Abstract
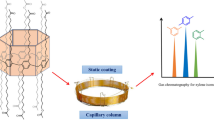
In this paper, an amphiphilic calix[8]arene with polyethylene glycol unit branches (C8A-PEG) was synthesized and applied for capillary gas chromatography (GC). The C8A-PEG was coated on the inner wall of a capillary column by a static method with the column efficiency of 3165 plates/m and polar nature. As demonstrated, the C8A-PEG column has excellent physicochemical properties and separation performance since it has π-electron-rich 3D cavity which combines with polar PEG units. Compared with two columns corresponding to the construction units C8A and PEG, the C8A-PEG column shows distinctly advantageous performance for the mixture of 22 components with diverse types. Impressively, it shows satisfactory resolution for positional isomers and cis-/trans- isomers, especially the challenging isomers of toluidine and dimethylaniline. The outstanding distinguishing capability of the C8A-PEG stationary phase is mainly attributed to the abundant molecular recognition interactions, including van der Waals, dipole–dipole, H-bonding and π–π stacking interactions. This work has proved that the new GC stationary phases constructed by different units can complement each other's advantages, improve their physicochemical properties and separation performance, and have broad application prospects in chromatographic analysis.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Capillary gas chromatography (GC) has been widely applied in analytical science due to the characteristics of low cost, high sensitivity and rapid speed [1,2,3,4]. The selection of suitable stationary phase determines the efficiency of GC analysis, thus diverse stationary phases with high selectivity, such as polysiloxane [5, 6], polyethylene glycol (PEG) [7,8,9], macrocycles [10, 11] and ionic liquids [12, 13] have been investigated recently to meet the demand of GC separations for a wide range of samples. PEG, is a kind of polyether polymer containing repeating oxyethylene subunits (–CH2–CH2–O–), and has been widely used as commercial and laboratory-made stationary phases in GC owing to the support-deactivating, low melting point and good film-forming property [8, 14]. It exhibits high selectivity for polar hydrogen-bonded compounds with similar boiling points, which can be credit to the ether bond together with terminal hydroxyl groups greatly increase the polarity [9]. For instances, Shende et al. reported a sol–gel chemistry-based stationary phase by surface-bonded with PEG, and it exhibited high chromatographic performances such as high number of theoretical plates and pronounced selectivity for acid and aniline [8]. Qi et al. introduced the three-dimensional (3D) rigid triptycene scaffold into the PEG backbone, exhibiting high selectivity for polar aromatics and improving the distinguishing capability for anilines and phenols isomers [15]. However, PEG is not as widely used as polysiloxane in GC and the major problems are the unfavorable characteristics of easy oxidation originating from a central cleavage around the ether bonds [16]. Moreover, there are lesser density domains and holes with molecular size in polymer, which is caused by the irregular molecular stacking in the amorphous phase and molecular relaxation [17,18,19]. These holes form the free volume and have an impact on the physicochemical properties including viscosity and gas permeabilities, etc. [19]. To overcome the shortcomings of PEG for application in GC, and with the continuous exploring and developing of supramolecules in chromatographic separation, this paper proposes a strategy that incorporation of PEG chains into the calixarene.
