Abstract
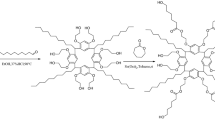
In this work, a novel calix[4]resorcinarene-based ionic liquid (C4RA-2IL) was synthesized, structurally characterized, and statically coated on capillary column as stationary phase for capillary gas chromatography (GC). The column efficiency of the C4RA-2IL column is 3345 plates m−1, which are determined by n-dodecane at 120 °C. Based on its McReynolds constants, the C4RA-2IL column showed moderate polarity. Particularly, the C4RA-2IL column show high separation performance for a wide range of analytes and some difficult separation of meta/para-isomers. Moreover, it exhibited excellent selectivity for critical aromatic isomers of chloroaniline, bromaniline, iodoaniline, toluidine and xylidine isomers and shows advantageous separation capability over the commercial polysiloxane stationary phase. This work presents a promising future of calixarene-based ionic liquid as a new type of stationary phase in GC separations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Capillary gas chromatography (GC) is widely used in medicament analysis, environmental analysis, food analysis, petroleum and petroleum chemicals analysis due to its high selectivity, sensitivity, fast, low cost and relatively simplicity [1,2,3,4,5,6]. In GC analysis, the stationary phase with special selectivity is the key to high resolution of analytes. To meet the requirement on GC separations of complex samples in diverse fields, many researchers focused their attention on the development of new stationary phases. In the past decades, diverse types of materials as GC stationary phases have been investigated, such as polysiloxanes, poly(ethylene glycol), macrocyclic compounds, ionic liquids and metal–organic frameworks (MOFs) [7,8,9,10,11,12,13,14,15]. Recently, macrocyclic molecules (cyclodextrins, crown ethers and calixarenes), which are capable of forming multiple interactions with numerous compounds because their unique structures, are widely used in chromatographic analysis [16,17,18,19,20,21,22].
Calixarenes represent the third generation of host molecules after cyclodextrins and crown ethers [23, 24]. Calixarenes are cavity-shaped cyclic oligomers composed of four or more phenolic units. Due to their conformational rigidity and easy derivatization in terms of cavity size and functional group on the upper or lower rims, calixarenes as the supramolecular platforms have attracted growing attention in supramolecular chemistry, coordination chemistry, sensor chemistry and molecular recognition [25,26,27,28,29,30,31]. From the perspective of chromatographic separations, their unique recognition ability of host to guest also endows them good candidates as separation materials [32, 33]. Currently, calixarene separation materials with unique structure and multiple molecular interactions involving hydrophobic, π–π, H-bonding, dipole–dipole, and dispersion interactions were successfully used to separate polycyclic aromatic hydrocarbons (PAHs), amino acids, nucleosides, sulfonamides, phenols, alkylbenzenes, aromatics positional isomers, and so on [34,35,36,37,38]. It is worth noting that there are many reports on calixarene stationary phases in liquid chromatography, and the reports on application for gas chromatography are by far less than them. This may be because of the poor column efficiency caused by low film-forming ability, which have greatly limited researches and applications of calixarenes in GC. Ionic liquids (ILs) are a special group of classical molten salts with low melting points. The outstanding characteristics of ILs, such as the negligible vapor pressure, dual natural polarity, a wide range of viscosities, excellent stability and good solvating properties, render they are usable in GC separations [39]. Calix[4]resorcinarene (C4RA) is a type of calixarene that has eight phenolic hydroxyl groups [40, 41]. By introducing functional ILs groups onto the three-dimensional calix[4]resorcinarene skeleton, it is possible to produce a mixed-mode stationary phase with different properties to yield multiple interactions with analytes, resulting in high separation performance for GC separations.
In this work, the calixarene ionic liquid (C4RA-2IL, Fig. 1), which contains a 3D aromatic cavity with ionic liquid groups, was synthesized and used as stationary phase for capillary GC separations. FT-IR, 1HNMR, MS and thermal gravimetric analysis (TGA) were used to characterize the C4RA-2IL composition and structure. Its separation performance was evaluated by employing a wide range of analytes and their structural, positional, and cis-/trans-isomers. To our knowledge, this is the first report on employing the calixarene ionic liquid as GC stationary phase.
