Abstract
In this study, octylbenzimidazolium-modified silica (BeImC8-Sil) was prepared by covalent attachment of 1-octylbenzimidazole to γ-chloropropyl silica. The synthesized materials were characterized by the elemental analysis, IR spectrum, and thermogravimetric analysis. Due to the introduction of phenyl and octyl groups on the quaternary imidazolium, the developed BeImC8-Sil column can function via both reversed-phase and anion-exchange retention mechanisms. The chromatographic properties of the synthesized material were investigated by the separations of polycyclic aromatic hydrocarbons, mono-substituted derivatives of benzene, anilines, and phenols, revealing the existence of multiple interactions, including hydrogen bonding, π–π stacking, electrostatic forces, and hydrophobic interactions in reversed-phase mode; inorganic and organic anions were also separated mainly through anion-exchange interaction. The proposed BeImC8-Sil is a promising mixed-mode stationary phase for the separation of complex samples in high-performance liquid chromatography.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High-performance liquid chromatography (HPLC) has widely been used as an essential analysis tool in the fields of pharmaceutical quality control, food analysis and inspection, environmental analysis, and biomolecular separation and detection [1, 2]. The most commonly used HPLC stationary phases often possess unique interaction mechanism, such as hydrophobic interaction for separating non-polar compounds on reversed-phase packing and ion-exchange interaction for separating ions on ion-exchange packing. However, some complex samples from pharmacology, environment, and food are often mixtures with different properties, thus cannot be separated efficiently on stationary phases possessing single separation mechanism.
Mixed-mode stationary phase, relative to single-mode, is a kind of promising stationary phase which can be applied in different modes on single column based on the multiple interaction mechanisms. This kind of stationary phase can reduce the testing cost and improve work efficiency to a great extent, especially for the analysis of some complex samples [3, 4]. These stationary phases took advantage of the functional groups with charged or chargeable moieties. Ionic or electrostatic interaction was involved in the separation of ionic analytes with the ion-exchange chromatographic condition [4]. For example, Lämmerhofer and his co-workers prepared the mixed-mode reversed-phase/weak anion-exchange separation materials by functionalizing thiol-modified silica with N-(10-undecenoyl)-3-amino quinuclidine and N-(10-undecenoyl)-3-α-aminotropane, respectively [5–7]. In addition, the mixed-mode phases have distinct interaction sites on a single chromatographic ligand, such as a hydrophobic alkyl chain strand (hydrophobic domain), embedded polar functional groups (hydrophilic domains), and a terminal bicyclic quinuclidine or tropane ring which accommodate the anion-exchange site (ionic domain). Many other nitrogen compounds, such as amine [8, 9], pyridine [10–13], 8-quinolinol [14], and imidazolium [15] were also immobilized to silica gel through a coupling carbon chain to achieve a mixed-mode reversed-phase/anion-exchange stationary phase. Since the phase combines hydrophobic-binding domains with terminal anion-exchange moiety, it exhibits both RP and anion-exchange chromatographic modes to achieve complementary selectivity. Multiple retention mechanisms, including hydrogen bonding, hydrophobic, electrostatic, and π–π interactions were coexisted.
Imidazolium is a widely used cation in ionic liquids (ILs), which constitutes novel solvents or materials with excellent physical and chemical properties. ILs can also be supported on silica, and they have been used for novel column packing materials termed surface-confined ILs (SCILs) or IL-modified silica stationary phases [15]. Because of the unique properties of SCILs, they have been used for the separation of various solutes, such as inorganic and organic anions, cations, bases, phenols, polycyclic aromatic hydrocarbons (PAHs), steroids, etc. A remarkable advantage of ILs is its tunability, i.e., the diversity of the species that can be generated by varying the organic cation, the inorganic or organic anion, and the length of the side chain attached to the organic cation. Imidazolium coupling with different functional groups (such as aliphatic chains, aromatic rings, sulfonic acid, etc.) was often immobilized to silica gel to achieve new stationary phases with special performances [16–26]. To our best knowledge, there is no report on imidazolium stationary phase simultaneously embedded phenyl and aliphatic group.