Calix[n]arene, as the third class of supramolecules after crown ether and cyclodextrin (CD), having π-electron-rich structure with highly thermal and chemical stability. It is a kind of cavity-shape oligomer synthesized by the oligomerization of formaldehyde and 4-substituted phenols [20,21,22]. Due to the variable numbers of polymeric aryl units, the calix[n]arene owns a hydrophobic cavity with higher degree of steric flexibility in contrast to the crown ether and cyclodextrin (CD) [23, 24]. In a case, 4-tertButylcalix[8]arene (C8A) contains a cavity with inner size of 8.6 Å (regular cone conformation) larger than the γ-CD (8.5 Å) [11, 24], signifying it can provide a larger ‘molecular pocket’ to include more guest molecules, and form entities among the compounds of complementary topology via non-covalent interactions [25, 26]. Owing to the enchanting aromatic scaffold and valuable features, the C8A enables to act as a precursor for the design, synthesis and application in GC and high-performance liquid chromatography (HPLC). Li et al. prepared a stationary phase named CABS using the calix[8]arene bonded silica gel, and it exhibited high selectivity for some aromatic carboxylic acids in HPLC [27]. Liu et al. reported the separation mechanism of steroids on calix[8]arene bonded silica stationary phase in HPLC, and it was ascribed to π-π, H-bonding and hydrophobic interactions [28]. Mangia et al. first used the C8A as a GC stationary phase supported on Chromosorb W. Silanized, and it successfully realized the separation of alcohols, chlorinated hydrocarbons and aromatics [29]. In our previous work, a stationary phase (C8A–NO2) was synthesized by modifying the upper and low rim of C8A, and it revealed high resolving capacity for diverse isomers in GC [11]. Based on these, this work constructed amphiphilicity architecture consisting of hydrophobic C8A and hydrophilic PEG chains. It can resolve the disadvantages of C8A used in GC such as poor film-forming property and high melting point [11, 30], while the 3D rigid C8A units can restrict the oxidative degradation and molecular stacking of PEG [17, 19]. More importantly, as the designed platform, the ‘molecular pocket’ of calix[8]arene was conductive to the forming of PEG branches, and it not only integrates the chromatographic properties of each individual but also multiplies the interaction sites with guest molecules.
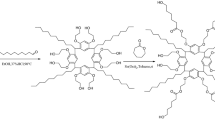
In general, the C8A-PEG was synthesized by chemically bonding the C8A with PEG (Scheme 1). Up to now, this is the first example that the calix[8]arene-based PEG stationary phase applied in GC, even though there were some reports on coupling calix[4]/[6]arene with PEG for GC separation [31,32,33]. For investigating its chromatographic performance, a mixture of 22 components were employed on C8A-PEG column and compared with that on C8A, PEG, commercial HP-35 and PEG-20 M columns, and discussing the influence of combining two different fragments having on the selectivity by injecting aromatics and alcohols. Furthermore, various positional isomers and cis-/trans-isomers were utilized to challenge its distinguishing capacity.
Experimental section
Materials and instruments
All the reagents and solvents were analytical grade and without further purification. 4-tert-Butylcalix[8]arene (C8A) and polyethylene glycol (PEG, M.W. 2000) were bought from Energy Chemical (Shanghai, China), and tosyl chloride (TsCl) was bought from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Untreated fused-silica capillary columns (0.25 mm, i.d.) were bought from Yongnian Ruifeng Chromatogram Apparatus Co., Ltd. (Hebei, China). The commercial capillary column HP-35 (5 m × 0.25 mm, i.d., 0.25 µm film thickness) was purchased from Agilent Technologies Co. Ltd. (Palo Alto, California, USA) and capillary column PEG-20 M (5 m × 0.25 mm, i.d., 0.25 μm film thickness) was obtained from Lanzhou Atech Technologies Co., Ltd. (Gansu, China). GC separations were operated on an Agilent 7890A GC system equipped with a split injector and a flame ionization detector (FID). High purity nitrogen (N2, 99.999%) was used as the carrier gas. IR spectrum and 1H NMR spectrum were recorded on a Tensor ΙΙ FT-IR spectrometer (Bruker Platinum ART, Karlsruhe, Germany) and a Bruker Biospin 400 MHz instrument (Bruker Biospin, Rheinstetten, Germany), respectively.
Synthesis of the PEG-OTs [15]
Polyethylene glycol (2.00 g, 10.00 mmol), tosyl chloride (TsCl) (0.19 g, 10.00 mmol) and triethylamine (TEA, 10 mL) were dissolved in dichloromethane (DCM, 20 mL). The mixture was stirred for 2 h at 25 ℃ under N2. Then, several drops of HCl (1 mol/L) were added into the mixture, and the anhydrous Na2CO3 was added into the solution. After filtering and evaporating in vacuo, the crude product was purified by column chromatography [CH2Cl2/MeOH (v/v = 1:9)] to obtain the oil (74% yield). IR (Fig S1, KBr, cm−1): 1095.13 (C–O–C), 1175.97 (S = O), 1188.83 (C–O–C), 1452.41 (C=C), 1597.63 (C=C), 2868.38 (CH2), 2943.79 (CH2), 3498.34 (OH).