Experimental
Materials and Instrument
All reagents and solvents were purchased from Sun Chemical Technology Co. Ltd (Shanghai, China), Aladdin Chemistry Co. Ltd. (Shanghai, China), J & K. Scientific. Ltd. (Beijing, China) and Sinopharm Chemical Reagent Co. Ltd. (Beijing, China). All the chemicals were of analytical grade and dissolved in ethanol for the mixtures at a concentration of 15 mg mL−1 for the separations. The bare fused-silica tubing capillary column with a protecting polyimide coating (0.25 mm, i.d.) were purchased from Yongnian Ruifeng Chromatogram Apparatus Co., Ltd. (Hebei, China). The commercial column DB-1701 capillary column (30 m long × 0.25 mm i.d. × 0.25 μm film thickness) was bought from Agilent Technologies Co. Ltd. (Palo Alto, CA, USA) for comparison.
All GC separations were performed using an Agilent 7890A gas chromatograph equipped with a split/splitless injector system and a flame ionization detector (FID) managed with the Agilent ChemStation software. The injector and detector temperatures were maintained at 300 °C using a split ratio of 60:1. Nitrogen (N2, 99.999% purity) was used as the carrier gas with a flow rate of 0.6 mL min−1 for 10- and 30-m-long columns. TGA was performed using a DTG-60AH instrument. FT-IR spectra were recorded on a Bruker Platinum ART Tensor II FT-IR spectrometer using KBr pellets. 1H NMR spectra were recorded on a Bruker Biospin 400 MHz instrument and MALDI-TOF mass spectra were recorded on a Bruker BIFLEX III mass spectrometer.
Preparation of the C4RA-2IL Capillary Column
The untreated fused-silica capillary column (10 m long × 0.25 mm, i.d.) was washed with 5-mL methanol and dried under nitrogen at 200 °C for 2 h. Then, the C4RA-2IL capillary column was prepared by the static coating method [42, 43]. Finally, the coated column was purged with N2 gas and conditioned under the following temperature program: 40 °C for 1 h first, then increased to 180 °C at a ramp rate of 1 °C min−1, and maintained at 180 °C for 7 h.
Results and Discussion
Characterization of C4RA-2IL Stationary Phase and Capillary Column
C4RA-2IL was synthesized following the procedure described in Fig. S1 [40, 41]. The thermal stability of the C4RA-2IL stationary phase has been measured by thermal gravimetric analysis (TGA). To evaluate the intrinsic thermal stability of C4RA-2IL, it was analyzed via TGA with operation temperatures from 40 to 600 °C at a rate of 10 °C min−1. The TGA curve for C4RA-2IL is shown in Fig. 2. As a result, C4RA-2IL is thermally stable up to 310 °C, suggesting its good thermal stability and feasibility as a GC stationary phase. The column efficiencies of C4RA-2IL column were determined through the number of plates per meter for n-dodecane at 120 °C. The Golay curve was further evaluated the column efficiencies of C4RA-2IL column at different flow rates, which relates the height equivalent to a theoretical plate (HETP or H) of the analyte against the flow rate of carrier gas. As presented in Fig. 2, C4RA-2IL column achieved the minimum HETP of 0.299 mm at 0.25 mL min−1, corresponding to the column efficiency of 3345 plates m−1, suggesting its good column efficiency as stationary phase for GC separations.
The polarity of the new stationary phases is an important GC parameter and selection in practical use. The McReynolds constant of C4RA-2IL stationary phase have been quantitatively measured at 120 °C by the five probe solutes of benzene, 1-butanol, 2-pentanone, 1-nitropropane and pyridine [44, 45]. Table 1 shows the McReynolds constants determined for C4RA-2IL stationary phase; the McReynolds constants of commercial DB-1701 stationary phase are also provided for reference. The average value of 257 for C4RA-2IL stationary phase suggested its moderate polarity larger than that of the commercial DB-1701 stationary phase.
