Abstract
Herein, we describe a simple and inexpensive protocol for the hydrothiolation of alkynes. In this context, water extract of rice straw ash (WERSA) has been proven to be a green, mild and efficient solvent for the preparation of vinyl thioethers. Generally, it was found that alkyne and thiol derivatives were excellent reaction partners, producing the corresponding products with good yields and good stereoselectivity with the predominant formation of the Z isomer. Moreover, WERSA was recovered and reused for further catalytic reactions without a significant loss of activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water extract of agro-waste ashes (AWEs) have recently attracted considerable attention in modern organic synthesis since these solvents are non-toxic, inexpensive and biodegradable (Sarmah et al. 2017). In this context, AWE have been successfully used as green catalytic media in several organic transformations (Konwar et al. 2016; Chia et al. 2018), such as the Suzuki–Miyaura (Boruah et al. 2015a, b; Sarmah et al. 2016) and Sonogashira reactions (Dewan et al. 2016). More specifically, water extract of rice straw ash (WERSA) has become the most powerful solvent for organic synthesis (Saikia et al. 2015). This solvent is primarily constituted by SiO2 (74.31%), K2O (11.30%), P2O5 (2.65%), MgO (1.89%), Na2O (1.85%), CaO (1.61%), Al2O3 (1.40%), Fe2O3 (0.73%) and TiO2 (0.02%) (Jenkins et al. 1996). The unique properties of WERSA make this solvent particularly valuable in a series of transformations (Mahanta et al. 2016; Boruah et al. 2015a, b), including the Dakin (Saikia and Borah 2015) and Henry reactions (Surneni et al. 2016).
Furthermore, organosulfur derivatives have become a valuable class of compounds that are useful intermediates in the preparation of target molecules with synthetic (Silveira et al. 2017) as well as biological applications (Li et al. 2013). In this regard, vinyl thioethers have been efficiently applied in several transformations (Palomba et al. 2016), including total synthesis (Pearson et al. 2004). Due to the extremely wide range of applications of vinyl thioethers, several research groups have reported suitable methodologies for obtaining vinyl thioethers (Singh et al. 2013; Rodygin et al. 2017). For example, these compounds can be prepared through metal-catalyzed cross-coupling reactions of vinyl halides with either thiols (Reddy et al. 2009) or diorganyl disulfides (Kundu et al. 2013; Gonçalves et al. 2013). However, the most useful and atom-economical method for the preparation of vinyl thioethers is the hydrothiolation of alkynes (Strydom et al. 2017). Among these, catalytic hydrothiolation techniques have emerged as a conveniently alternative to afford vinyl thioethers which can be obtained by either anti-Markovnikov- or Markovnikov-type addition (Malyshev et al. 2006; Beletskaya and Ananikov 2007, 2011). Generally, this strategy can yield the respective products at different levels of stereo- and regioselectivity under different conditions (Dondoni and Marra 2014; Palacios et al. 2016). In particular, the selective preparation of vinyl thioethers through the anti-Markovnikov pathway has received special attention and has been prominently described in the literature (Kondoh et al. 2005; Liao et al. 2013; Silva et al. 2008; Chun et al. 2016). In this context, vinyl thioethers have been efficiently obtained by transition-metal-catalyzed reactions (Modem et al. 2016; Zhao et al. 2012), for instance, using iron (Rocha et al. 2017), copper (Riduan et al. 2012; Yang and Rioux 2014; Trostyanskaya and Beletskaya 2012), indium (Sarma et al. 2012) and gold (Corma et al. 2010).
In spite of these advances, most of these protocols have different drawbacks such as air sensitivity and the use of expensive metals and toxic solvents. Very recently, we have reported an effective method for the hydrothiolation of alkenes and alkynes under more green conditions (Rosa et al. 2017). Nevertheless, the development of an alternative method for obtaining vinyl thioethers with high selectivity is still highly desirable.