Synthesis of the C8A-PEG
PEG-OTs (1.36 g, 0.63 mmol), C8A (0.10 g, 0.075 mmol) and K2CO3 (0.5 g, 3.62 mmol) were added in acetonitrile (ACN, 30 mL), the mixture was stirred for 24 h at 85 ℃ under nitrogen. Afterwards, the solution was washed with deionized water and NaOH solution, then the organic phase was evaporated in vacuo and the obtained crude product was purified by column chromatography [CH2Cl2/MeOH (v/v = 1:9)]. The tan oil was obtained at 40 ℃ in a vacuum (70% yield). 1H NMR (Fig S2, 400 MHz, CDCl3) δ: 7.02 (s, 16H, Ha), 3.74–3.60 (m, 720H, Hb + Hc + Hd + He + Hf), 1.23 (s, 72H, Hg). IR (Fig S3, KBr, cm−1): 1101.43 (C–O–C), 1497.70 (C=C), 1647.45 (C=C), 2878.76 (CH2), 2957.63 (CH2), 3338.92 (OH).
Fabrication of the C8A-PEG capillary column
Using untreated fused-silica capillary column (5 m × 0.25 m, i.d.) to fabricate the C8A-PEG column. First, the column was pretreated with a saturated solution of NaCl in Methanol (MeOH) and conditioned up to 200 ℃ (held 3 h) under N2 to rough its inner wall. Then, a solution of C8A-PEG in DCM (0.3%, w/v) was injected and the stationary phase was coated onto the capillary column by a static method [10, 34]. Specifically, one end of the column was sealed and the other was connected to the vacuum system, then the solvent was slowly evaporated at 40 ℃. Finally, the coated C8A-PEG column was conditioned from 40 ℃ (held for 30 min) to 160 ℃ at the rate of 1 ℃/min and maintained for 7 h under N2. C8A and PEG columns were fabricated with the same procedure.
Results and discussion
Characterization of the C8A-PEG stationary phase and its capillary column
The inherent thermal stability of C8A-PEG stationary phase was investigated by carrying out thermogravimetric analysis (TGA). As shown in Fig. 1a, a 5% weight loss of C8A-PEG remained at 312 ℃ which is superior to that of PEG (191 ℃), reflecting the greatly heightened features by covalently bonding with thermotolerant C8A (5% weight loss at 355 ℃). To comprehensively evaluate the column efficiency of C8A-PEG column, Golay curves were plotted by measuring the height equivalent to a theoretical plate (HETP) under different flow rates at 120 ℃, using naphthalene as a solute. As shown in Fig. 1b, the minimum HETP of C8A-PEG column remained 0.32 mm at 0.40 mL/min, corresponding to a column efficiency of 3165 plates/m, and it was greatly improved compared to the 1748 plates/m of PEG column. The result revealed the functionalization not only efficiently restricted the molecular stacking of PEG chains but also improved the film-forming ability of calix[8]arene.
Moreover, the McReynolds constants were determined by injecting five probes including benzene (X′), 1-butanol (Y′), 2-pentanone (Z′), 1-nitropropane (U′) and pyridine (S′) into the capillaries at 120 ℃ [35, 36]. As shown in Table 1, the average value of C8A-PEG stationary phase (402) suggested the high polarity, and it was lower than that of PEG and PEG-20 M stationary phases and higher than that of HP-35 and C8A stationary phases. The result can be attribute to the molecular distribution of PEG chains, which greatly affect the retention interactions between C8A-PEG stationary phase and analytes. Especially, the greatest values of Y′, U′ and S′ indicated that C8A-PEG column had strong dipolar and H-bonding interactions with testing probes due to the presence of PEG chains [13], and it may affect the selectivity of C8A-PEG column for polar analytes.