Separation Performance and Retention Behavior
To explore the separation performance of C4RA-2IL stationary phase for analytes of wide variety, the mixture of 30 analytes including n-alkanes, aldehydes, ketones, esters, alcohols, bromoalkanes, alkylbenzenes, and halogenated benzenes was separated on C4RA-2IL column. As shown in Fig. 3, the C4RA-2IL column achieved baseline resolution (R > 1.5) all analytes with good peak shapes within 13 min, showing high selectivity and resolution for aliphatic and aromatic analytes of wide polar nature. The relevant chromatographic parameters are provided in Table 1. Notably, the C4RA-2IL column displays stronger retention for substituted benzenes (toluene, ethylbenzene, propylbenzene, 1,2,4-trichlorobenzene) and alcohols (octanol, 1-dodecanol). The prolonged retention may stem from π–π interactions between the 3D cavity and the imidazolium ionic liquids moieties of C4RA-2IL and the aromatic group of the analytes, which result in the elution order of n-octane/toluene (peaks 1/2; b.p., 125 °C/110.6 °C), n-nonane/ethylbenzene (peaks 3/4; b.p., 150.8 °C/136.2 °C), n-decane/propylbenzene (peaks 6/7; b.p., 174.1 °C/159.2 °C), and n-tridecane/1,2,4-trichlorobenzene (peaks 19/20; b.p., 235.4 °C/221 °C) on the C4RA-2IL column. Most interestingly, it shows prolonged retention for alcohols over adjacent analytes mainly due to its stronger H-bonding and dipole–dipole interactions, giving rise to the elution order of methyl heptanoate/1-octanal (peaks 12/13; b.p., 172 °C/163.4 °C) and 1-bromodecane/1-dodecanol (peaks 29/30; b.p., 276 °C/255 °C). It can be found that the contribution of H-bonding and dipole–dipole interactions to its separation process [46]. This finding suggests the different retention behavior of the C4RA-2IL stationary phase for the aliphatic and aromatic analytes of wide nature due to its unique amphiphilic architecture and multiple molecular interactions. The imidazolium cation units enhanced the H-bonding and dipole–dipole interactions between the C4RA-2IL stationary phase with polar compounds and the π–π interactions with aromatic analytes. The above results demonstrate high resolving capability and special retention behavior of the C4RA-2IL stationary phase for diverse types of analytes.
Chromatogram of the mixture of 30 analytes with different types on the C4RA-2IL capillary column. Peaks: (1) n-octane; (2) toluene; (3) n-nonane; (4) ethylbenzene; (5) o-xylene; (6) n-decane; (7) propylbenzene; (8) heptaldehyde; (9) 1,3,5-trimethylbenzene; (10) 1,2,4-trimethylbenzene; (11) n-undecane; (12) methyl heptanoate; (13) 1-octanal; (14) 1-heptanol; (15) n-dodecane; (16) methyl octanoate; (17) nonanal; (18) 1-octanol; (19) n-tridecane; (20) 1,2,4-trichlorobenzene; (21) 2-undecanone; (22) n-tetradecane; (23) 1-bromodecane; (24) 2-undecanone; (25) n-pentadecane; (26) 1-bromoundecane; (27) 2-dodecanone; (28) n-hexadecane; (29) 1-bromodecane; (30) 1-dodecanol. GC conditions on C4RA-2IL column: 40 °C (1 min)–160 °C at a rate of 10 °C min−1
The Grob test mixture containing 12 probing analytes was well recognized for comprehensive evaluation of the separation performance of a GC stationary phase. Figure S1 shows the separations of the Grob test mixture on the C4RA-2IL column and the DB-1701 column, respectively. Most of the analytes in Grob test mixture were well separated on C4RA-2IL column. Unfortunately, there are a small number of chromatographic peaks overlapped, such as 2,6-dimethylphenol/dicyclohexylamine (peak 6/7) and 2-ethylhexanoic acid/methyl decanoate (peak 8/9). Notably, the C4RA-2IL column exhibited different retention and elution order for some of the analytes from the DB-1701 column. As shown, the C4RA-2IL column shows longer retention for 2,3-butanediol, 1-octanol, 2-ethylhexanoic acid, 2,6-dimethylaniline, indicating its stronger H-bonding and dipole–dipole interactions with the analytes through its phenolic hydroxyl and ILs moieties. The above results demonstrate the different interaction mechanisms of the C4RA-2IL stationary phase from the DB-1701 stationary phase.
Subsequently, the C4RA-2IL column was investigated for its separation capability to separate different types of isomers, including structural isomers (propylbenzene and butylbenzene), positional isomers (trimethylbenzene, trichlorobenzene, methylnaphthalene, dimethylnaphthalene, nitrobenzaldehyde, dichlorobenzaldehyde, carvacrol/thymol and xylenol) and cis-/trans-isomers. Figure 4 shows that eight structural and positional isomers of wide polar nature were baseline separated (R > 1.5) with good sharp peaks on the C4RA-2IL column. Usually, benzaldehydes and phenols are tough analytes prone to peak tailing are liable to peak tailing in GC separations, but the C4RA-2IL column showed symmetric peak shapes. The above results evidenced the high resolving performance of C4RA-2IL column for aromatic isomers of high similarity. The unique 3D aromatic skeleton of calixarene is conducive for enhancing π–π interactions among the aromatic analytes. Next, the cis–trans isomer mixtures with highly similar structures and physicochemical properties were used to confirm the superior separation capability of the C4RA-2IL column. The chromatograms of 11 cis–trans isomer mixtures are illustrated in Fig. 5. As a result, the C4RA-2IL column exhibits high selectivity and separation performance for cis–trans isomer mixtures of diverse types. The obtained results indicate the high separation capability of the C4RA-2IL column for structural, positional, and cis-/trans-isomers of wide nature by multiple molecular interactions.
