Abstract
As an indirect defense to herbivore attack, plants release many types of volatile organic compounds (VOCs), which guide parasitoids to their herbivore hosts. In the present study, VOCs were categorized as those released passively from undamaged plants and herbivore-induced plant volatiles (HIPVs). HIPVs were further categorized into: (1) volatiles released by fresh damage plants, and (2) volatiles released by old damage plants. We used as models, two parasitoids with different degree of host specificity, Microplitis croceipes (specialist) and Cotesia marginiventris (generalist), to address the evolutionary and mechanistic question of whether specialist and generalist parasitoids differ in their use of VOCs for host location. Both species are solitary larval endoparasitoids in the same family (Hymenoptera: Braconidae) and are important parasitoids of caterpillar pests of cotton. Based on the results of previous studies, α-pinene, (Z)-3-hexenol, and (Z)-3-hexenyl acetate were selected as representatives of the different types of VOCs in cotton. The attraction of both parasitoid species to synthetic components and a binary mixture of the above VOCs was tested in four-choice olfactometer bioassays. Female M. croceipes showed the greatest attraction to the HIPVs while female C. marginiventris could not discriminate among the three VOCs. Conspecific males showed similar responses with a few exceptions. When presented with the choices; α-pinene, (Z)-3-hexenol and a binary mixture (50:50v/v) of the two compounds, the specialist showed the greatest attraction to the mixture. However, the mixture did not elicit such an additive effect on the attraction of the generalist. Overall response latency (time taken to choose VOCs) indicated species and sexual (in the specialist) differences. Using a simple model, this study provides a fundamental insight into odor preferences and discriminatory ability of the test parasitoids. The ecological significance and practical implications of these results are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural enemies such as parasitoids; herbivore insects and their host plants interact in a complex tritrophic system in which herbivore infested plants release volatile organic compounds (VOCs) that can attract parasitoids. Host-induced plant volatiles (HIPVs) are released by plants in response to herbivore infestation and may be used for host location by natural enemies such as parasitoids (Turlings et al. 1990; De Moraes et al. 1998; Pare and Tumlinson 1999; Mumm and Hilker 2005; Wei and Kang 2006; Ngumbi and Fadamiro 2012). Plants may release constitutive volatiles or synthesize new ones as an induced response to attack (mechanical/herbivore damage) (Alborn et al. 1997; Pare and Tumlinson 1997; Boland et al. 1998; Rose and Tumlinson 2004). Only certain components of natural volatile blends are attractive or ecologically relevant to parasitic wasps, making the identification of specific VOCs that inform parasitoid behaviors a critical task (D’Alessandro and Turlings 2005; Hoballah and Turlings 2005; Schnee et al. 2006; van Dam et al. 2010). Therefore, parasitoids must fine tune their olfactory system to discriminate among several odors to exploit certain VOCs for host location. The degree of host specificity required may determine to what extent a parasitoid species may have to discriminate among plant VOCs. Previous studies have demonstrated the attraction of some parasitoid species to components of plant VOCs both in the laboratory (Wei et al. 2007) and in the field (James and Grasswitz 2005). Others have reported the positive role of synthetic VOCs in recruiting natural enemies for plant defense (Thaler 2002; James and Price 2004). Indeed, most olfactory receptor neurons in insects only respond to one or very few chemical compounds (Kaissling 1986; Meiners et al. 2002; De Bruyne and Baker 2008).
However, natural odors from plants are rarely emitted as single compounds (Bargmann 2006). VOCs that are not attractive to a parasitoid species may still contribute to the olfactory contrast that enhances attraction to other VOCs of interest in the mixture/blend (D’Alessandro et al. 2009). Thus, a mixture of plant VOCs may be more attractive than a single compound because it presents an odor context more similar to what obtains in nature (van Wijk et al. 2011). It is believed that the differences in various VOC blends may serve as important host recognition codes for natural enemies (De Moraes et al. 1998; Smith 1998; De Bruyne and Baker 2008). At the simplest level, the effect of natural plant volatile blends on the attraction of parasitoids can be demonstrated with binary mixtures of synthetic VOCs.