Separation performance and selectivity of the C8A-PEG capillary column
Selectivity for a mixture of 22 components
To more comprehensively investigate the selectivity of C8A-PEG stationary phase, the chromatogram of separating a mixture of 22 components was obtained with C8A-PEG column and compared to these obtained with PEG, C8A, commercial HP-35 and PEG-20 M columns. As illustrated in Fig. 2, C8A-PEG column had outstanding separation capability over its two bonded units (C8A and PEG) columns and two commercial columns, and achieved baseline separation (R ˃ 1.5) for all the components while C8A column exhibited seriously tailing peaks and other columns co-eluted or overlapped some analytes. Especially, some were co-eluted or overlapped on PEG column, such as the adjacent pairs of n-butylbenzene/n-tridecane (peaks 6/7 b.p. 183 ℃/235 ℃), 2,3-butanediol/1,2,4-trichlorobenzene/n-hexadecane (peaks 13/14/15 b.p. 183 ℃/211 ℃/287 ℃) and 1,4-dibromobenzene/2-dodecanone (peaks 16/17 b.p. 219 ℃/247 ℃). This finding revealed that the C8A-PEG and PEG stationary phases had some differences in retention behaviors even though these exhibited high similarity in retention order, and it can be credit to the introduction of π-electron-rich C8A. More than these, using C8A-PEG column also attained an interestingly later elution for the alcohols and anilines compared with using HP-35 column. In particular, it reversed the order of alcohols/n-alkanes with huge difference in boiling points (> 46 ℃), most probably due to the molecular distribution of PEG increased the polarity of stationary phase and enhance the dipole–dipole and H-bonding interactions with polar hydrogen-bonded compounds. This finding can be confirmed as follows: the adjacent pairs of n-tridecane/methyl octanoate/1,4-dichlorobenzene/1-heptanol (peaks 7/8/9/10 b.p. 253 ℃/179 ℃/174 ℃/156 ℃), n-pentadecane/2,3-butanediol (peaks 12/13 b.p. 268 ℃/183 ℃), 2-dodecanone/1-decanol/2,6-dimethylaniline (peaks 17/18/19 b.p. 247 ℃/233 ℃/216 ℃) and n-hexadecane/2-dodecanone/1-undecanol (peaks 15/17/20 b.p. 287 ℃/247 ℃/241 ℃) were eluted against their boiling point but relevance with polarity on C8A-PEG column, and the result was consistent with that on PEG and PEG-20 M columns but contrary to that on HP-35 column.
Chromatograms of a mixture of 22 components with diverse types obtained using C8A-PEG, C8A, PEG, HP-35 and PEG-20 M columns. Peaks: (1) ethylbenzene, (2) 1-bromohexane, (3) methyl hexanoate, (4) 1,3,5-trimethylbenzene, (5) 1-bromoheptane, (6) n-butylbenzene, (7) n-tridecane, (8) methyl octanoate, (9) 1,4-dichlorobenzene, (10) 1-heptanol, (11) 1,3,5-trichlorobenzene, (12) n-pentadecane, (13) 2,3-butanediol, (14) 1,2,4-trichlorobenzene, (15) n-hexadecane, (16) 1,4-dibromobenzene, (17) 2-dodecanone, (18) 1-decanol, (19) 2,6-dimethylaniline, (20) 1-undecanol, (21) 2,6-dimethylnaphthalene and (22) 1-dodecanol. Temperature program: 40 ℃ (keep 1 min) up to 160 ℃ (keep 5 min) at 10 ℃/min and flow rate: 0.6 mL/min
Selectivity for the π-conjugated analytes
Furthermore, the elution of trimethylbenzene isomers, dichlorobenzene isomers and alcohols on C8A-PEG and PEG columns were performed to clarify the selectivity of C8A-PEG stationary phase for π-conjugated analytes. As shown in Fig. 3a, the C8A-PEG column still retained the 1,2,4-trimethylbenzene and 1,2-dichlorobenzene more strongly than the adjacent alcohols if compared to the PEG column, even though both of them allowed strong dipole–dipole and H-bonding interactions with alcohols. The same phenomenon can be obtained from Fig. 3b, the increasing value of retention difference (Δk) reflected that the prolonged retention time on C8A-PEG column, in particular the more extended trend of trimethylbenzene and dichlorobenzene. This selectivity for the aromatics can be credit to the aromatic cavities to enhance the π-π stacking interaction between them.
