Abstract
In this study, a mixed mode stationary phase for capillary liquid chromatography (cLC) was prepared by chemical modification of glycol diglycidyl ether (EGDE) and γ-aminobutyric acid (GABA) bonded to the silica, named as Sil–EGDE–GABA. The Sil–EGDE–GABA was characterized by Fourier transform infrared spectroscopy and elemental analysis. The retention of nucleotide bases at different acetonitrile levels in the mobile phase indicated that the stationary phase possessed both hydrophilic chromatography (HILIC) and reversed-phase chromatography (RPLC) characteristics. Positional isomers, aniline compounds and a mixture of polar–nonpolar analytes were separated in RPLC mode. The stationary phase exhibited different separation mechanisms in the separation of positional isomers, compared with a commercial ODS column. Sulfonamides and biogenic amines were successfully separated in HILIC mode. These results demonstrated the application possibilities of the prepared Sil–EGDE–GABA as packing materials in cLC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Capillary liquid chromatography (cLC) is an important analytical method due to the merits of low solvent consumption, high column efficiency, and good compatibility with mass spectrometry [1,2,3]. Stationary phases are the core of chromatographic separation for cLC. Various types of chromatographic stationary phases, such as reversed phase stationary phase, hydrophilic stationary phase and ion-exchange stationary phase, have been obtained by the surface modification of silica gel and have played important roles in the separation of nonpolar, polar and ionic solutes. However, prepared columns have limitations in the separation of complex sample mixtures including hydrophobic, hydrophilic and ionic solutes through a single retention mechanism. Mixed-mode chromatography (MMC) is a desirable approach to improve the separation performance for complex sample matrices.
MMC is a promising chromatographic technology in which multiple retention mechanisms are used during the separation. Based on the multiple interactions, different types of analytes can be separated on a single column, which results in lower cost and improved separation efficiency, particularly for complex samples [4, 5]. Undeniably, MMC loses the robustness of a LC method. Minor changes of mobile phase or other conditions might change the retention order of analytes. Thus it consumes more time and efforts of the users to obtain good separation of a complex sample. Until now, there have been many reports on MMC including reversed-phase liquid chromatography (RPLC)/hydrophilic chromatography (HILIC) [6,7,8,9], HILIC/ion-exchange chromatography (IEC) [10, 11], RPLC/IEC [12,13,14] and RPLC/HIILC/IEC [15, 16]. Among these methods, HILIC/RPLC is a MMC technique that utilizes hydrophobic or hydrophilic interactions to achieve good separation for different solutes. RPLC is suitable for the separation of weakly polar and moderately polar analytes, while HILIC is complementary to RPLC and has great advantages in the separation of polar analytes [17, 18]. Therefore, RPLC/HILIC can overcome the deficiencies of both modes and separate a wider range of analytes. For example, Aral et al. [6] prepared a novel multifunctional stationary phase for RPLC/HILIC and reported good separation performance for a mixture of polar and nonpolar compounds. Wang et al. [9] prepared a C18-diol stationary phase and used it to simultaneously separate acids, bases and neutral compounds.
Zwitterion-based stationary phases (sulfobetaine, phosphorylcholine), which have a zwitterionic structure with an equal number of positive and negative charges, have been developed due to their good hydrophilicity and selectivity. For example, hydrophilic polymeric monoliths containing choline phosphate [19, 20] showed good selectivities for polar analytes. A zwitterionic stationary phase modified with a negatively charged phosphate group and a positively charged amino group was used for the rapid of acidic and alkaline proteins from egg white [21]. A gemini-type sulfobetaine-based hybrid monolith showed good separation of small molecules [22]. Similarly, γ-aminobutyric acid (GABA), which is widely present in organisms as an active substance, possesses a positively charged amine and a negatively charged carboxyl, as well as hydrophilicity, but it has not been used for the preparation of a stationary phase.