Despite the high effectiveness of WERSA in a wide range of reactions, to the best our knowledge, it has not been applied as a solvent in the hydrothiolation transformations. Thus, herein, we report a straightforward and useful methodology for the synthesis of vinyl thioethers using WERSA as a natural feedstock solvent (Scheme 1).
Experimental
General procedure
Thiol 1 (0.5 mmol) and terminal acetylene 2 (0.6 mmol) were placed in a round-bottom flask, followed by the addition of the solvent (1 mL). The mixture was stirred at room temperature for 2 h, and the progress of the reaction was monitored by thin-layer chromatography (TLC). After the reaction was completed, the product was extracted with diethyl ether and water (3 × 5 mL). The organic phase was dried over MgSO4 and filtered, and the volatiles were completely removed under a vacuum. The crude product was purified by column chromatography with a mixture of ethyl acetate/hexane (01:99) to afford the desired vinyl thioether 3.
Preparation of WERSA 1 g of rice straw ash was suspended in 10 mL of distilled water in an Erlenmeyer and it was stirred for 1 h at room temperature. Subsequently, this mixture was filtered through a sintered glass crucible and the filtrate was used as WERSA. Recyclability experiments After completion of the synthesis, the reaction mixture was extracted with diethyl ether (5 × 2 mL) and the organic phase, containing the crude product, was separated from WERSA. The residue of WERSA was reused directly for the next experiment.
Results and discussion
To optimize the reaction conditions, 4-methylbenzenethiol 1a and phenylacetylene 2a were selected as standard substrates (Table 1). Initially, the reaction was carried out under inert atmosphere conditions in the presence of water extract of rice straw ash (WERSA), affording thioether 3a with a 69% yield and very poor stereoselectivity (entry 1). However, when the reaction was carried out in the air atmosphere, the desired product was achieved with an 89% yield and a Z/E ratio of 76:24 (entry 2). Next, we investigated the most appropriate temperature for the reaction. Room temperature proved to be the best choice since no improvement in the chemical yield of 3a was achieved when this parameter was modified (entries 3–5).

After identifying the best temperature, we then evaluated the influence of the reaction time on the reaction system (entries 6–8). A screening of this parameter revealed that 2 h was the best option, furnishing the desired product with a 93% yield and Z/E ratio of 83:17 (entry 6). Having determined the optimal time and temperature, the influence of several solvents was next investigated in detail (entries 11–16). However, no enhancement in the yield value of product 3a was achieved in the presence of any other solvent. It was also observed that the basic nature of WERSA (pH 12) seems to be essential for this hydrothiolation reaction, since better results were obtained under alkaline conditions (compare entries 6, 15 and 16).
Furthermore, we also evaluated the activity of different ash water extracts such as water extract of banana peel ash (WEB) and water extract of papaya peel ash (WEPAB). However, a slight decrease in the yield value of vinyl thioether 3a was observed by using these ash water extract variants (entries 17 and 18).
Having optimized the reaction conditions, we then evaluated the substrate scope using different thiols 1 and alkynes 2 (Table 2). Initially, we focused particularly on the influence of several aromatic thiols on the reaction (entries 1–5). Regarding the electron effects, it was found that the reaction proceeded efficiently in the presence of electron-donating groups, affording the respective products with good yields and appreciable stereoselectivity (entries 1 and 2). Nevertheless, when an aromatic thiol containing a withdrawing group attached at the para position of the aromatic ring was used, a significant decrease in the yield value was observed (entry 3). To our delight, it was also observed that thiophenol reacted very smoothly with phenyl acetylene, furnishing the corresponding product 3d with a 92% yield and Z/E ratio of 81:19 (entry 4). Regarding the steric effects, it was observed that steric hindrance at the ortho-substituted thiol affected the reaction course since compound 3e was achieved with a lower yield and stereoselectivity under optimized conditions (entry 5).