a Chromatograms of trimethylbenzene isomers, dichlorobenzene isomers and alcohols: 1-pentanol, 1-hexanol, 1-heptanol obtained using C8A-PEG and PEG columns, b the retention difference of retention factor (Δk = kC8A-PEG-kPEG) between C8A-PEG and PEG columns. GC conditions on two columns: 40 ℃ (1 min) to 160 ℃ at 10 ℃/min. Flow rate at 0.6 mL/min
Resolving capacity for the aniline isomers
Aniline is widely applied in the chemical industry, and it was commonly considered to be high toxicity and carcinogenicity [37]. However, the separation of aniline isomers is a challenge due to their extremely high similarity in physical and chemical properties, and researches usually perform the detection with pre-treatment method including derivatization and microextraction in practice [37, 38]. It is meaningful to develop a high-performance stationary phase for the rapid and efficient separation, to simplify the operational steps. As above mentioned, the amphiphilic C8A-PEG integrated the chromatographic properties of each unit, in a case the presence of PEG and cavity-shaped C8A was conductive to the special selectivity for indicated compounds. This complementary advantage is more prominent, if separating dimethylaniline or toluidine isomer mixtures with high similarity in structure, boiling point and dipole moment (Fig. 4 and Table S1). As can be seen, the C8A-PEG column revealed higher resolution for all the critical pairs of isomers and a better peak shape for most components (Table S2) than the bonded unit (C8A, PEG) columns, especially it achieved baseline separation (R ˃ 1.5) for all the mixtures while PEG column overlapped critical pair of 2,3-/3,4-dimethylaniline (R2,3-/3,4- = 1.41 on PEG column). In view of the unique structure, the high-performance might be conductive to synergetic effect of molecular dipole–dipole, H-bonding and π–π stacking interactions.
Resolving capacity for various positional and cis-/trans-isomers
Furthermore, various positional isomers and cis-/trans-isomers were employed to evaluate the separation capability of C8A-PEG stationary phase. Figure 5 showed the chromatograms of using C8A-PEG separated aromatic isomers containing methylnaphthalene (Fig. 5a) and dimethylnaphthalene (Fig. 5b), halogenated benzenes (Fig. 5c–f), benzaldehydes (Fig. 5g and h) and dimethylphenol (Fig. 5i). All the isomers ranging from nonpolar to polar obtained baseline separation (R ˃ 1.5) on C8A-PEG column, which can be attribute to C8A-PEG allowed molecular van der Waals, π–π, H-bonding and dipole–dipole interactions with guest molecules. Figure 6 presents the separations of the nine cis-/trans-isomers on the C8A-PEG column. As shown, all the isomer mixtures were completely separated (R ˃ 1.5) with satisfactory chromatographic peak shapes on C8A-PEG column. The above findings indicated that the C8A-PEG column can separate different types of isomers and have good resolution ability for highly similar analytes.
Chromatograms of isomer mixtures: a methylnaphthalene, b dimethylnaphthalene, c dibromobenzene, d trichlorobenzene, e nitrochlorobenzene, f nitrobromobenzene, g nitrobenzaldehyde, h dichlorobenzaldehyde and i dimethylphenol obtained using C8A-PEG column. Temperature program: 40 ℃ (keep 1 min) up to 160 ℃ (keep 5 min) at 10 ℃/min and flow rate: 0.6 mL/min
Chromatograms of cis-/trans-isomers: a acetaldoxime, b nerol/geraniol, c nerolidol, d 2-methyl-4-propyl-1,3-oxathiane, e 2,5-dihydro-2,5-dimethoxyfuran, f 2,5-dimethyltetrahydrofuran, g 2,5-dimethoxytetrahydrofuran, h decahydronaphthalene and i 4-tert-butylcyclohexanol obtained using C8A-PEG column. Temperature program: 40 ℃ (keep 1 min) up to 160 ℃ (keep 5 min) at 10 ℃/min and flow rate: 0.6 mL/min
Conclusion
This work presents employing amphiphilic calix[8]arene as a new type of stationary phase for GC separations. An amphiphilic calix[8]arene polymer stationary phase was constructed by covalent bonding of calixarene and polyethylene glycol units, which inherited their respective performance advantages and selectivity characteristics. First, the separation capacity of the C8A-PEG column for complex samples clearly outperformed that of the C8A and PEG columns. Second, the C8A-PEG column has special retention interactions on aromatic and H-bonded analytes. As demonstrated, the C8A-PEG column exhibited high-resolution performance for different types of analytes and their isomers, especially the isomers of toluidine and dimethylaniline, which are difficult to separate in GC. The above work proves that the new GC stationary phases based on different material compositions can give full play to their respective advantages, and inspires us to develop more stationary phases with special structures to meet the requirements for separating complex samples.