Chromatogram of isomer mixtures of a propylbenzene and butylbenzene, b trimethylbenzene, c trichlorobenzene, d methylnaphthalene, e dimethylnaphthalene, f nitrobenzaldehyde, g dichlorobenzaldehyde, h carvacrol/thymol, and i xylenol on the C4RA-2IL capillary column. GC conditions on C4RA-2IL column (a–i): 40 °C (1 min)–160 °C at a rate of 10 °C min−1
Chromatogram of cis-/trans-isomers of a 1,2,3-trichloropropene, b 1,2-dimethylcyclohexane, c 1,3-dimethylcyclohexane, d 1,4-dimethylcyclohexane, e 2,5-dimethyltetrahydrofuran, f 2,5-dimethoxytetrahydrofuran, g decahydronaphthalene, h citral, i nerol/geraniol, j methyl dihydrojasmonate, and k nerolidol on the C4RA-2IL capillary column. GC conditions on C4RA-2IL column (a–k): 40 °C (1 min)–160 °C at a rate of 10 °C min−1
Aromatic amines are important industrial chemicals, which are widely used for manufacturing of drugs, dyes, rubber, pesticides, and other miscellaneous chemical products. Aromatic amines are also major environmental pollutants due to their carcinogenic nature. o-Toluidine, 2,6-dimethylaniline, and 4-chloroaniline have been listed in carcinogenic aromatic amine. Figure 6 presents the separations of the chloroaniline, bromaniline, iodoaniline, toluidine and xylidine isomers on the C4RA-2IL column (10 m long) in comparison with the commercial DB-1701 column (30 m long). As can be seen, the C4RA-2IL column well separated resolved all the aromatic aniline isomers. However, some of aromatic anilines partially overlapped or even coeluted on the commercial DB-1701 column. They showed advantageous separation capability for aromatic aniline isomers, over the commercial DB-1701 column. As for their retention behavior, the halogenated aniline isomers were eluted according to the order of their polarity (ortho < meta < para) on the C4RA-2IL column. These findings substantiated that the H-bonding interaction between the polar compounds, and C4RA-2IL was crucial in the separation mechanism.
Afterwards, the challenging isomer mixtures of toluidine and xylidine were used to investigate the separation capability of the C4RA-2IL column in comparison to the commercial DB-1701 column. As shown in Fig. 7, C4RA-2IL column baseline resolved for all the analytes, and exhibited much higher separation capability than the DB-1701 column that overlapped or coeluted some of them. Peak pairs, such as o-toluidine (b.p., 200.7 °C; dipole moments, 1.76 D)/o-toluidine (b.p., 200.4 °C; dipole moments, 1.49 D) and 2,6-dimethylaniline (b.p., 214 °C; dipole moments, 1.77 D)/2,5-dimethylaniline (b.p., 218 °C; dipole moments, 1.60 D) were separated by the baseline on the C4RA-2IL column despite they have similar boiling points and polarity. This finding suggested the high selectivity and separation capability of the C4RA-2IL column for aromatic amines with similar structures and properties. This capability was owing to its unique amphiphilic structure and multiple molecular interactions involving dispersion, π-π stacking, H-bonding, and dipole–dipole interactions.
