojas PS, Velasco G (1995) Releases of biological control agents of insect pests on Easter Island (Pacific Ocean). Entomophaga 40:427–440
Rix RR, Ayyanath MM (2004) Cutler GC (2016) Sublethal concentrations of imidacloprid increase reproduction, alter expression of detoxification genes, and prime Myzus persicae for subsequent stress. J Pest Sci 89:581–589
Roditakis E (2018) Insecticide mode of action and resistance management: lessons learned from the case of T. absoluta. In XI European Congress of Entomology, pp 56–57, Napoli 2–6 July
Roditakis E, Skarmoutsou C, Staurakaki M (2013a) Toxicity of insecticides to populations of tomato borer Tuta absoluta (Meyrick) from Greece. Pest Manag Sci 69:834–840
Roditakis E, Skarmoutsou C, Staurakaki M et al (2013b) Determination of baseline susceptibility of European populations of Tuta absoluta (Meyrick) to indoxacarb and chlorantraniliprole using a novel dip bioassay method. Pest Manag Sci 69:217–227
Roditakis E, Vasakis E, Grispou M et al (2015) First report of T. absoluta resistance to diamide insecticides. J Pest Sci 88:9–16
Roditakis E, Mavridis K, Riga M, Vasakis E, Morou E, Rison JL, Vontas J (2017a) Identification and detection of indoxacarb resistance mutations in the para sodium channel of the tomato leafminer, T. absoluta. Pest Manag Sci 73:1679–1688
Roditakis E, Steinbach D, Moritz G et al (2017b) Ryanodine receptor point mutations confer diamide insecticide resistance in tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). Insect Biochem Mol Biol 80:11–20
Roditakis E, Vasakis E, García-Vidal L, del Rosario Martínez-Aguirre M, Rison JL, Haxaire-Lutun MO, Nauen R, Tsagkarakou A, Bielza P (2018) A four-year survey on insecticide resistance and likelihood of chemical control failure for tomato leaf miner T. absoluta in the European/Asian region. J Pest Sci 91:421–435
Salazar ER, Araya JE (1997) Deteccin de resistencia a insecticides en la polilla del tomate. Simiente 67:8–22
Salazar ER, Araya JE (2001) Tomato moth, Tuta absoluta (Meyrick) response to insecticides in Arica, Chile. Agric Técnica 61:1–7
Sankarganesh E, Firake DM, Sharma B et al (2017) Invasion of the South American Tomato Pinworm, Tuta absoluta, in northeastern India: a new challenge and biosecurity concerns. Entomol Gen 1:335–345
Santana PA Jr, Kumar L, Da Silva RS, Picanço MC (2019) Global geographic distribution of Tuta absoluta as affected by climate change. J Pest Sci. https://doi.org/10.1007/s10340-018-1057-y
Scott JG (2008) Unraveling the mystery of spinosad resistance in insects. J Pestic Sci 33:221–227
Sharma PL, Gavkare O (2017) New distributional record of invasive pest Tuta absoluta (Meyrick) in North-Western Himalayan Region of India. Natl Acad Sci Lett 40:217–220
Silva GA, Picanço MC, Bacci L et al (2011) Control failure likelihood and spatial dependence of insecticide resistance in the tomato pinworm, Tuta absoluta. Pest Manag Sci 67:913–920
Silva WM, Berger M, Bass C et al (2015) Status of pyrethroid resistance and mechanisms in Brazilian populations of Tuta absoluta. Pestic Biochem Physiol 122:8–14
Silva JE, Assis CPO, Ribeiro LMS, Siqueira HAA (2016a) Field-evolved resistance and cross-resistance of Brazilian Tuta absoluta (Lepidoptera: Gelechiidae) populations to diamide insecticides. J Econ Entomol 109:2190–2195
Silva TBM, Silva WM, Campos MR et al (2016b) Susceptibility levels of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) to minor classes of insecticides in Brazil. Crop Prot 79:80–86
Silva WM, Berger M, Bass C et al (2016c) Mutation (G275E) of the nicotinic acetylcholine receptor α6 subunit is associated with high levels of resistance to spinosyns in Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Pestic Biochem Physiol 131:1–8
Silva JE, Ribeiro LMS, Vinasco N et al (2019) Field-evolved resistance to chlorantraniliprole in the tomato pinworm Tuta absoluta: inheritance, cross-resistance profile, and metabolism. J Pest Sci. https://doi.org/10.1007/s10340-018-1064-z
Siqueira HAA, Guedes RNC, Picanço MC (2000a) Insecticide resistance in populations of Tuta absoluta (Lepidoptera: Gelechiidae). Agric For Entomol 2:147–153
Siqueira HAA, Guedes RNC, Picanço MC (2000b) Cartap resistance and synergism in populations of Tuta absoluta (Lep., Gelechiidae). J Appl Entomol 124:233–238
Siqueira HAA, Guedes RNC, Fragoso DB, Magalhaes LC (2001) Abamectin resistance and synergism in Brazilian populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Int J Pest Manag 47:247–251
Soares MA, Campos MR, Passos LC, Carvalho GA (2019) Botanical insecticide and natural enemies: a potential combination for pest management against Tuta absoluta. J Pest Sci. https://doi.org/10.1007/s10340-018-01074-5