Furthermore, we also investigated whether the reaction could be applied for the preparation of vinyl thioethers starting from aliphatic thiols (entries 6–7). Gratifyingly, when cyclohexanethiol was used, product 3f was obtained with a 71% yield and a Z/E ratio of 87:13 (entry 6). However, a decrease in the yield value was observed when 1-dodecanethiol was employed as the sulfur source (entry 7).
Next, we evaluated the influence of different alkynes on the reaction system (entries 8–13). Generally, 4-methylbenzenethiol reacted well with different alkynes derivatives, furnishing the corresponding products with satisfactory yields and reasonable stereoselectivities. It was found that terminal alkynes bearing electron-releasing substituents were less reactive than their withdrawing analogs (entries 8–9). For instance, when 1-chloro-4-ethynylbenzene 2b was treated with 4-methylbenzenethiol, the desired product 3h was achieved with a 96% yield and a Z/E ratio of 71:29 (entry 8). However, when a phenylacetylene derivative containing a methyl group at the para position was used, the respective product was obtained with a lower yield and stereoselectivity (entry 9).
However, 3-ethynylanisole 2d reacted smoothly with 4-methylbenzenethiol, affording the respective product with a reasonable yield and very high stereoselectivity (entry 10). Nevertheless, the reaction was not applicable for the hydrothiolation of aliphatic alkynes since only traces of the products 3k and 3l were observed under similar conditions (entries 11 and 12).
It is well recognized that the preparation of thioethers through the hydrothiolation of alkenes via the anti-Markovnikov addition has also received particular attention and has been well described (Banerjee et al. 2010). Thus, inspired by the results obtained in the preparation of vinyl thioethers, we also attempted to treat thiols with alkenes under similar conditions (entries 13–14). Gratifyingly, the reaction of phenylstyrene with thiols 1a and 1c afforded the corresponding products with reasonable yields (entries 13–14).
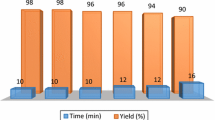
To investigate whether the present approach is an environmentally benign strategy for the preparation of vinyl thioethers, we also studied the recyclability of WERSA (Fig. 1).
Therefore, after carrying out the reaction under optimized conditions, the solvent was easily recovered from the reaction media and reused for further transformations. Notably, WERSA conserved its activity up to the fourth cycle, furnishing the respective thioether 3a with a very good yield and high stereoselectivity (Fig. 1).
Conclusion
In summary, we successfully developed an efficient and easy-to-perform method for obtaining vinyl thioethers by the hydrothiolation of alkynes with thiols using WERSA as a recyclable solvent. Generally, the corresponding products were obtained with good to excellent yields and good stereoselectivity. Remarkably, WERSA was easily recovered from the reaction media and reused for up to four cycles without a significant loss of activity. We believe that the chemistry described herein represents an environmentally friendly alternative for the hydrothiolation of alkynes. Studies on elucidating the mechanism of this transformation are still in progress in our laboratory.