Octylbenzimidazole, formed from the simultaneous introduction of phenyl and octyl on the imidazolium, has displayed special physicochemical properties [27]. As a chromatographic ligand, octylbenzimidazole has distinct interaction sites. First, the aliphatic octyl on the ligand can interact with hydrophobic solutes in analogy with the conventional C18 columns. Second, the aromatic imidazolium and benzene group could lead to an enhanced selectivity to aromatic compounds, such as PAHs by π–π interactions. Third, the polar cationic imidazolium can give anion-exchange, hydrogen bonding, and electrostatic repulsion effect to specific solutes. In this work, the benzimidazole-embedded C8-based-modified silica (BeImC8-Sil) was facilely prepared and further used for mixed-mode chromatographic separation. The new material could be utilized as a reversed-phase stationary phase for the separation of neutral solutes, as well as an anion-exchange stationary phase for the separation of inorganic anions, which indicates its multi-interaction mechanism and mixed-model characteristic, and its analytical prospect for complex samples.
Experimental
Apparatus and Materials
Silica gel (with particle size of 5 μm, pore size of 100 Å, and specific surface area of 300 m2 g−1) was provided by the Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences (Lanzhou, China). 3-Chloropropyltrimethoxysilane was purchased from Aladdin reagent Company (Shanghai, China). HPLC-grade acetonitrile and methanol were purchased from Kermel Chemical Reagent (Tianjin, China). Organics, including PAHs, mono-substituted benzenes, isomers of disubstituted benzene, anilines, and phenols, used in reversed-phase chromatographic tests, as well as inorganic salts, including potassium iodate, potassium bromide, potassium iodide, potassium bromate, and potassium thiocyanate used in anion-exchange chromatographic tests were analytical-grade reagents (AR). Potassium chloride used as a buffer salt in anion-exchange chromatographic tests was guaranteed reagent (GR). All the other compounds used in experiments were of analytical grade without further purification. Deionized water was obtained on Milli-Q purification equipment. C18, C8, home-made 1-methylimidazolium column (Key Laboratory for Natural Medicine of Gansu Province, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, 150 mm), and phenyl column (IBM commercial column, 150 mm) were used as referenced columns.
HPLC analysis was performed using an Agilent 1260 series system equipped with a 1200 model quaternary pump, a G1315A model DAD detector, and a G1316A model thermostatic column compartment. The elemental analysis was performed on a Flash EA 1112 elemental analyzer (Thermo Electron Corporation). IR spectra were performed on a Brucker IFS120HR Fourier-transform spectrometer (Ettlingen, Germany). The thermogravimetric analysis was carried out with a Shimadzu DT-40 thermal analyzer, and the analysis was performed from 40 to 650 °C at heating rate of 10 °C min−1 in argon atmosphere with a gas flow rate of 20 mL min−1.
Preparation of BeImC8-Sil
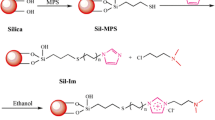
1-Octylbenzimidazole was synthesized according to the reported procedure [27]. The developed stationary phase was synthesized via two steps (Fig. 1). First, chloropropyl silica (SilprCl) was prepared based on the previous reports [24]. In brief, 3.0 g silica was placed in a 250 mL flask. After, it was suspended in 80 mL dried toluene; 3 mL 3-chloropropyltrimethoxysilane was added, followed by refluxing and mechanical agitation for 24 h at 110 °C under nitrogen atmosphere. The resulting product, chloropropyl silica (SilprCl), was cooled to room temperature, and washed with methanol and methanol/water (1:1, v/v) repeatedly. Finally, SilprCl was dried under vacuum and used as a precursor in the following reaction.
Second, octylbenzimidazole-bonded stationary phase BeImC8-Sil was prepared. 3.0 g of SilprCl (3-chloropropyl silica) and 80 mL 1,4-dioxane were placed in a 250 mL reaction flask. Then, 1.0 g of octylbenzimidazole was added into the mixture. The reaction mixture was stirred under nitrogen at 100 °C for 48 h. Then, the reaction was stopped, and the modified silica was collected and washed with ethanol, water, and methanol successively. BeImC8-Sil was thus obtained and dried under vacuum at 75 °C for 10 h, before column packing and characterizations.