Parasitic wasps have been considered good models for insect olfaction studies (Meiners et al. 2002; Rains et al. 2004; Harris et al. 2012). Based on their relative host range, they can be broadly categorized as specialist or generalist. The question of whether the degree of host specificity affects odor discriminatory ability in parasitoids is yet to be fully answered. This question has serious ecological and evolutionary significance as it concerns the fitness of the two groups of parasitoids. In this study, the specialist parasitoid, Microplitis croceipes (Cresson) and the generalist parasitoid, Cotesia marginiventris (Cresson) were used as models to test the hypothesis that specialist and generalist parasitoids differ in their use of VOCs for host location. Both wasps are koinobiont, solitary larval endoparasitoids (Hymenoptera: Braconidae) of Heliothis virescens (Fab.) (Lepidoptera: Noctuidae), an important pest of cotton. Microplitis croceipes and C. marginiventris, have been used in many behavioral olfactometer bioassays to study parasitoid attraction to plant VOCs (Navasero and Elzen 1989; Meiners et al. 2002; Olson et al. 2003; Turlings et al. 2004; Sobhy et al. 2012; Ngumbi and Fadamiro 2012).
In the present study, VOCs were categorized as those released passively from undamaged plants and herbivore-induced plant volatiles (HIPVs). HIPVs were further categorized into: (1) volatiles released by fresh damage plants, and (2) volatiles released by old damage plants. In making the selection of test VOCs, results from previous studies (Loughrin et al. 1994; Mc Call et al. 1994; Rose et al. 1996, 1998; De Moraes et al. 1998; Rose and Tumlinson 2004; Ngumbi et al. 2009; Magalhães et al. 2012) that have collected, identified and quantified VOCs from cotton headspace were considered. α-Pinene (undamaged plant volatile), (Z)-3-hexenol (fresh damage plant volatile), and (Z)-3-hexenyl acetate (old damage plant volatile) were selected as representatives of broader categorizations of plant volatiles. α-Pinene is a monoterpene that is constitutively released by cotton but plant induction via herbivory results in higher emissions (Loughrin et al. 1994). (Z)-3-hexenol is generally considered host induced in cotton. Like many green leaf volatiles (GLVs), this VOC is usually released by cotton starting during the early stages (2–6 h) of herbivore damage (Mc Call et al. 1994; Penaflor et al. 2011). (Z)-3-hexenyl acetate is also induced by herbivore damage in cotton. Mc Call et al. (1994) reported that (Z)-3-hexenyl acetate was the only GLV that was significantly detected in cotton during the late stages (16–24 h) of host infestation. α-Pinene (Lozano et al. 2000; Ozawa et al. 2008), (Z)-3-hexenol (Wei et al. 2007; Ngumbi and Fadamiro 2012) and (Z)-3-hexenyl acetate (Ozawa et al. 2008; Yu et al. 2010; Uefune et al. 2013) have been associated with the attraction of parasitoids.
In this study, parasitoid attraction to select synthetic VOCs and a binary mixture of cotton volatiles was tested. Based on previous studies from our group (Chen and Fadamiro 2007; Ngumbi et al. 2009, 2010, 2012), it is hypothesized that the two parasitoid species will discriminate among single VOCs to varying extent, and that binary mixtures will generally be more attractive than single VOCs. In addition to testing parasitoid attraction, the time taken to choose different VOCs (response latency) was also recorded in this study. The concept of behavioral response latency to semiochemicals in insects has only been investigated in a few studies (Baker and Vogt 1988; Ngumbi et al. 2012). The ecological significance and practical implications of the results are discussed.
Materials and methods
Insects
M. croceipes and C. marginiventris were reared in our laboratory (Auburn University AL, USA) on H. virescens larvae. The rearing procedures were similar to those described by Lewis and Burton (1970) and Ngumbi et al. (2009). Upon emergence, adult wasps were transferred to aerated plastic cages (~30 × 30 × 30 cm) and supplied with 10 % sugar water. For parasitization, female wasps (2–5 days old) were supplied with 2nd–3rd instar larvae (caterpillars) of H. virescens in the ratio 1 female to 20 larvae. Mated, naïve (untrained) parasitoids (aged 2–5 days old) were used in the behavioral bioassays to test innate responses of parasitoids to plant volatiles. Most behavioral studies have utilized mated parasitoids because changes in the physiological state of mated females presumably increase the probability of host searching behavior (Chen and Fadamiro 2007). Larvae of H. virescens were reared on pinto bean artificial diet (Shorey and Hale 1965). The general rearing conditions for all insects were 25 ± 1 °C, 75 ± 5 % RH and 14:10 h (L:D) photoperiod.