References
Banerjee S, Das J, Alvarez RP, Santra S (2010) Silica nanoparticles as a reusable catalyst: a straightforward route for the synthesis of thioethers, thioesters, vinyl thioethers and thio-Michael adducts under neutral reaction conditions. New J Chem 34:302–306. https://doi.org/10.1039/b9nj00399a
Beletskaya IP, Ananikov VP (2007) Unusual influence of the structures of transition metal complexes on catalytic C–S and C–Se bond formation under homogeneous and heterogeneous conditions. Eur J Org Chem 2007:3431–3444. https://doi.org/10.1002/ejoc.200700119
Beletskaya IP, Ananikov VP (2011) Transition-metal-catalyzed C–S, C–Se, and C–Te bond formation via cross-coupling and atom-economic addition reactions. Chem Rev 11:1596–1636. https://doi.org/10.1021/cr100347k
Boruah PR, Ali AA, Chetia M, Saikia B, Sarma D (2015a) Pd(OAc)2 in WERSA: a novel Green catalytic system for Suzuki-Miyaura cross-coupling reactions at room temperature. Chem Commun 51:11489–11492. https://doi.org/10.1039/c5cc04561d
Boruah PR, Ali AA, Saikia B, Sarma D (2015b) A novel Green protocol for ligand free Suzuki-Miyaura cross-coupling reactions in WEB at room temperature. Green Chem 17:1442–1455. https://doi.org/10.1039/c4gc02522a
Chia PW, Lim BS, Yong FSJ, Poh S-C, Kan S-Y (2018) An efficient synthesis of bisenols in water extract of waste onion peel ash. Environ Chem Lett 16:1–7. https://doi.org/10.1007/s10311-018-0764-1
Chun S, Chung J, Park JE, Chung YK (2016) Hydrothiolation of alkenes and alkynes catalyzed by 3,4-dimethyl-5-vinylthiazolium iodide and poly (3,4-dimethyl-5-vinylthiazolium) iodide. ChemCatChem 8:2476–2481. https://doi.org/10.1002/cctc.201600363
Corma A, González-Arellano C, Iglesias M, Sánchez F (2010) Efficient synthesis of vinyl and alkyl sulfides via hydrothiolation of alkynes and electron-deficient olefins using soluble and heterogenized gold complexes catalysts. Appl Catal A Gen 375:49–54. https://doi.org/10.1016/j.apcata.2009.12.016
Dewan A, Sarmah M, Bora U, Thakur AJ (2016) A green protocol for ligand, copper and base free Sonogasfira cross-coupling reaction. Tetrahedron Lett 57:3760–3763. https://doi.org/10.1016/j.tetlet.2016.07.021
Dondoni A, Marra A (2014) Metal-catalyzed and metal-free alkyne hydrothiolation: synthetic aspects and application trends. Eur J Org Chem 2014:3955–3969. https://doi.org/10.1002/ejoc.201301879
Gonçalves LCC, Lima DB, Borba PMY, Perin G, Alves D, Jacob RG, Lenardão EJ (2013) Glycerol/CuI/Zn as a recyclable catalytic system for synthesis of vinyl sulfides and tellurides. Tetrahedron Lett 54:3475–3480. https://doi.org/10.1016/j.tetlet.2013.04.119
Jenkins BM, Bakker RR, Wei JB (1996) On the properties of washed straw. Biomass Bioenergy 10:177–200. https://doi.org/10.1016/0961-9534(95)00058-5
Kondoh A, Takami K, Yorimitsu H, Oshima K (2005) Stereoselective hydrothiolation of alkynes catalyzed by cesium base: facile access to (Z)-1-alkenyl sulfides. J Org Chem 70:6468–6473. https://doi.org/10.1021/jo050931z
Konwar M, Ali AA, Sarma D (2016) A green protocol for peptide bond formation in WEB. Tetrahedron Lett 57:2283–2285. https://doi.org/10.1016/j.tetlet.2016.04.041
Kundu D, Chatterjee T, Ranu BC (2013) Magnetically separable CuFe2O4 nanoparticles catalyzed ligand-free C–S coupling in water: access to (E)- and (Z)-styrenyl-, heteroaryl and sterically hindered aryl sulfides. Adv Synth Cat 355:2285–2296. https://doi.org/10.1002/adsc.201300261