Data availability
The data can be available from the corresponding author upon reasonable request.
References
Y. Mogi, Y. Kebukawa, K. Kobayashi, Anal. Sci. (2022). https://doi.org/10.2116/analsci.21P188
K. Shigeta, H. Tao, K. Nakagawa, T. Kondo, T. Nakazato, Anal. Sci. (2018). https://doi.org/10.2116/analsci.34.227
Z. Zhou, Y. Wang, F. Peng, F. Meng, J. Zha, L. Ma, Y. Du, N. Peng, L. Ma, Q. Zhang, L. Gu, W. Yin, Z. Gu, C. Tan, Angew. Chem. Int. Ed. (2022). https://onlinelibrary.wiley.com/doi/https://doi.org/10.1002/anie.202115939
H. Iwai, M. Yamamoto, M. Matsuo, D. Liu, M. Fukushima, Anal. Sci. (2021). https://doi.org/10.2116/analsci.20P304
T. Sun, Q.C. Huang, R.N. Chen, W. Zhang, Q. Li, A.P. Wu, G.X. Wang, S.Q. Hu, Z.Q. Cai, New J. Chem. (2021). https://doi.org/10.1039/D1NJ03893A
X. Han, X.X. He, H.A. Wang, B. Wang, B. Wu, J. Chromatogr. A (2016). https://doi.org/10.1016/j.chroma.2016.04.073
T. Sun, X.M. Shuai, K.X. Ren, X.X. Jiang, Y.J. Chen, X.Y. Zhao, Q.Q. Song, S.Q. Hu, Z.Q. Cai, Molecules (2019). https://doi.org/10.3390/molecules24173158
C. Shende, A. Kabir, E. Townsend, A. Malik, Anal. Chem. (2003). https://doi.org/10.1021/ac0207224
C.F. Poole, Q.L. Li, W. Kiriden, W.W. Koziol, J. Chromatogr. A (2000). https://doi.org/10.1016/S0021-9673(00)00829-3
T. Sun, Q.C. Huang, W. Zhang, R.N. Chen, W. Li, H.P. Chen, S.Q. Hu, Z.Q. Cai, J. Chromatogr. A (2022). https://doi.org/10.1016/j.chroma.2022.463008
X.M. Shuai, Z.Q. Cai, Y.J. Chen, X.Y. Zhao, Q.Q. Song, K.X. Ren, X.X. Jiang, T. Sun, S.Q. Hu, Microchem. J. (2020). https://doi.org/10.1016/j.microc.2020.105124
X.M. Shuai, Z.Q. Cai, X.Y. Zhao, Y.J. Chen, Q. Zhang, Z.W. Ma, J.J. Hu, T. Sun, S.Q. Hu, Chromatographia (2021). https://doi.org/10.1007/s10337-021-04018-x
H. Nan, J.L. Anderson, TrAC, Trends Anal. Chem. (2018). https://doi.org/10.1016/j.trac.2018.03.020
F.R. Mansour, L. Zhou, N.D. Danielson, Chromatographia (2015). https://doi.org/10.1007/s10337-015-2983-y
Y.R. He, M.L. Qi, J. Chromatogr. A (2020). https://doi.org/10.1016/j.chroma.2020.460928
J. Glastrup, Polym. Degrad. Stab. (2003). https://doi.org/10.1016/S0141-3910(03)00097-1
S. Eceolaza, M. Iriarte, C. Uriarte, J. del Rio, A. Etxeberria, Eur. Polym. J. (2012). https://doi.org/10.1016/j.eurpolymj.2012.04.018
K. Hagiwara, T. Ougizawa, T. Inoue, K. Hirata, Y. Kobayashi, Radiat. Phys. Chem. (2000). https://doi.org/10.1016/S0969-806X(00)00211-5
A.W. Thornton, K.M. Nairn, A.J. Hill, J.M. Hill, J. Membr. Sci. (2009). https://doi.org/10.1016/j.memsci.2009.03.053
T. Sun, X.M. Shuai, Y.J. Chen, X.Y. Zhao, Q.Q. Song, K.X. Ren, X.X. Jiang, S.Q. Hu, Z.Q. Cai, RSC Adv. (2019). https://doi.org/10.1039/C9RA07798G
J. Zhang, G. Podoprygorina, V. Brusko, V. Bohmer, A. Janshoff, Chem. Mater. (2005). https://doi.org/10.1021/cm047980x