Four-choice olfactometer
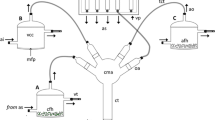
The set-up of the four-choice olfactometer used for behavioral bioassays is as shown in Fig. 1. Consideration for the new design was partly due to studies by Turlings et al. (2004) and Ngumbi and Fadamiro (2012). The olfactometer used was made of glass and supported with a retort stand. The main piece has a spherical bulb 75 mm diameter from which four horizontally inclined arms 10 cm long projected upwards. At the base of the bulb, a central tube 17 cm long extends downwards to form the entry route for insects. A 30 mm diameter hemispherical depression on top of the bulb (decision-making area) created a vantage position from which insects were evenly exposed to odor streams from all four arms. The VOCs tested were placed on filter paper strips (odor source) and inserted into the small connector tubes from which insects were physically excluded to avoid contamination. A white light bulb (20 W, 250 lux) hung about 40 cm above the olfactometer provided illumination. The entire set-up was placed in a white box (80 cm × 60 cm × 60 cm) to minimize visual distraction. An air delivery system (Analytical Research Systems, Gainesville, FL) passed humidified and purified air through Teflon ® tubes into the olfactometer arms.
Behavioral bioassays
Humidified and purified (charcoal filtered) air was passed into each of the olfactometer arms at 200 ml/min while the vacuum pump was set at 800 ml/min to avoid a mix-up of volatiles in the chamber. The synthetic VOCs used (purity 95–99 %) were purchased from Sigma® Chemical Co. (St. Louis, Missouri). The compounds were formulated in hexane (HPLC-grade) at 1 μg/μl concentration and delivered as 10 μl samples (10 μg dose) on Whatman No. 1 filter paper strips (25 × 7 mm). This dose was selected based on the results of a preliminary experiment and previous studies by our group (Ngumbi and Fadamiro 2012). The solvent was allowed to evaporate from the filter paper for about 10 s before insertion into the olfactometer arm.
In the first experiment, each sex of the specialist, M. croceipes and the generalist, C. marginiventris was presented with α-pinene, (Z)-3-hexenol, (Z)-3-hexenyl acetate, and hexane (control) in separate tests. α-Pinene elicited the greatest attraction in the generalist while (Z)-3-hexenol elicited the greatest attraction in the specialist. Consequently, a second experiment was set up in which the parasitoids were presented with four choices: α-pinene, (Z)-3-hexenol, a binary mixture of the both compounds, and hexane (control). The binary mixture tested was made by mixing equal volumes (50:50 v/v) of α-pinene and (Z)-3-hexenol at the same concentration (1 μg/μl). Individual insects were tested for odor preference and response latency. Response latency was defined as the duration from the time of insect release to the time insect crosses into the extension tube of an arm. After testing four insects, the odor sources were replaced and the olfactometer was rotated 90° to avoid any error due to position effect. The olfactometer arms were labeled so that the same compound was maintained in each arm after replacements. The entire set-up was cleaned (with acetone) after testing 20 insects. Wasps were used only once and discarded. A wasp that did not make a choice after 15 min of exposure was recorded as ‘No choice’ and not included in the data analysis (<10 % in all experiments). A parasitoid was recorded to have made a clear choice for the odor offered through an arm when it enters into the extension tube and remains there for at least 15 s. Bioassays of different sexes and species were carried out in a randomized block design on different days between 0900 h and 1700 h.
Data analyses
Attraction of parasitoids to each VOC was modeled as a binary response count and treatments were compared using Logistic Regression Analysis. The model adequacy for each set of experiment was confirmed with a Likelihood Ratio (Wajnberg and Haccou 2008). Slopes were separated using Proc Logistic Contrast in SAS. For data presentation, parasitoid attraction to VOCs was represented on charts as percentages of total wasps that responded due to varying sample sizes. Sexual difference in overall response latency was analyzed using two-sided Wilcoxon–Mann–Whitney test. All analyses were performed using SAS 9.2 with 0.05 level of significance.
Results
Attraction to Single VOCs
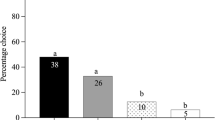
Female M. croceipes (specialist) were significantly (χ 2 = 18.17; P < 0.0004; N = 59) more attracted to the two HIPVs: (Z)-3-hexenol and (Z)-3-hexenyl acetate, than to α-pinene and hexane (control) (Fig. 2a). Males were also significantly (χ 2 = 10.97; P < 0.01; N = 49) more attracted to (Z)-3-hexenol than to the other treatments (Fig. 2b). Both sexes of C. marginiventris (generalist) could not significantly discriminate among the three VOCs (Fig. 3). These results suggest that the specialist parasitoid showed greater attraction to herbivore-induced VOCs, whereas the generalist did not show preference among the VOCs.