Results and Discussion
Characterization of BeImC8-Sil
In the infrared spectrum of SilprCl, the symmetric extension vibration of methylene (–CH2–) appeared at 790 cm−1, asymmetric extension vibration of –CH2– group at 2974 cm−1, and asymmetric extension vibration of –CH2–Cl at 1170 cm−1. After the covalent anchoring of benzimidazole, the IR spectrum of BeImC8-Sil indicated that the organic ligands are bonded onto silica gel. Peaks emerged at 2900 cm−1, which could be assigned to the aliphatic C–H stretching of the tetrahedral carbon. Peaks emerged around 1550 cm−1 were attributed to the aromatic C=C or C=N stretching vibration, and the new bands around 750 cm−1 were attributed to the aromatic C=C or C=N bending vibration, resulting from the presence of the benzimidazolium groups on BeImC8-Sil.
The quantitative information on the new packing was attained via the elemental analyses. The carbon content for SilprCl was 4.28 % (bonding density of chloropropyl ligand was approximately 4.1 µmol m−2), which elevated to 9.66 % after the immobilization of BeImC8; meanwhile the nitrogen content of BeImC8-Sil was 1.08 %. Based on the nitrogen content, the bonding amount of BeImC8 was ca. 1.5 µmol m−2. The relatively low density of BeImC8 was foreseeable, since the reaction was carried out heterogeneously, as well as the significant steric hindrance of benzene ring.
TG analysis curve showed that the weight loss temperature of BeImC8-Sil was higher than 330 °C, indicating that the packing material possessed reliable thermal stability at ambient temperature. The total weight loss was 8.32 % for BeImC8-Sil in the temperature range of 40–650 °C, which was attributed to the decomposition of organic species bonded on the silica surface together dehydration of silica from the condensation of silanols.
Tanaka Test
To comprehensively investigate the chromatographic properties and uniqueness of BeImC8-Sil, another four columns, including C18, Phenyl, C8, and a home-made 1-methylimidazolium stationary phase, were encompassed in the following chromatographic evaluations using the various types of test solutes, including PAHs, mono-substituted benzenes, phenols, anilines, inorganic anions, and organic acids (The chromatograms were not given). However, it was very important for selecting appropriate chromatographic conditions to perform separation and revealing the retention mechanism of the stationary phase. BeImC8-Sil was selected to separately investigate the chromatographic conditions on the retention factors.
The performance of BeImC8-Sil was first evaluated by the Tanaka test, a chromatographic standard test well established by Tanaka et al. [28, 29], which was commonly used to evaluate the hydrophobicity, and shape selectivity (capability to differentiate planar and non-planar solutes) of RP columns. The retention factor (k) of neutral solutes like alkylbenzenes on RP columns depended on the carbon content or ligands density of the stationary phases [29]. As shown in Table 1, BeImC8-Sil exhibits modest smaller k PB values than C18. These results indicate that the hydrophobicity of BeImC8-Sil is weaker than C18, which could be attributed to the lower carbon loading compared with C18. Retention factor ratio of pentylbenzene and butylbenzene, known as hydrophobic selectivity, is usually used to evaluate the hydrophobic potency of the stationary phase and surface coverage by accessing the ability of the phase to separate alkylbenzene solutes (with one methylene group difference). As shown in Table 1, both BeImC8-Sil and LiChrosorb C-18 phase share similar αCH2 value (1.32 for BeImC8-Sil and 1.42 for LiChrosorb C-18), which is higher than that of XTerra phenyl phase. It indicates that BeImC8better with the increase of benzene number-Sil possessed the capacity to separate the compounds differing by only a methylene group.
Retention factor ratio of n-pentylbenzene and o-terphenyl previously suggested by Lindner [30] reflects the selectivity of stationary phase toward separating aromatic solutes. Making the comparison of the results displayed in Table 2, it is evident that BeImC8-Sil gives a relatively small value of αPB/O, indicating that the solute of o-terphenyl with higher number of aromatic rings is retained longer on the BeImC8-Sil phase than that of pentylbenzene, which should be ascribed to the stronger π–π interaction.