Li Q, Dong T, Liu X, Lei X (2013) A bioorthogonal ligation enabled by click cycloaddition of o-quinolinone quinone methide and vinyl thioether. J Am Chem Soc 135:4996–4999. https://doi.org/10.1021/ja401989p
Liao Y, Chen S, Jiang P, Qi H, Deng G-J (2013) Stereoselective formation of Z- or E-vinyl thioethers from arylthiols and acetylenes under transition-metal-free condition. Eur J Org Chem 2013:6878–6885. https://doi.org/10.1002/ejoc.201300727
Mahanta A, Mondal M, Thakur AJ, Bora U (2016) An improved Suzuki-Miyaura cross-coupling reaction with the aid of in situ generated PdNPs: evidence for enhancing effect with biphasic system. Tetrahedron Lett 57:3091–3095. https://doi.org/10.1016/j.tetlet.2016.05.098
Malyshev DA, Scott NM, Marion N, Stevens ED, Ananikov VP, Beletskaya IP, Nolan SP (2006) Homogeneous nickel catalysts for the selective transfer of a single arylthio group in the catalytic hydrothiolation of alkynes. Organometallics 25:4462–4470. https://doi.org/10.1021/om060302v
Modem S, Kankala S, Balaboina R, Thirukovela NS, Jonnalagadda SB, Vadde R, Vasam CS (2016) Decarbonylation of salicylaldehyde activated by p-cymene ruthenium(II) dimer: implication for catalytic alkyne hydrothiolation. Eur J Org Chem 2016:4635–4642. https://doi.org/10.1002/ejoc.201600809
Palacios L, Giuseppe AD, Artigas MJ, Polo V, Lahoz FJ, Castarlenas R, Pérez-Torrente J, Oro LA (2016) Mechanistic insight into the pyridine enhanced α-selectivity in alkyne hydrothiolation catalysed by quinolinolate–rhodium(ǀ)–N-heterocyclic carbine complexes. Catal Sci Technol 6:8548–8561. https://doi.org/10.1039/c6cy01884j
Palomba M, Bagnoli L, Marini F, Santi C, Sancineto L (2016) Recent advances in the chemistry of vinylchalcogenides. Phosphorus, Sulfur Silicon Relat Elem 191:235–244. https://doi.org/10.1080/10426507.2015.1067212
Pearson WH, Lee IY, Mi Y, Stoy P (2004) Total synthesis of the Kopsia Lapidilecta alkaloid (±)-Lapidilectine B. J Org Chem 69:9109–9122. https://doi.org/10.1021/jo048917u
Reddy VP, Swapna K, Kumar AV, Rao KR (2009) Recyclable nano copper oxide catalyzed stereoselective synthesis of vinyl sulfides under ligand-free conditions. Synlett 17:2783–2788. https://doi.org/10.1055/s-0029-1217990
Riduan SN, Ying JY, Zhang Y (2012) Carbon dioxide mediated stereoselective copper-catalyzed reductive coupling of alkynes and thiols. Org Lett 14:1780–1783. https://doi.org/10.1021/ol3003699
Rocha MST, Rafique J, Saba S, Azeredo JB, Back D, Godoi M, Braga AL (2017) Regioselective hydrothiolation of terminal acetylene catalyzed by magnetite (Fe3O4) nanoparticles. Synth Commun 47:291–298. https://doi.org/10.1080/00397911.2016.1262421
Rodygin KS, Gyrdymova YV, Zarubaev VV (2017) Synthesis of vinyl thioethers and bis-thioethenes from calcium carbide and disulfides. Mendeleev Commun 27:476–478. https://doi.org/10.1016/j.mencom.2017.09.015
Rosa CH, Peixoto MLB, Rosa GR, Godoi B, Galetto FZ, D’Oca MGM, Godoi M (2017) Sulfamic acid: an efficient and recyclable catalyst for the regioselective hydrothiolation of terminal alkenes and alkynes with thiols. Tetrahedron Lett 58:3777–3781. https://doi.org/10.1016/j.tetlet.2017.08.051
Saikia B, Borah P (2015) A new avenue to the Dakin reaction in H2O2-WERSA. RSC Adv 5:105583–105586. https://doi.org/10.1039/c5ra20133k
Saikia E, Bora SJ, Chetia B (2015) H2O2 in WERSA: an efficient green protocol for ipso-hydroxylation of aryl/heteroarylboronic acid. RSC Adv 5:102723–102726. https://doi.org/10.1039/c5ra21354a