Attraction of M. croceipes to different types of VOCs: females (a), and males (b). Values (%) having no letter in common are significantly different (P < 0.05; Proc. Logistic Regression). Attraction to VOCs was modeled as binary response counts and represented on the chart as percentage of total responding wasps
Attraction of C. marginiventris to different types of VOCs: females (a), and males (b). Values (%) having no letter in common are significantly different (P < 0.05; Proc. Logistic Regression). Attraction to VOCs was modeled as binary response counts and represented on the chart as percentage of total responding wasps
Effect of binary VOC mixture
When females of M. croceipes were presented with a choice of α-pinene, (Z)-3-hexenol, and a mixture (50:50v/v) of both compounds, the mixture elicited the highest attraction (40 % of wasps) (χ 2 = 6.31; P < 0.01; N = 80) (Fig. 4a). Similarly, conspecific males showed a significantly (χ 2 = 8.99; P < 0.0027; N = 85) greater attraction to the mixture, compared to the single VOCs (Fig. 4b). In contrast, female C. marginiventris showed no preference among the three treatments (Fig. 5a), while males showed the greatest attraction to α-pinene (Fig. 5b).
Attraction of M. croceipes to single VOCs and a binary mixture: females (a), and males (b). Values (%) having no letter in common are significantly different (P < 0.05; Proc. Logistic Regression). Attraction to VOCs was modeled as binary response counts and represented on the chart as percentage of total responding wasps
Attraction of C. marginiventris to single VOCs and a binary mixture: females (a), and males (b). Values (%) having no letter in common are significantly different (P < 0.05; Proc. Logistic Regression). Attraction to VOCs was modeled as binary response counts and represented on the chart as percentage of total responding wasps
Response latency to single VOCs
Overall, a significantly shorter response latency (Z = 5.91; P < 0.0001; N = 108) was recorded for males (68.1 s) than for females (128.6 s) of M. croceipes (Fig. 6a). No significant sexual difference in overall response latency was recorded for C. marginiventris (Fig. 6b). Comparing the species, mean response time was significantly (Z = 2.48; P < 0.01; N = 116) shorter for female M. croceipes (128.6 s) compared to female C. marginiventris (231.2 s).
Discussion
In the present study, the attraction of M. croceipes (specialist) to (Z)-3-hexenol and (Z)-3-hexenyl acetate (both HIPVs) was consistent with the findings of van Poecke et al. (2003), Penaflor et al. (2011), and Ngumbi and Fadamiro (2012), which showed that specialist parasitoids were more attracted to induced plant volatiles than to constitutive volatiles. Arguably, there is a greater chance that HIPVs will provide more specific host recognition cues than constitutive plant volatiles. On the other hand, C. marginiventris (generalist) showed no preference among the tested constitutive plant volatile (α-pinene) and the two HIPVs [(Z)-3-hexenol and (Z)-3-hexenyl acetate]. The results are in support of the findings of Fontana et al. (2011) in which C. marginiventris was attracted to constitutive volatiles of maize. Although constitutively released in cotton, α-pinene is also released in higher amounts during early stages of herbivore damage (Loughrin et al. 1994). Ozawa et al. (2008) and Uefune et al. (2012, 2013) have also reported the attraction of other parasitoids in the genus Cotesia to α-pinene.
In previous electroantennogram studies by our group (Ngumbi et al. 2009, 2010), M. croceipes showed greater responses to HIPVs while C. marginiventris showed greater responses to GLVs. Furthermore, Ngumbi and Fadamiro (2012) conducted Y-tube olfactometer bioassays to test the attraction of the two parasitoid species to various VOCs using a series of two-choice tests. Since parasitoids are exposed to a wide array of odors in nature, the present study builds on the previous studies by testing preferential attraction of the parasitoids to synthetic VOCs and mixture in multiple-choice tests. In four-choice olfactometer bioassays, three VOCs representing undamaged, fresh damage, and old damage cotton volatiles were tested. Schroder and Hilker (2008) suggested that attraction of insects to specific odors used to locate resources may become enhanced due to the presence of other less attractive odors. Thus, M. croceipes showed significantly greater attraction to (Z)-3-hexenol in the present study, compared to the previous study (Ngumbi and Fadamiro 2012) probably because the parasitoids experienced the VOC in a more appropriate context. The importance of odor context was previously discussed by Mumm and Hilker (2005) and van Wijk et al. (2011).