In this study, GABA was bound to the surface of silica through a reaction between the epoxy groups of glycol diglycidyl ether (EGDE) and amine groups. EGDE were not only used as the coupling agent, but also provided interaction sites. The Sil–EGDE–GABA stationary phase developed here provides hydroxyl, amino and carboxylic groups as polar sites, as well as hydrophobic alkyl chains as nonpolar sites. Hence, this stationary phase possesses both RPLC and HILIC retention mechanisms and could be used for the separation of anilines, positional isomers and a mixture of polar-nonpolar analytes in RPLC, as well as for the separation of sulfonamides and biogenic amines in HILIC.
Experimental
Reagents and Materials
Spherical porous silica (diameter 5 µm, pore size 12 nm, specific surface area 300 m2 g−1) was purchased from Nano-Micro Technology Co., Ltd. (Suzhou, China). Fused silica capillaries (internal diameter 100 µm) were obtained from Reafine Chromatography Ltd. (Hebei, China). Three sulfonamides including sulfaguanidine (SG), sulfamerazine (SM1) and sulfadimethoxine (SDM) were a gift from Dr. Ehrenstorfer (Augsburg, Germany). Four biogenic amines including norepinephrine bitartrate, dopamine, tyramine and tryptamine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Structures of sulfonamides and biogenic amines mentioned were presented in Fig. S1. Positional isomers were purchased from Acros (Morris Plains, NJ, USA). Benzene, toluene, ethylbenzene and n-propylbenzene were provided by Chemical Reagent Plant (Shanghai, China). Glycol diglycidyl ether (EGDE) was purchased from Maya Reagent (Zhejiang, China). Adenine, cytosine, uracil, adenosine, cytidine, 3-aminopropyltrimethoxysilane (Aps), r-aminobutyric acid and sodium silicate were all obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
Preparation of the Sil–EGDE–GABA Stationary Phase
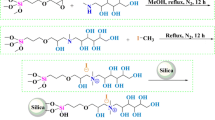
The Sil–EGDE–GABA stationary phase was synthesized as shown in Fig. 1. First, spherical silica was dispersed in 10% wt hydrochloric acid and refluxed by heating for 30 min [23]. The silica was then washed with ultrapure water and dried under vacuum at 120 °C for 6 h. The activated silica was further used to prepare Sil–Aps according to a previously published method. The activated silica (2 g) and APS (1.5 mL) were refluxed in dry toluene (20 mL) for 24 h. The mixture was then washed successively with toluene, ethanol and diethyl ether. Finally, the particles were dried under vacuum overnight [24].
Next, Sil–Aps (0.5 g) was suspended in ethanol, followed by the addition of glycol diglycidyl ether (1 mL). The mixture was stirred at 60 °C for 6 h. After successive washing with ethanol, then ultrapure water, the EGDE modified silica (Sil–EGDE) was dried under vacuum.
Finally, obtained Sil–EGDE was added to ultrapure water containing GABA (0.18 g) with stirring at 60 °C for 6 h. The final product (Sil–EGDE–GABA) was washed with ultrapure water several times and dried under vacuum.
Column Packing
The column was packed according to the method of Otsuka et al. [25]. Preparation of the Sil–EGDE–GABA column is illustrated in Fig. 2. A section of fused silica capillary was cut to a length of 50 cm and successively washed with 1M NaOH for 30 min, followed by ultrapure water for 30 min, 0.1 M HCl for 30 min, and then ultrapure water for 30 min. Finally, the capillary was dried under a nitrogen stream. A small amount of saturated sodium silicate solution was injected into the pretreated capillary from one end and quickly heated to form a temporary frit. Another end of the capillary was joined to a stainless steel tube containing a slurry of Sil–EGDE–GABA (0.2 g Sil–EGDE–GABA was dispersed in acetonitrile) and the packing was then forced into the capillary under a constant pressure (20 Mpa). The pumping solvent was methanol/water (v/v, 80:20). When the packings in capillary exceeded required length, slurry of Sil–EGDE–GABA in stainless steel tube was changed into water and sintered packed stationary phase at the selected position under high pressure. Permanent frits at both ends were all obtained by sintering the packed materials themselves. The pump was then turned off and the capillary was connected in the reverse direction to remove the excess packing materials at 10 Mpa. Finally, the detection window was prepared close to the frit by removing the polyimide coating on the fused silica capillary. Notably, the capillary was immersed in an ultrasonic water bath to obtain a uniform and dense column packing.