The retention factors ratio of triphenylene (planar) and o-terphenyl (non-planar) (α TR/O = k triphenylene/k o-terphenyl), an indicator for shape selectivity of the stationary phase, was then calculated to illustrate the capability of a stationary phase to differentially separate planar and non-planar analytes with similar molecular weights and sizes. As shown in Table 2, the higher shape selectivity (α TR/O = 2.58) was obtained for BeImC8-Sil compared with C18 (α TR/O = 1.66) and RP phenyl phases (α TR/O = 0.88), which indicates that BeImC8-Sil has a better capacity to differentiate compounds with spatial configuration. The possible reason can also be ascribed to π–π interaction as discussed above, as the planar triphenylene with a larger delocalization π electrons conjugated system than that of non-planar o-terphenyl, the π–π interaction was stronger between triphenylene and BeImC8-Sil, so it retained longer on the BeImC8-Sil. Furthermore, five PAHs were separated on BeImC8-Sil to evaluate its π–π interaction. As shown in Fig. 2, under the same chromatographic conditions, the retention times increase with the extension of the π-electron system in five PAHs, which indicates the presence of the π–π interaction between BeImC8-Sil and PAHs. In addition, under the adjustment of π–π interaction, the selectivity also became better with the increase of benzene number.
Separation of Mono-Substituted Derivatives of Benzene
Baseline separation of the mono-substituted derivatives of benzene with high column efficiency (21000 plates m−1) and high selectivity was achieved on the BeImC8-Sil packed column within 20 min (Fig. 3). The octanol/water partition coefficient (log P) is often used as a measurement of the molecular hydrophobicity. Compounds with larger log P values are usually more hydrophobic. Comparing with the retentions of six mono-substituted derivatives of benzene, the solutes with larger log P values were retained longer, which illustrate the important role of hydrophobic interaction on BeImC8-Sil. In addition, as the log P values of toluene and chlorobenzene are closer, they cannot be separated on C18 due to the similar hydrophobicity [31]. However, baseline separation can be obtained on BeImC8-Sil, which should be ascribed to the dipole–dipole interaction due to the existence of polar imidazolium groups.
Separation of Polar Phenols and Anilines
The test polar mixture composed of p-dihydroxyhenzene (log P, 0.59; pKa, 10.85), m-dihydroxybenzene (log P, 0.8; pKa, 9.32), phenol (log P, 1.46; pKa, 9.99), o-dihydroxyhenzene (log P, 0.88; pKa, 9.45), o-chlorophenol (log P, 2.15; pKa, 8.56), and 1-naphthol (log P, 2.85; pKa, 9.34) was used to investigate the chromatographic performance of BeImC8-Sil. As shown in Fig. 4, the separation of six phenols is obtained with 50 % (v/v) methanol–water. Different from benzimidazolium-boned counterpart without aliphatic substituent [24], the hydrophobicity of BeImC8-Sil which resulted from the introduction of C8 group shows an important role in the separation of phenols. The stronger retention of o-dihydroxybenzene than phenol may be attributed to the hydrogen-bonding interaction. For 1-naphthol, it was more strongly retained than other phenols, which may be resulted from stronger π–π and hydrophobic interactions. In a word, the hydrophobic interaction, as well as π–π interaction, electrostatic repulsion, and hydrogen bond interaction were all involved in the separation mechanism of phenols on BeImC8-Sil. Under the comprehensive adjustment of the multiple interactions, the mixture of anilines was also well separated (Fig. 5). All pKa and log P data in this paper were taken from SRC PhysProp database.
Separation of Inorganic and Organic Anions
Based on the cation composed of nitrogen ring atom with a localized pair, imidazole was often used as the anion-exchange chromatography ligand to separate organic or inorganic anions. Using KCl solution as the mobile phase, BeImC8-Sil can also be applied as an anion-exchange stationary phase. Inorganic anions composed of IO3 −, BrO3 −, Br−, I−, and SCN− were chosen to investigate the anion-exchange characteristic of the BeImC8-Sil phase in anion-exchange mode. As shown in Fig. 6, it is easy to understand that the retentions of inorganic anions decrease with the increase of KCl concentration. It was observed in Fig. 7 that all components of the test mixture were fully separated on BeImC8-Sil using 25 mmol L−1 KCl-methanol (95/5, v/v) as mobile phase. The test organic anions mixture composed of benzoic acid (log P, 1.87; pKa, 4.19), p-aminobenzoic acid (log P, 0.83; pKa, 2.38), p-hydroxybenzoic acid (log P, 1.58; pKa, 4.54), p-methylbenzoic acid (log P, 2.27; pKa, 4.37), and p-chlorobenzoic acid (log P, 2.65; pKa, 3.98) were well separated on BeImC8-Sil column using 35 % (v/v) methanol in 10 mmol L−1 KH2PO4 solution as a mobile phase in Fig. 8. There is neither hydrophobic nor electrostatic correlation observed for the retention of benzoic acid derivatives on the BeImC8-Sil which indicates that the synergistic interaction based on the multiple interactions controls their chromatographic behaviors.