Comparing species, M. croceipes females were significantly more attracted to (Z)-3-hexenol than C. marginiventris females, suggesting that the specialist may depend more on induced volatiles for host location. More importantly, the specialist was able to discriminate HIPVs from constitutive VOC of cotton while the generalist could not, possibly indicating a more specialized olfactory mechanism. In contrast, C. marginiventris females were significantly more attracted to α-pinene than M. croceipes females, suggesting the likelihood of the generalist to frequent plants more. From an ecological perspective, parasitoids that show greater attraction to undamaged plant volatiles may be recruited early, possibly making the first contact with caterpillar hosts on the plant. On the other hand, parasitoids that show greater attraction to HIPVs may arrive much later. Conceivably, the timing of parasitoid recruitment largely dependent on the relative attractiveness of plant VOCs may determine the outcome of interspecific competitions (see De Moraes and Mescher 2005).
A narrowly tuned olfactory mechanism has the advantage of saving valuable energy resources while searching for specific hosts. However, when extrinsic interspecific competition exists, a broadly tuned olfactory mechanism may present an ecological edge. The results corroborate the prediction of previous studies (Smid et al. 2002; Chen and Fadamiro 2007; Ngumbi et al. 2009, 2010, 2012) that the degree of host specificity in parasitoids may affect their use of various plant volatiles for host location. Generally, similar trends were recorded for conspecific males (as their females), suggesting that male parasitoids may be able to exploit certain VOCs as cues to enhance mate location (Chen and Fadamiro 2007; Ngumbi and Fadamiro 2012).
In the bioassays with M. croceipes, the mixture of α-pinene and (Z)-3-hexenol elicited a greater attraction than either compound—an additive effect that was not recorded in bioassays with C. marginiventris. There are two general models that may explain how an animal’s olfactory system processes odor mixtures, leading to behavioral responses: the elemental and the configural models (Erickson et al. 1990; Alvarado and Rudy 1992; Kay et al. 2005). A classic review of the central processing of odor blends in insects was provided by Lei and Vickers (2008). In the simplest terms, the elemental model holds that responses to odor mixtures resemble that of individual components while the configural model holds that odor blends present an entirely new identity and they elicit responses that are different from those of individual components. In this study, the binary mixture used has highly dissimilar components [α-pinene and (Z)-3-hexenol]. The components differ in chemical class, pathway of production (terpenoid and lipoxygenase pathways), and the timing of release by plants. Linster and Cleland (2004) explained that the more dissimilar the components of an odor mixture, the less overlap the signals generated, and the more the response to the mixture becomes a linear summation of the responses to both components (elemental processing). Thus, the greater attraction elicited by the mixture suggests an elemental processing of the binary mixture in the specialist. However, the mixture did not elicit an additive effect in the attraction of the generalist. A possible explanation is that the generalist could not discriminate among the component VOCs of the mixture in the initial bioassays with single compounds. Conceivably, the less apparent the difference in the components, the less likely it is for the odor mixture to elicit an additive effect (Linster and Cleland 2004). It should be noted that the above is considered a possible explanation of the present results from the perspective of neural processing, and that other factors may influence insect behavior. Another plausible explanation is that the specialist may have evolved an olfactory mechanism that is more tuned to VOC mixtures than to single components, as would be expected in nature.
There was no correlation between response latency and attraction of parasitoids to each VOC, suggesting that response latency to VOCs may be more related to a species’ olfactory architecture rather than to functional behavioral responses. Furthermore, Ngumbi et al. (2012) reported no significant differences in the response latencies of trained versus untrained M. croceipes and C. marginiventris to various host-related plant volatiles, indicating that response latency may be innate in these parasitoids. In the present study, M. croceipes generally made choices faster than C. marginiventris in the olfactometer, and male M. croceipes made choices faster than conspecific females, similar to the report of Ngumbi et al. (2012). Interestingly, Das et al. (2011) reported that sensilla placodea (olfactory sensilla) were significantly more abundant on the antennae of M. croceipes than in C. marginiventris, and that the same sensilla were significantly more abundant in male M. croceipes than in conspecific females. This trend corresponds to the response latency of the parasitoids presently reported, suggesting a possible relationship between the abundance of olfactory sensilla and the response latency of these parasitoids. We are not aware of any previous study relating this morphological characteristic to behavioral responses of parasitoids to host-related odors. It has been shown that adult parasitoids have limited ability to synthesize lipids. Thus, a reduced activity rate in some female parasitoids has been linked to energy conservation (Denis et al. 2013). Further studies with other parasitoids are needed to establish if host specificity affects the response latency of parasitoids to host-related plant volatiles.