T. Sun, L.R. Qi, W.W. Li, Y. Li, X.M. Shuai, Z.Q. Cai, H.O. Chen, X.G. Qiao, L.F. Ma, J. Chromatogr. A (2019). https://doi.org/10.1016/j.chroma.2019.04.068
Y. Xue, Y. Guan, A.N. Zheng, H.N. Xiao, Colloids Surf. B (2013). https://doi.org/10.1016/j.colsurfb.2012.06.022
N.Q. Li, B. Zhou, H.X. Luo, W.J. He, Z.J. Shi, Z.N. Gu, X.H. Zhou, J. Solid State Electrochem. (1998). https://doi.org/10.1007/s100080050096
D. Budurova, D. Momekova, G. Momekov, P. Shestakova, H. Penchev, S. Rangelov (2021). https://doi.org/10.3390/pharmaceutics13122025
C. Meenakshi, P. Sangeetha, V. Ramakrishnan, J. Lumin. (2013). https://doi.org/10.1016/j.jlumin.2012.12.055
L.S. Li, M. Liu, S.L. Da, Y.Q. Feng, Talanta (2004). https://doi.org/10.1016/j.talanta.2003.09.015
M. Liu, L.S. Li, S.L. Da, Y.Q. Feng, Talanta (2005). https://doi.org/10.1016/j.talanta.2004.09.022
A. Mangia, A. Pochini, Ŕ Ungaro, G.D. Andreetti, Anal. Lett. (2006). https://doi.org/10.1080/00032718308067960
X.H. Lai, L. Lin, C.Y. Wu, Chromatographia (1999). https://doi.org/10.1007/BF02493674
R.N. Chen, Z.Q. Cai, W. Li, Q.C. Huang, D. Nardiello, M. Quinto, X.M. Liu, S.Q. Hu, T. Sun, Chem. Biodiversity (2022). https://doi.org/10.1002/cbdv.202200829
G. Delahousse, V.P. Agasse, J.C. Debray, M. Vaccaro, G. Cravotto, I. Jabin, P. Cardinael, J. Chromatogr. A (2013). https://doi.org/10.1016/j.chroma.2013.10.007
Q.C. Huang, Z.Q. Cai, W. Li, R.N. Chen, W. Zhang, K.Y. Jin, Y. Zhao, Y.W. Li, Tao Sun. Anal. Lett. (2022). https://doi.org/10.1080/00032719.2022.2143794
J. Bouche, M. Verzele, J. Chromatogr. Sci. (1968). https://doi.org/10.1093/chromsci/6.10.501
I.G. Zenkevich, A.A. Makarov, J. Anal. Chem. (2005). https://doi.org/10.1007/s10809-005-0193-8
W.O. McReynolds, J. Chromatogr. Sci. (1970). https://doi.org/10.1093/chromsci/8.12.685
J.M.P. Wasylka, C. Morrison, M. Biziuk, J. Namiesnik, Chem. Rev. (2015). https://doi.org/10.1021/cr4006999
M.M. Chen, G.H. Zhu, J.X. Xu, H.R. Zhang, J.S. Liu, K.Z. Jiang, Rapid Commun. Mass Spectrom. (2017). https://doi.org/10.1002/rcm.8043
Acknowledgements
The work was supported by the National Natural Science Foundation of China (No. 21705072), Natural Science Foundation of Liaoning Province (20180550016), the Training Project for Youth Backbone Teachers in Colleges and Universities of Luoyang normal university, and the Colleges and Universities in Henan Province Key Science and Research Project (No. 23A150008).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Q., Cai, Z., Chen, R. et al. Separation performance of the calix[8]arene functionalized with polyethylene glycol units for capillary gas chromatography. ANAL. SCI. 39, 989–998 (2023). https://doi.org/10.1007/s44211-023-00307-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44211-023-00307-7