Instruments and Chromatographic Conditions
All chromatographic separations were carried out on a TriSep-2100 pressurized capillary electrochromatography system (Unimicro Technologies Inc., Shanghai, China) equipped with a UV detector. The system can also be used for capillary liquid chromatography. The IR spectra data were acquired from a NEXUS 670 Fourier-transform spectrometer (Perkin Elmer, USA). Elemental analyses were performed on a Vario EL III elemental analyzer (Hanau, Germany). Ultrapure water was obtained from Milli-Q ultra-pure water system (Millipore, Bedford, MA, USA). A P600 infusion pump was from LabTech (Beijing, China).
The Sil–EGDE–GABA column (total length 50 cm, effective length 25 cm) was prepared as described above and a commercial ODS column (total length 45 cm, effective length 20 cm, Unimicro Technologies Inc., Shanghai, China) was used as a comparison. The mobile phases were prepared using HPLC-grade methanol and acetonitrile. All analytes were dissolved in methanol or ultra-pure water. Biogenic amines were measured at 230 nm, and all other analytes were measured at 254 nm.
Results and Discussion
Characterizations
Infrared Spectroscopy
Infrared spectroscopy is a useful approach for the identification of chemical modifications. The spectra of the bare silica, Sil–Aps, Sil–EGDE and Sil–EGDE–GABA are shown in Fig. 3. The broad absorption band around 1110 cm−1 in spectra was attributed to Si–O–Si stretching vibration. There was an absorption band around 3500 cm−1 in spectra of silica, stretching and deformation of O–H were also observed at 974 cm−1, 1640 cm−1. The absorbance at 974 cm−1, 1640 cm−1 were weaker in spectra of Sil–Aps and Sil-EGDE, indicating successful bonding. Stretching vibrations of N–H was around 3300–3500 cm−1 which could be seen in spectra of Sil–Aps, Sil–EGDE and Sil–EGDE–GABA. Absorption bands at 2940 cm−1 were attributed to C–H stretching vibrations. Additionally, lower intensity in spectra of Sil–EGDE–GABA at 3500 cm−1 indicated the modification of GABA. All these results demonstrated successful modification of the silica to include EGDE and GABA.
Elemental Analysis
Elemental composition of the silica, Sil–Aps, Sil–EGDE and Sil–EGDE–GABA was determined by elemental analysis, and the resultant data are listed in Table 1. Compared with activated silica and Sil–Aps, there was a significant increase in the content of carbon and hydrogen for Sil-EGDE, which was due to the attachment of EGDE. The carbon, hydrogen and nitrogen contents increased further after modifying GABA on the surface of Sil-EGDE. The results of the elemental analysis confirmed the presence of EGDE and GABA. The contents of surface coverage for Sil–Aps, Sil–EGDE and Sil–EGDE–GABA were 2.87 mmol g−1, 0.59 mmol g−1, 0.35 mmol g−1, respectively. Related calculations were listed in Supplementary material. In addition, the yields in each step are very important for a multiple-step synthesis procedure. According to the calculation based on the percentage of carbon, the actual percentage of Sil–EGDE–GABA in stationary phase is about 25.6%. Although the yield in each step is not ideal, the aim of performance evaluation could be achieved on the prepared Sil–EGDE–GABA stationary phase under this surface concentration.