In summary, results of the present study showed that key differences exist in the responses (attraction and response latency) of M. croceipes and C. marginiventris to select synthetic VOCs and mixture. The degree of host specificity is believed to be one of the key factors affecting parasitoids’ odor discriminatory ability as well as the use of various VOCs for host location (Smid et al. 2002; Chen and Fadamiro 2007; Ngumbi et al. 2009, 2010, 2012). Since parasitoids are often exposed to blends rather than single VOCs emitted by herbivore-damaged plants (van Wijk et al. 2011), it is reasonable to gain fundamental knowledge of how parasitoids respond to odor mixtures containing attractive VOCs that signal resource location. In the present study, a simple binary mixture was tested so as to gain a fundamental understanding of more complex ratio-specific odor recognition in parasitoids (see Bruce et al. 2009; Uefune et al. 2013). Further behavioral studies comparing the attraction of M. croceipes and C. marginiventris to more complex VOC blends are needed, as reported for C. vestalis by Uefune et al. (2013). In addition, further studies investigating the attraction of various parasitoids to plant VOCs based on other differences in life strategy are needed. These studies are expected to yield results that could inform the identification of attractive VOCs and mixtures that may enhance the performance of the parasitoids as biocontrol agents.
References
Alborn T, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH (1997) An elicitor of plant volatiles from beet armyworm oral secretion. Science 276:945–949
Alvarado MC, Rudy JW (1992) Some properties of configural learning—an investigation of the transverse-patterning problem. J Exp Psychol Anim Behav Process 18:145–153
Baker TC, Vogt RG (1988) Measured behavioural latency in response to sex-pheromone loss in the large silk moth Antheraea polyphemus. J Exp Biol 137:29–38
Bargmann CI (2006) Comparative chemosensation from receptors to ecology. Nature 444:295–301
Boland W, Hopke J, Piel J (1998) Induction of plant volatile biosynthesis by jasmonates. In: Schreier P, Herderich M, Humpf H, Schwab W (eds) Natural product analysis: chromatography, spectroscopy, biological testing. Viehweg, Braunschweig, pp 255–269
Bruce TJA, Midega CAO, Birkett MA, Pickett JA, Khan ZR (2009) Is quality more important than quantity? Insect behavioural responses to changes in a volatile blend after stemborer oviposition on an African grass. Biol Lett 6:314–317
Chen L, Fadamiro HY (2007) Differential electroantennogram response of females and males of two parasitoid species to host-related green leaf volatiles and inducible compounds. Bull Entomol Res 97:515–522
D’Alessandro M, Turlings TCJ (2005) In situ modification of herbivore-induced plant odors: a novel approach to study the attractiveness of volatile organic compounds to parasitic wasps. Chem Senses 30:739–753
D’Alessandro M, Brunner V, von Mérey G, Turlings TCJ (2009) Strong attraction of the parasitoid Cotesia marginiventris towards minor volatile compounds of maize. J Chem Ecol 35:999–1008
Das P, Chen L, Sharma KR, Fadamiro HY (2011) Abundance of antennal chemosensilla in two parasitoid wasps with different degree of host specificity, Microplitis croceipes and Cotesia marginiventris may explain sexual and species differences in their response to host-related volatiles. Microsc Res Tech 74:900–909
De Bruyne M, Baker TC (2008) Odor detection in insects: volatile codes. J Chem Ecol 34:882–897
De Moraes CM, Mescher MC (2005) Intrinsic competition between larval parasitoids with different degrees of host specificity. Ecol Entomol 30:564–570
De Moraes CM, Lewis WJ, Pare PW, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573
Denis D, van Baaren J, Pierre J, Wajnberg E (2013) Evolution of a physiological trade-off in a parasitoid wasp: how best to manage lipid reserves in a warming environment. Entomol Exp Appl 148:27–38
Erickson RP, Priolo CV, Warwick ZS, Schiffman SS (1990) Synthesis of tastes other than the primaries—implications for neural coding theories and the concept of suppression. Chem Senses 15:495–504
Fontana A, Held M, Fantaye C, Turlings T, Degenhardt J, Gershenzon J (2011) Attractiveness of constitutive and herbivore-induced sesquiterpene blends of maize to the parasitic wasp Cotesia marginiventris (Cresson). J Chem Ecol 37:582–591