Chromatogram of toluidine and xylidine isomers on the C4RA-2IL capillary column in comparison to the DB-1701 column. GC conditions for toluidine isomers on C4RA-2IL column: 100–160 °C at a rate of 10 °C min−1. GC conditions for xylidine isomers on C4RA-2IL column: 120–160 °C at a rate of 10 °C min−1. GC conditions for toluidine and xylidine isomers on DB-1701 column: 100–180 °C at a rate of 10 °C min−1
Column Repeatability, Long-Term Stability, Thermal Stability and Loadability
The repeatability and reproducibility of the C4RA-2IL column were measured by separation of the isomer mixtures of trimethylbenzene and trichlorobenzene and evaluated by the RSD% values on their retention times. As shown in Table S2, the RSD% values in the range of 0.01–0.03% for run-to-run, 0.03–0.30% for day-to-day and 2.23–4.98% for column-to-column proved its good column repeatability and reproducibility. In addition, the long-term stability of the C4RA-2IL column was evaluated by the separation of the same isomer mixtures over 12 months. As shown in Table S3, the RSD% values for the analytes were in the range of 0.36–0.50% over the time period, indicating its good long-term stability for GC analyses. In addition, we determined the column bleeding profile of the C4RA-2IL column. As shown in Fig. S2, the C4RA-2IL column was used up to its maximum allowable operating temperature of 235 °C, wherein the increase in background signal measured is 30 pA [47]. The above results demonstrate the maximum allowable operating temperature of the C4RA-2IL column is limited. In the following work, the introduction of ILs units with longer alkyl chains on the calixarene molecules may improve the thermal stability and film-forming properties of the calixarene stationary phase. The column loadability was determined by the mass of an analyte injected on a column, wherein the column efficiency drops to 80% of the maximum value (N/Nmax = 0.80) and the peak width at half height (w1/2) value dramatically increases [48]. In this work, effect of analyte mass on the N/Nmax and w1/2 values on the C4RA-2IL column was measured by injecting different amount of p-toluidine. Figure S3a, b shows the effect of the analyte mass on the column efficiency, showing noticeable efficiency decrease, peak width broadening and peak asymmetry rise with the mass over 20 ng due to column overloading. Figure S3c shows the effect of the analyte mass on its peak area and retention time on the C4RA-2IL column, thereby suggesting a good linearity between the mass and its peak area over the range and the potential for quantitative analysis.
Conclusion
This work presents a novel calixarene-based stationary phase for capillary GC separations by combining the 3D aromatic cavity with the polar ionic liquids. The calixarene ionic liquid with unique amphiphilic architecture shows high separation performance for different types of analytes. Moreover, the C4RA-2IL column baseline resolved the challenging isomers of benzaldehydes, phenols, and anilines. Most interestingly, the C4RA-2IL column exhibits preferential retention for aromatic and H-bonding analytes, inherently originating from its unique 3D aromatic cavity with polar ionic liquids. The present work will encourage more research and applications of calixarene ionic liquid in chromatographic analysis.
References
Farmani Z, Schrader W (2019) Energies 12:3455–3466
Qian JN, Zhang YD, Liu XH, Xia JB (2019) Talanta 204:592–601
García-Valcárcel AI, Martínez-Ferrer MT, Campos-Rivela JM, Guil MDH (2019) Talanta 204:153–162
Ortega-Gavilán F, Valverde-Som L, Rodríguez-García FP, Cuadros-Rodríguez L, Bagur-González MG (2020) Food Chem 322:126743
Rivera-Pérez A, López-Ruiz R, Romero-González R, Garrido Frenich A (2020) Food Chem 321:126727
Zhang CY, Li MH, Guo MQ (2020) J Chromatogr A 1621:461024–461030
He XX, Han X, Wang H, Wang B, Wu B (2016) RSC Adv 6:76514–76523