Chromatographic Evaluation of Sil–EGDE–GABA in HILIC/RPLC Mixed Mode
Due to the existence of polar and nonpolar sites of the prepared Sil–EGDE–GABA stationary phase, it should be able to be used for separation in both HILIC and RPLC modes. Three nucleotide bases including adenine, cytosine, uracil were chosen to evaluate the chromatographic performance of the Sil–EGDE–GABA column. As shown in Fig. 4, the retention behaviors of the three bases at different acetonitrile levels in the mobile phase were investigated. When the content of acetonitrile in mobile phase varied from 10 to 40%, the retention factors of the three bases were decreased, which was in accordance with RPLC retention characteristics. Furthermore, when the level of acetonitrile in mobile phase increased to 90%, the retention factors of the three bases were all increased, which was in accordance with HILIC retention characteristics. Retention of the three bases was also investigated under the same conditions on the commercial ODS column, they could not be separated, as shown in Figs. S2 and S3. These results indicated that the Sil–EGDE–GABA column possessed both HILIC and RPLC chromatographic properties.
Chromatographic Evaluation of Sil–EGDE–GABA in RPLC Mode
Various positional isomers of substituted benzene are important industrial materials and the main chemical substances that affect environmental and human health. Therefore, the separation and detection of these compounds is of great significance. Four positional isomers including nitroaniline, nitrobromobenzene, dihydroxybenzene and bromoaniline were used to evaluate the separation performance of the Sil–EGDE–GABA column in RPLC mode. As shown in Fig. 5a–d, the four positional isomers were well separated using 20% methanol as the mobile phase. Retention time (t), retention factor (k) and resolution (R) are shown in Table 2. Furthermore, the retention of four positional isomers was also studied under the same mobile phase on the commercial ODS column as a comparison. They exhibited stronger retention on the ODS column when using 20% methanol as the mobile phase and were not eluted until 90 min except for nitroaniline. Additionally, bromoaniline isomers were not separated effectively on the ODS column. Chromatograms of the isomers under optimal conditions on commercial ODS column were presented in Fig. S4. Chromatographic data including t, k, R and N are shown in Table S1. Column efficiencies (N) of the four positional isomers on the two columns were listed in Table S2. Generally, the results showed that Sil–EGDE–GABA column had better selectivity for the four positional isomers than the ODS column. Notably, elution order of the four positional isomers was different on the two columns. Retention of the positional isomers was determined by hydrophobic interactions on the ODS column, which suggests that multiple interaction mechanisms were in use on the Sil–EGDE–GABA column for the separation of positional isomers.
Aniline compounds have moderate polarity and can be separated in RPLC mode [26]. A mixture of anilines including p-phenylenediamine, p-nitroaniline, p-bromoaniline, p-methylaniline, p-chloroaniline, m-iodoaniline were used to further evaluate the properties of the Sil–EGDE–GABA column in RPLC mode. As shown in Fig. 6, the six anilines eluted within 15 min using 30% methanol as a mobile phase. Chromatographic data including k, R and N for the six anilines are shown in Table S3. It was found that p-nitroaniline eluted next to p-phenylenediamine, while the elution order of the other anilines corresponded to their increasing hydrophobicity. Additionally, p-nitroaniline and p-phenylenediamine eluted at the complete opposite side of the chromatogram. Their retention was probably determined by the difference in pKa. The pKa and LogP of six anilines are summarized in Table 3. This phenomenon indicated that the retention of anilines on Sil–EGDE–GABA column was determined by multiple interactions.
As mentioned above, the prepared Sil–EGDE–GABA possesses both hydrophobic and hydrophilic groups. To evaluate the separation performance of the Sil–EGDE–GABA column for a mixture of polar–nonpolar analytes, eight analytes, including adenine, adenosine, cytosine, cytidine, benzene, toluene, ethylbenzene and n-propylbenzene, were separated. As shown in Fig. 7, the eight analytes were separated within 40 min using 30% methanol as a mobile phase. Chromatographic data including k, R and N for the eight analytes are given in Table S4. In addition, the retention behaviors of the mixture on commercial ODS column were also investigated under the same condition. The polar analytes were not separated; the nonpolar analytes exhibited a stronger retention and were not eluted until 45 min, as shown in Fig. S5. The separation of a mixture of polar and nonpolar analytes is difficult on RPLC or HILIC columns, however, they were simultaneously separated using a Sil–EGDE–GABA column. The results indicate that the Sil–EGDE–GABA column has wider applicability than a single mode column.