Harris CM, Ruberson JR, Meagher R, Tumlinson J (2012) Host suitability affects odor association in Cotesia marginiventris: implications in generalist parasitoid host-finding. J Chem Ecol 38:340–347
Hoballah ME, Turlings TCJ (2005) The role of fresh versus old leaf damage in the attraction of parasitic wasps to herbivore-induced maize volatiles. J Chem Ecol 31:2003–2018
James DG, Grasswitz TR (2005) Synthetic herbivore-induced plant volatiles increase field captures of parasitic wasps. Biocontrol 50:871–880
James DG, Price TS (2004) Field-testing of methyl salicylate for recruitment and retention of beneficial insects in grapes and hops. J Chem Ecol 30:1595–1610
Kaissling K (1986) Chemo-electrical transduction in insect olfactory receptors. Ann Rev Neurosci 9:121–145
Kay LM, Crk T, Thorngate J (2005) A redefinition of odor mixture quality. Behav Neurosci 119:726–733
Lei H, Vickers N (2008) Central processing of natural odor mixtures in insects. J Chem Ecol 34:915–927
Lewis WJ, Burton RL (1970) Rearing Microplitis croceipes in the laboratory with Heliothis zea as host. J Econ Entomol 63:656–658
Linster C, Cleland TA (2004) Configurational and elemental odor mixture perception can arise from local inhibition. J Comput Neurosci 16:39–47
Loughrin JH, Manukian A, Heath RR, Turlings CJ, Tumlinson JH (1994) Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plants. Proc Nati Acad Sci 91:11836–11840
Lozano C, Gonzalez E, Pena A, Campos M, Plaza MT et al (2000) Response of parasitoids Dendrosoter protuberans and Cheiropachus quadrum to attractants of Phloeotribus scarabaeoides in an olfactometer. J Chem Ecol 26:791–799
Magalhães DM, Borges M, Laumann RA, Sujii ER, Mayor P et al (2012) Semiochemicals from herbivory induced cotton plants enhance the foraging behavior of the cotton boll weevil, Anthonomus grandis. J Chem Ecol 38:1528–1538
Mc Call PJ, Turlings CJ, Loughrin J, Proviouex AT, Tumlinson JH (1994) Herbivore-induced volatiles from cotton (Gossypium hirsutum L.) seedlings. J Chem Ecol 20(12):3039–3050
Meiners T, Wackers F, Lewis WJ (2002) The effect of molecular structure on olfactory discrimination by the parasitoid Microplitis croceipes. Chem Senses 27:811–816
Mumm R, Hilker M (2005) The significance of background odour for an egg parasitoid to detect plants with host eggs. Chem Senses 30:337–343
Navasero RC, Elzen GW (1989) Responses of Microplitis croceipes to host and non-host plants of Heliothis virescens in a wind tunnel. Entomol Exp Appl 53:57–63
Ngumbi E, Fadamiro H (2012) Species and sexual differences in behavioral responses of a specialist and generalist parasitoid species to host-related volatiles. Bull Entomol Res 102:710–718
Ngumbi E, Chen L, Fadamiro HY (2009) Comparative GC-EAD responses of a specialist (Microplitis croceipes) and a generalist (Cotesia marginiventris) parasitoid to cotton volatiles induced by two caterpillar species. J Chem Ecol 35:1009–1020
Ngumbi E, Chen L, Fadamiro H (2010) Electroantennogram (EAG) responses of Microplitis croceipes and Cotesia marginiventris and their lepidopteran hosts to a wide array of odor stimuli: correlation between EAG response and degree of host specificity? J Insect Physiol 56:1260–1268
Ngumbi E, Jordan M, Fadamiro HY (2012) Comparison of associative learning of host-related plant volatiles in two parasitoids with different degrees of host specificity, Cotesia marginiventris and Microplitis croceipes. Chemoecology 22:207–215
Olson DM, Rains GC, Meiners T, Takasu K, Tertuliano M et al (2003) Parasitic wasps learn and report diverse chemicals with unique conditionable behaviors. Chem Senses 28:545–549
Ozawa R, Shiojiri K, Sabelis MW, Takabayashi J (2008) Maize plants sprayed with either jasmonic acid or its precursor, methyl linolenate, attract armyworm parasitoids, but the composition of attractants differs. Entomol Exp Appl 129:189–199
Pare PW, Tumlinson JH (1997) De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol 114:1161–1167
Pare PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant Physiol 121:325–331
Penaflor MFGV, Erb M, Miranda LA, Werneburg AG, Bento JMS (2011) Herbivore-induced plant volatiles can serve as host location cues for a generalist and a specialist egg parasitoid. J Chem Ecol 37:1304–1313
Rains GC, Tomberlin JK, D’Alessandro M, Lewis WJ (2004) Limits of volatile chemical detection of a parasitoid wasp, Microplitis croceipes, and an electronic nose: a comparative study. Trans ASAE 47:2145–2152