Conclusions
The preparation and characterization of a reversed-phase/anion-exchange mixed-mode stationary phase based on octylbenzimidazolium-functionalized silica were described. Different series of analytes, including PAHs, anilines, phenols, and inorganic anions, were successfully separated on this novel multi-modal stationary phase. In comparison with other stationary phases such as C8, C18, phenyl, and 1-methylimidazolium-based columns, the existence of multi-interaction mechanisms, including π–π, hydrophobic, electrostatic, and anion-exchange interactions was confirmed between BeImC8-Sil and analytes. With such characteristics of multi-interaction mechanism and mixed-mode separation potency, this stationary phase has a promising application in the analysis of complex samples.
References
Snyder LR, Kirkland JJ, Dolan JW (2010) Introduction to modern liquid chromatography. Wiley, New Jersey
Fekete S, Oláh E, Fekete J (2012) Fast liquid chromatography: the domination of core-shell and very fine particles. J Chromatogr A 1228:57–71
Yang Y, Geng X (2011) Mixed-mode chromatography and its applications to biopolymers. J Chromatogr A 1218:8813–8825
Mansour FR, Danielson ND (2013) Multimodal liquid chromatography of small molecules. Anal Methods 5:4955–4972
Apfelthaler E, Bicker W, Lämmerhofer M, Sulyok M, Krska R, Lindner W, Schuhmacher R (2008) Retention pattern profiling of fungal metabolites on mixed-mode reversed-phase/weak anion exchange stationary phases in comparison to reversed-phase and weak anion exchange separation materials by liquid chromatography–electrospray ionisation-tandem mass spectrometry. J Chromatogr A 1191:171–181
Bicker W, Lämmerhofer M, Lindner W (2008) Mixed-mode stationary phases as a complementary selectivity concept in liquid chromatography–tandem mass spectrometry-based bioanalytical assays. Anal Bioanal Chem 390:263–266
Dietrich MA, Adamek M, Bilińska B, Hejmej A, Steinhagen D, Ciereszko A (2011) Multi-modal applicability of a reversed-phase/weak-anion exchange material in reversed-phase, anion-exchange, ion-exclusion, hydrophilic interaction and hydrophobic interaction chromatography modes. Anal Bioanal Chem 400:2517–2530
Kiseleva MG, Nesterenko PN (2000) Phenylaminopropyl silica—a new specific stationary phase for high-performance liquid chromatography of phenols. J Chromatogr A 898:23–34
Kiseleva MG, Radchenko LV, Nesterenko PN (2001) Ion-exchange properties of hypercrosslinked polystyrene impregnated with methyl orange. J Chromatogr A 920:79–85
Takeuchi T, Kawasaki T, Lim LW (2010) Separation of inorganic anions on a pyridine stationary phase in ion chromatography. Anal Sci 26:511–514
Auler LMLA, Silva CR, Collins KE, Collins CH (2005) New stationary phase for anion-exchange chromatography. J Chromatogr A 1073:147–153
Auler L, Silva CR, Bottoli C, Collins CH (2011) Anion separations for liquid chromatography using propylpyridinium silica as the stationary phase. Talanta 84:1174–1179
Sun M, Feng J, Liu S, Xiong C (2011) Polydopamine supported preparation method for solid-phase microextraction coatings on stainless steel wire. J Chromatogr A 1218:3601–3607
Muenter MM, Stokes KC, Obie RT, Jezorek JR (1999) Simultaneous separation of inorganic ions and neutral organics on ion-exchange stationary phases. J Chromatogr A 844:39–51
Zhang M, Liang X, Jiang S, Qiu H (2014) Preparation and applications of surface-confined ionic-liquid stationary phases for liquid chromatography. TrAC Trends Anal Chem 53:60–72
Qiu H, Jiang S, Liu X, Zhao L (2006) Novel imidazolium stationary phase for high-performance liquid chromatography. J Chromatogr A 1116:46–50