Chromatographic Evaluation of Sil–EGDE–GABA in HILIC Mode
Efficient separation of highly polar substances in HILIC is easily accomplished compared to RPLC [27, 28]. Four biogenic amines including tryptamine, tyramine, dopamine and norepinephrine bitartrate were selected as probes to evaluate the chromatographic performance of the Sil–EGDE–GABA stationary phase in HILIC mode. Figure 8 shows the retention of four biogenic amines on the Sil–EGDE–GABA stationary phase. Chromatographic data for the four biogenic amines are shown in Table S5. The four biogenic amines were separated within 8 min in the order of tryptamine, tyramine, dopamine and norepinephrine bitartrate, and they were eluted according to their polarities from low to high. These results confirmed the HILIC separation based on a pure partitioning mechanism, and the retention times were enhanced with the increasing polarity of each analyte.
Similarly, the Sil–EGDE–GABA column showed good performance for the separation of three sulfonamides. The chromatogram is shown in Fig. 9. SG, SM1 and SDM were separated using 80% acetonitrile as an eluent. This also shows the further application of the Sil–EGDE–GABA column in HILIC mode. Chromatographic data for the three sulfonamides are shown in Table S6.
Repeatability and Stability
In this work, adenine, cytosine and uracil were used as test analytes to evaluate repeatability of the Sil–EGDE–GABA column with 80% acetonitrile as the mobile phase. The results were based on the relative standard deviations (RSD) of the retention times for the three bases. As listed in Table 4, the RSDs for intra-day (n = 5), inter-day (n = 5) and column–column (n = 4) reproducibility were in the range of 1.34–2.37%, 2.35–3.23% and 3.50–4.74%, respectively. Additionally, during the whole period of the experiment, peak shapes and separation efficiency of the three bases were not obviously changed. All these results illustrated the reproducibility and stability of the Sil–EGDE–GABA column.
Conclusions
In this work, a mixed mode stationary phase (Sil–EGDE–GABA) was successfully prepared and used as a packing material for a capillary column. Due to the combination of polar groups and nonpolar chains, the prepared column exhibited good performance in HILIC and RPLC modes. Various analytes including positional isomers, anilines, a mixture of polar, nonpolar analytes and sulfonamides, and biogenic amines were all separated in RPLC or HILIC mode. The prepared Sil–EGDE–GABA column showed different retention mechanisms compared with an ODS column in RPLC. Based on the multiple retention mechanisms, it is expected that Sil–EGDE–GABA could be valuable for the analysis of increasingly complicated mixtures of compounds.
References
Huang XA, Zhang S, Schultz GA, Henion J (2002) Anal Chem 74:2336–2344. https://doi.org/10.1021/ac011202w
Jiang ZJ, Smith NW, Ferguson PD, Taylor MR (2007) J Biochem Biophys Methods 70:39–45. https://doi.org/10.1016/j.jbbm.2006.08.009
Oberacher H, Huber CG (2002) Trends Anal Chem 21:166–174. https://doi.org/10.1016/s0165-9936(02)00304-7
Mansour FR, Danielson ND (2013) Anal Methods 5:4955–4972. https://doi.org/10.1039/c3ay40302e
Yang Y, Geng XD (2011) J Chromatogr A 1218:8813–8825. https://doi.org/10.1016/j.chroma.2011.10.009