Han X, Liu JC, Wang B, Du AQ, Xu L, Wu B (2018) J Chromatogr A 1578:76–82
Sun T, Shuai XM, Ren KX, Jiang XX, Chen YJ, Zhao XY, Song QQ, Hu SQ, Cai ZQ (2019) Molecules 24:3158
Nan H, Zhang C, O’Brien RA, Benchea A, Davis JH, Anderson JL (2017) J Chromatogr A 1481:127–136
Nan H, Anderson JL (2018) TrAC Trends Anal Chem 105:367–379
Fan J, Wang ZZ, Li Q, Qi ML, Shao SJ, Fu RN (2014) J Chromatogr A 1362:231–240
Zhang P, Qin SJ, Qi ML, Fu RN (2014) J Chromatogr A 1334:139–148
Xie SM, Zhang XH, Wang BJ, Zhang M, Zhang JH, Yuan LM (2014) Chromatographia 77:1359–1365
Xie SM, Zhang M, Fei ZX, Yuan LM (2014) J Chromatogr A 1363:137–143
Zhang WF, Zhang YH, Zhang YM, Lan C, Miao Y, Deng ZF, Ba X, Zhao WD, Zhang SS (2019) Talanta 193:56–63
Lu J, Zhang W, Zhang Y, Zhao W, Hu K, Yu A, Liu P, Wu Y, Zhang S (2014) J Chromatogr A 1350:61–67
Sun T, Li B, Li Y, Zhao XY, Song QQ, Jiang XX, Shuai XM, Li YY, Cai ZQ, Hu SQ (2019) Chromatographia 82:1697–1708
Zátopková R, Aturki Z, Bednář P (2020) J Sep Sci 43:3382–3390
Sun WY, Jin Y, Wang CY, Zhao SS, Wang X, Luo M, Yan JZ, Tong SQ (2020) J Chromatogr A 1617:460834–460843
Feng H, Ye X, Liu Y, Wang Z, Gao T, Cheng A, Wang X, Chen J (2020) J Chromatogr A 1624:461234
Shinkai S (1993) Tetrahedron 49:8933–8968
Kim H, Lee MH, Mutihac L, Vicens J, Kim JS (2012) Chem Soc Rev 41:1173–1190
Yilmaz M, Erdemir S (2013) Turk J Chem 37:558–585
Edwards NY, Possanza AL (2013) Supramol Chem 25:446–463
Zhao BT, Zhu XM, Chen XH, Yan ZN, Zhu WM (2013) Chin Chem Lett 24:573–577
Zhao BT, Peng QM, Zhu XM, Yan ZN, Zhu WM (2015) J Org Chem 80:1052–1058
Yan Z, Lu Y, Wang H, Wu S, Zhao B (2013) J Mol Liq 183:72–78
Yang BC, Wang CB, Zhao BT, Zhang SS, Ye BX (2012) Int J Environ Anal Chem 92:1776–1785
Liu M, Liao W, Hu C, Du S, Zhang H (2012) Angew Chem Int Ed 51:1585–1588
Mokhtari B, Pourabdollah K (2012) J Incl Phenom Macrocycl Chem 73:1–15
Mokhtari B, Pourabdollah K, Dalali N (2011) J Incl Phenom Macrocycl Chem 69:1–55
Mokhtari B, Pourabdollah K, Dalali N (2011) Chromatographia 73:829–847
Śliwka-Kaszyńska M, Ślebioda M (2014) J Sep Sci 37:543–550
Delahousse G, Lavendomme R, Jabin I, Agasse V, Cardinael P (2015) Curr Org Chem 19:2237–2249
Hu K, Zhao W, Wen F, Liu J, Zhao X, Xu Z, Niu B, Ye B, Wu Y, Zhang S (2011) Talanta 85:317–324
Hu K, Deng Z, Wang B, Cui Y, Miao M, Liu W, Jiang Q, Zhao W, Huang Y, Zhang S (2015) J Sep Sci 38:60–66
Zadmard R, Tabar-Heydar K, Imani M (2014) J Chromatogr Sci 53:702–707
Poole CF, Lenca N (2014) J Chromatogr A 1357:87–109
Pashirova TN, Gibadullina EM, Burilov AR, Kashapov RR, Zhiltsova EP, Syakaev VV, Habicher WD, Rümmeli MH, Latypov SK, Konovalov AI, Zakharova LY (2014) RSC Adv 4:9912–9919
Padnya PL, Andreyko EA, Gorbatova PA, Parfenov VV, Rizvanov IK, Stoikov II (2017) RSC Adv 7:1671–1686
Sun T, Jiang XX, Song QQ, Shuai XM, Chen YJ, Zhao XY, Cai ZQ, Li K, Qiao XG, Hu SQ (2019) RSC Adv 9:28783–28792
Peng J, Sun T, Wu L, Qi M, Huang X (2017) RSC Adv 7:45408–45415
Sun T, Chen H, Qiao X, Ma L, Hu S, Liu X (2018) RSC Adv 8:34102–34109
Lv Q, Zhang Q, Qi M, Bai H, Ma Q, Meng X, Fu R (2015) J Chromatogr A 1404:89–94
Zhang YF, Qi ML, Fu RN (2016) Chin Chem Lett 27:88–90
Roeleveld K, David F, Lynen F (2016) J Chromatogr A 1451:135–144
Shashkov MV, Sidelnikov VN (2013) J Chromatogr A 1309:56–63
Acknowledgements
The work was supported by the National Natural Science Foundation of China (No. 21705072), Natural Science Foundation of Liaoning Province (20180550016), Scientific Research Fund Liaoning Provincial Education Department of China (LJGD2020015), Henan Province Science and Technology Attack Plan Foundation (No. 202102310485).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shuai, X., Cai, Z., Zhao, X. et al. A New Stationary Phase for Capillary Gas Chromatography: Calix[4]resorcinarene Functionalized with Imidazolium Cationic Units. Chromatographia 84, 325–333 (2021). https://doi.org/10.1007/s10337-021-04018-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-021-04018-x