Soliman T, Mourits MCM, Lansink AGJMO, Van Der Werf W (2015) Quantitative economic impact assessment of invasive plant pests: what does it require and when is it worth the effort? Crop Prot 69:9–17
Sparks TC, Nauen R (2015) IRAC: mode of action classification and insecticide resistance management. Pestic Biochem Physiol 121:122–128
Sparks TC, Dripps JE, Watson GB, Paroonagian D (2012) Resistance and cross-resistance to the spinosyns—a review and analysis. Pestic Biochem Physiol 102:1–10
Steinbach D, Gutbrod O, Lümmen P et al (2015) Geographic spread, genetics and functional characteristics of ryanodine receptor based target-site resistance to diamide insecticides in diamondback moth, Plutella xylostella. Insect Biochem Mol Biol 63:14–22
Sylla S, Brevault T, Bal AB, Chailleux A, Diatte M, Desneux N et al (2017) Rapid spread of the tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae), an invasive pest in Sub-Saharan Africa. Entomol Gen 36:269–283
Tonnang HEZ, Mohamed SF, Khamis F, Ekesi S (2015) Identification and risk assessment for worldwide invasion and spread of Tuta absoluta with a focus on Sub-Saharan Africa: implications for phytosanitary measures and management. PLoS ONE 10:e0135283
Troczka B, Zimmer CT, Elias J et al (2012) Resistance to diamide insecticides in diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) is associated with a mutation in the membrane-spanning domain of the ryanodine receptor. Insect Biochem Mol Biol 42:873–880
Troczka BJ, Williams AJ, Williamson MS et al (2015) Stable expression and functional characterisation of the diamondback moth ryanodine receptor G4946E variant conferring resistance to diamide insecticides. Sci Rep 5:14680
Ugurlu Karaağaç S (2015) Enzyme activities and analysis of susceptibility levels in Turkish T. absoluta populations to chlorantraniliprole and metaflumizone insecticides. Phytoparasitica 43:693–700
Urbaneja A, Vercher R, Navarro V et al (2007) La polilla del tomate, Tuta absoluta. Phytoma Espana 194:16–23
Visser D, Uys VM, Nieuwenhuis RJ, Pieterse W (2017) First records of the tomato leaf miner Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae) in South Africa. Bio Invasions Rec 6:301–305
Vontas J et al (2018) Rapid selection of a pyrethroid metabolic enzyme CYP9K1 by operational malaria control activities. Proc Natl Acad Sci 115:4619–4624
Wang X, Wu Y (2012) High levels of resistance to chlorantraniliprole evolved in field populations of Plutella xylostella. J Econ Entomol 105:1019–1023
Wang X-L, Su W, Zhang J-H et al (2016) Two novel sodium channel mutations associated with resistance to indoxacarb and metaflumizone in the diamondback moth, Plutella xylostella. Insect Sci 23:50–58
Yalçin M, Mermer S, Kozaci LD, Turgut C (2015) Insecticide resistance in two populations of T. absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae) from Turkey. Turkiye Entomoloji Dergisi 39:137–145
Zibaee I, Mahmood K, Esmaeily M et al (2018) Organophosphate and pyrethroid resistances in the tomato leaf miner T. absoluta (Lepidoptera: Gelechiidae) from Iran. J Appl Entomol 142:181–191
Zimmer CT (2018) Characterization and monitoring of target-site resistance in the Ryanodine Receptor of the tomato leafminer, T. absoluta. In: XI European Congress of Entomology, pp 54–55, Napoli 2–6 July
Acknowledgements
We thank Drs. A. Biondi and N. Desneux for the invitation to prepare the present review and to the several funding agencies that have been providing financial support for the authors’ research on insecticide resistance in the tomato pinworm.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and institutional guidelines for the care and use of animals were considered in the present study.
Informed consent
The authors of this manuscript accept that the paper is submitted for publication in the Journal of Pest Science, and report that this paper has not been published or accepted for publication in another journal, nor is under consideration at another journal.
Additional information
Communicated by A. Biondi and N. Desneux.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special Issue on Advances in the Management of Tuta absoluta Review article.
Rights and permissions
About this article
Cite this article
Guedes, R.N.C., Roditakis, E., Campos, M.R. et al. Insecticide resistance in the tomato pinworm Tuta absoluta: patterns, spread, mechanisms, management and outlook. J Pest Sci 92, 1329–1342 (2019). https://doi.org/10.1007/s10340-019-01086-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-019-01086-9