Aral H, Celik KS, Altindag R, Aral T (2017) Talanta 174:703–714. https://doi.org/10.1016/j.talanta.2017.07.014
Li YY, Xu ZG, Feng YY, Liu XY, Chen T, Zhang HX (2011) Chromatographia 74:523–530. https://doi.org/10.1007/s10337-011-2120-5
Wang HZ, Zhang L, Ma T, Zhang LY, Qiao XQ (2016) J Sep Sci 39:3498–3504. https://doi.org/10.1002/jssc.201600448
Wang Q, Ye M, Xu L, Shi ZG (2015) Anal Chim Acta 888:182–190. https://doi.org/10.1016/j.aca.2015.06.058
Wang L, Wu MH, Wang QX, Zhan JJ, Chen HB (2016) Chromatographia 79:1263–1269. https://doi.org/10.1007/s10337-016-3150-9
Yang BB, Liu HM, Chen J, Guan M, Qiu HD (2016) J Chromatogr A 1468:79–85. https://doi.org/10.1016/j.chroma.2016.09.021
Bo CM, Wang CZ, Wei YM (2017) J Sep Sci 40:4700–4708. https://doi.org/10.1002/jssc.201700719
Jiang Q, Zhao WJ, Qiu HD, Zhang SS (2016) Chromatographia 79:1437–1443. https://doi.org/10.1007/s10337-016-3166-1
Sun M, Feng JJ, Luo CN, Liu X, Jiang SX (2013) Talanta 105:135–141. https://doi.org/10.1016/j.talanta.2012.11.077
Li Y, Yang JJ, Jin J, Sun XL, Wang LX, Chen JP (2014) J Chromatogr A 1337:133–139. https://doi.org/10.1016/j.chroma.2014.02.044
Qiu HD, Mallik AK, Takafuji M, Jiang SX, Ihara H (2012) Analyst 137:2553–2555. https://doi.org/10.1039/c2an35348b
Moravcova D, Planeta J, Kahle V, Roth M (2012) J Chromatogr A 1270:178–185. https://doi.org/10.1016/j.chroma.2012.11.005
Shao W, Liu J, Liang Y, Yang K, Min Y, Zhang X, Liang Z, Zhang L, Zhang Y (2018) Anal Bioanal Chem 410:1019–1027. https://doi.org/10.1007/s00216-017-0626-x
Jiang ZJ, Reilly J, Everatt B, Smith NW (2009) J Chromatogr A 1216:2439–2448. https://doi.org/10.1016/j.chroma.2009.01.028
Wang QQ, Wu HH, Peng K, Jin HY, Shao HK, Wang YQ, Crommen J, Jiang ZJ (2018) Anal Chim Acta 999:184–189. https://doi.org/10.1016/j.aca.2017.11.032
Cheng XD, Peng XT, Yu QW, Yuan BF, Feng YQ (2013) Chromatographia 76:1569–1576. https://doi.org/10.1007/s10337-013-2534-3
Tan WM, Chang F, Shu Y, Chen Y, Liu JJ, Chen YZ, Ma M, Chen B (2017) Talanta 173:113–122. https://doi.org/10.1016/j.talanta.2017.05.072
Zhang Y, Zhang Y, Wang G, Chen WJ, He PG, Wang QJ (2016) Analyst 141:1083–1090. https://doi.org/10.1039/c5an02021b
Ray S, Takafuji M, Ihara H (2012) J Chromatogr A 1266:43–52. https://doi.org/10.1016/j.chroma.2012.10.004
Otsuka K, Mikami C, Terabe S (2000) J Chromatogr A 887:457–463. https://doi.org/10.1016/s0021-9673(99)01205-4
Aral H, Aral T, Celik KS, Ziyadanogullari B, Ziyadanogullari R (2014) Chromatographia 77:771–781. https://doi.org/10.1007/s10337-014-2678-9
Zeng J, Liu SQ, Wang ML, Yao SZ, Chen YZ (2017) Electrophoresis 38:1325–1333. https://doi.org/10.1002/elps.201600526
Zhang Y, Zhang Y, Wang G, Chen WJ, He PG, Wang QJ (2016) Talanta 161:762–768. https://doi.org/10.1016/j.talanta.2016.09.022
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21575042).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, S., Luo, F., Zhang, Y. et al. Glycol Diglycidyl Ether and γ-Aminobutyric Acid Functionalized Silica as a Mixed Mode Stationary Phase for Capillary Liquid Chromatography. Chromatographia 82, 661–669 (2019). https://doi.org/10.1007/s10337-019-03687-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-019-03687-z