Sarma R, Rajesh N, Prajapati D (2012) Indium(III) catalysed substrate selective hydrothiolation of terminal alkynes. Chem Commun 48:4014–4016. https://doi.org/10.1039/c2cc30350g
Sarmah M, Dewan A, Mondal M, Thakur AJ, Bora U (2016) Analysis of the water extract of waste papaya bark ash and its implications as an in situ base in the ligand-free recyclable Suzuki-Miyaura coupling reaction. RSC Adv 6:28981–28985. https://doi.org/10.1039/c6ra00454g
Sarmah M, Mondal M, Bora U (2017) Agro-waste extract based solvents: emergence of novel green solvent for the design of sustainable processes in catalysis and organic chemistry. ChemistrySelect 2:5180–5188. https://doi.org/10.1002/slct.201700580
Silva MS, Lara RG, Marczewski JM, Jacob RG, Lenardão EJ, Perin G (2008) Synthesis of vinyl sulfides via hydrothiolation of alkynes using Al2O3/KF under solvent-free conditions. Tetrahedron Lett 49:1927–1930. https://doi.org/10.1016/j.tetlet.2008.01.093
Silveira GD, Carvalho LM, Montoya N, Domenech-Carbó A (2017) Solid state electrochemical behavior of organosulfur compounds. J Electroanal Chem 806:180–190. https://doi.org/10.1016/j.jelechem.2017.10.055
Singh R, Raghuvanshi DS, Singh KN (2013) Regioselective hydrothiolation of alkynes by sulfonyl hydrazides using organic ionic base—Bronsted acid. Org Lett 16:4202–4205. https://doi.org/10.1021/ol401925u
Strydom I, Guisado-Barrios G, Fernández I, Liles DC, Peris E, Bezuidenhout DI (2017) A hemilabile and cooperative N-donor-functionalized 1,2,3-triazol-5-ylidene ligand for alkyne hydrothiolation reactions. Chem Eur J 23:1393–1401. https://doi.org/10.1002/chem.201604567
Surneni N, Barua NC, Saikia B (2016) Application of natural feedstock extract: the Henry reaction. Tetrahedron Lett 57:2814–2817. https://doi.org/10.1016/j.tetlet.2016.05.048
Trostyanskaya IG, Beletskaya IP (2012) Regio- and stereoselective copper-catalyzed addition of aromatic and aliphatic thiols to terminal and internal nonactivated alkynes. Synlett 23:535–540. https://doi.org/10.1055/g-0031-1290345
Yang Y, Rioux RM (2014) Highly stereoselective anti-Markovnikov hydrothiolation of alkynes and electron-deficient alkenes by a supported Cu–NHC complex. Green Chem 16:3916–3925. https://doi.org/10.1039/c4gc00642a
Zhao H, Peng J, Cai M (2012) Heterogeneous hydrothiolation of alkynes with thiols catalyzed by diphosphino-functionalized MCM-41 anchored rhodium complex. Catal Lett 142:138–142. https://doi.org/10.1007/s10562-011-0732-x
Acknowledgements
We gratefully acknowledge the National Council for Scientific and Technological Development—CNPq, CAPES and FAPERGS-Pronex for financial support. The authors are also grateful to CIA-FURG for the NMR analysis and the Program of Support for the Publication of Academic Production/PROPESP/FURG/2018. CAPES and PET-FURGSAP are also acknowledged for the fellowship for A. L. and L. M. C. B., respectively. Funding was provided by Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (Grant No FAPERGS/CNPq 12/2014 - PRONEX) and National Council for Scientific and Technological Development-CNPq (Grant No 428494/2018-8).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Godoi, M., Leitemberger, A., Böhs, L.M.C. et al. Rice straw ash extract, an efficient solvent for regioselective hydrothiolation of alkynes. Environ Chem Lett 17, 1441–1446 (2019). https://doi.org/10.1007/s10311-019-00882-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-019-00882-0