Bi W, Zhou J, Row KH (2010) Separation of xylose and glucose on different silica-confined ionic liquid stationary phases. Anal Chim Acta 677:162–168
Qian W, Baker GA, Baker SN, Colón LA (2006) Surface confined ionic liquid as a stationary phase for HPLC. Analyst 131:1000–1005
Qiu H, Jiang Q, Wei Z, Wang X, Liu X, Jiang S (2007) Preparation and evaluation of a silica-based 1-alkyl-3-(propyl-3-sulfonate) imidazolium zwitterionic stationary phase for high-performance liquid chromatography. J Chromatogr A 1163:63–69
Qiu H, Jiang Q, Liu X, Jiang S (2008) Comparison of anion-exchange and hydrophobic interactions between two new silica-based long-chain alkylimidazolium stationary phases for LC. Chromatographia 68(3–4):167–171
Sun Y, Cabovska B, Evans CE, Ridgway TH, Stalcup AM (2005) Retention characteristics of a new butylimidazolium-based stationary phase. Anal Bioanal Chem 382:728–734
Chitta KR, Meter DSV, Stalcup AM (2009) Separation of peptides by HPLC using a surface-confined ionic liquid stationary phase. Anal Bioanal Chem 396:775–781
Meter DSV, Oliver NJ, Carle AB, Dehm S, Ridgway TH, Stalcup AM (2009) Characterization of surface-confined ionic liquid stationary phases: impact of cation and anion identity on retention. Anal Bioanal Chem 393:283–294
Sun M, Feng J, Luo C, Liu X, Jiang S (2013) Benzimidazole modified silica as a novel reversed-phase and anion-exchange mixed-mode stationary phase for HPLC. Talanta 105:135–141
Qiu H, Mallik AK, Takafuji M, Liu X, Jiang S, Ihara H (2012) A new imidazolium-embedded C18 stationary phase with enhanced performance in reversed-phase liquid chromatography. Anal Chim Acta 738:95–101
Qiao X, Ye M, Xiang C, Wang Q, Liu CF, Miao WJ (2012) Analytical strategy to reveal the in vivo process of multi-component herbal medicine: a pharmacokinetic study of licorice using liquid chromatography coupled with triple quadrupole mass spectrometry. J Chromatogr A 1258:84–93
Shannon MS, Hindman MS, Danielsen SPO, Tedstone JM, Gilmore RD, Bara JE (2012) Properties of alkylbenzimidazoles for CO2 and SO2 capture and comparisons to ionic liquids. Sci China Chem 55:1638–1647
Kimata K, Iwaguchi K, Onishi S, Jinno K, Eksteen R, Hosoya K, Araki M, Tanaka N (1989) Chromatographic characterization of silica C18 packing materials. Correlation between a preparation method and retention behavior. of stationary phase. J Chromatogr Sci 27:721–728
Tanaka N, Tokuda Y, Iwaguchi K, Araki M (1982) Effect of stationary phase structure on retention and selectivity in reversed-phase liquid chromatography. J Chromatogr A 239:761–772
Horak J, Maier NM, Lindner W (2004) Investigations on the chromatographic behavior of hybrid reversed-phase materials containing electron donor-acceptor systems II. Contribution of π–π aromatic interactions. J Chromatogr A 1045:43–58
Zhao Q, Wei F, Xiao N, Yu Q-W, Yuan B-F, Feng Y-Q (2012) Dispersive microextraction based on water-coated Fe3O4 followed by gas chromatography-mass spectrometry for determination of 3-monochloropropane-1,2-diol in edible oils. J Chromatogr A 1240:45–51
Acknowledgments
The authors express their thanks to the support of the “Hundred Talents Program” of the Chinese Academy of Sciences, and the National Natural Science Foundation of China (No. 21275133).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Jiang, Q., Zhao, W., Qiu, H. et al. Silica-Based Phenyl and Octyl Bifunctional Imidazolium as a New Mixed-Mode Stationary Phase for Reversed-Phase and Anion-Exchange Chromatography. Chromatographia 79, 1437–1443 (2016). https://doi.org/10.1007/s10337-016-3166-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-016-3166-1