Rose USR, Tumlinson JH (2004) Volatiles released from cotton plants in response to Helicoverpa zea feeding damage on cotton flower buds. Planta 218:824–832
Rose USR, Manukian A, Heath RR, Turlings CJ, Tumlinson JH (1996) Volatile semiochemicals released from undamaged cotton Leaves. A systemic response of living plants to caterpillar damage. Plant Physiol 111:487–495
Rose USR, Lewis WJ, Tumlinson JH (1998) Specificity of systemically released cotton volatiles as attractants for specialist and generalist parasitic wasps. J Chem Ecol 24:303–319
Schnee C, Kollner TG, Held M, Turlings TCJ, Gershenzon J et al (2006) The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci USA 103:1129–1134
Schroder R, Hilker M (2008) The relevance of background odor in resource location by insects: a behavioral approach. Bioscience 58:308–316
Shorey HH, Hale RL (1965) Mass rearing of the larvae of nine noctuid species on a simple artificial medium. J Econ Entomol 58:55–68
Smid HA, van Loon JJA, Posthumus MA, Vet LEM (2002) GC-EAG-analysis of volatiles from Brussels sprouts plants damaged by two species of Pieris caterpillars: olfactory receptive range of a specialist and a generalist parasitoid wasp species. Chemoecology 12:169–176
Smith BH (1998) Analysis of interaction in binary mixtures. Physiol Behav 65:397–407
Sobhy IS, Erb M, Sarhan AA, El-Husseini MM, Mandour NS, Turlings T (2012) Less is more: treatment with BTH and laminarin reduces herbivore-induced volatile emissions in maize but increases parasitoid attraction. J Chem Ecol 38:348–360
Thaler JS (2002) Effect of jasmonate-induced plant responses on the natural enemies of herbivores. J Anim Ecol 71:141–150
Turlings TCJ, Tumlinson JH, Lewis WJ (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250:1251–1253
Turlings TCJ, Davisony AC, Christina T (2004) A six-arm olfactometer permitting simultaneous observation of insect attraction and odour trapping. Physiol Entomol 29:45–55
Uefune M, Kugimiya S, Sano K, Takabayashi J (2012) Herbivore-induced plant volatiles enhance the ability of parasitic wasps to find hosts on a plant. J Appl Entomol 136:133–138
Uefune M, Kugimiya S, Ozawa R, Takabayashi J (2013) Parasitic wasp females are attracted to blends of host-induced plant volatiles: do qualitative and quantitative differences in the blend matter? F1000Research 2:57. doi:10.12688/f1000research.2-57.v2
van Dam NM, Qiu B, Hordijk CA, Vet LEM, Jansen JJ (2010) Identification of biologically relevant compounds in aboveground and belowground induced volatile blends. J Chem Ecol 36:1006–1016
van Poecke RMP, Roosjen M, Pumarino L, Dicke M (2003) Attraction of the specialist parasitoid Cotesia rubecula to Arabidopsis thaliana infested by host or non-host herbivore species. Entomol Exp Appl 1075:229–236
van Wijk M, De Bruijn PJA, Sabelis MW (2011) Complex odor from plants under attack: herbivore’s enemies react to the whole, not its parts. PLoS ONE 6:e21742. doi:10.1371/journal.pone.0021742
Wajnberg É, Haccou P (2008) Statistical tools for analyzing data on behavioral ecology of insect parasitoids. In: Wajnberg E, Bernstein C, van Alphen J (eds) Behavioral ecology of insect parasitoids: from theoretical approaches to field applications. Blackwell Publishing Ltd., Oxford, pp 402–429
Wei J, Kang L (2006) Electrophysiological and behavioral responses of a parasitic wasp to plant volatiles induced by two leaf miner species. Chem Senses 31:467–477
Wei J, Wang L, Zhu J, Zhang S, Nandi OI, Kang L (2007) Plants attract parasitic wasps to defend themselves against insect pests by releasing hexenol. PLoS ONE 2:e852. doi:10.1371/journal.pone.0000852
Yu H, Zhang Y, Wyckhuys AG, Wu K, Gao X, Gou Y (2010) Electrophysiological and behavioral responses of Microplitis mediator (Hymenoptera: Braconidae) to caterpillar-induced volatiles from cotton. Environ Entomol 39:600–609
Acknowledgments
We thank Erica Williams, Matthew McTernan and Savannah Duke for rearing the insects used for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Michael Heethoff.
Rights and permissions
About this article
Cite this article
Morawo, T., Fadamiro, H. Attraction of two larval parasitoids with varying degree of host specificity to single components and a binary mixture of host-related plant volatiles. Chemoecology 24, 127–135 (2014). https://doi.org/10.1007/s00049-014-0154-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-014-0154-5