Abstract
Stereotactic Radiosurgery (SRS) delivers a high dose of radiation to a specific brain area while limiting radiation to nearby healthy tissue. While most SRS has traditionally been performed with a stereotactic frame-based approach, this study aims to investigate the safety and efficacy of frameless radiosurgery in patients with brain metastases. Our study followed the recommended guidelines summarized in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist. The electronic databases of PubMed/Medline, Scopus, Embase, and Web of Science (WOS) were searched from inception to 10 October 2023. The pooled rate of outcomes was calculated using random effect model and Restricted maximum–likelihood (REML) method. All statistical analysis was performed by STATA V.17. A total of 499 studies were recruited from the electronic databases. After removing duplicates (n = 117), 382 studies were used for title/abstract, and 329 were removed from the study selection process. A total of 53 articles were used for full-text assessment, and 35 studies were included for data extraction. Our analysis revealed a significant increase across all pooled survival rates and local control rates by initiating the radiosurgery for patients, estimating the pooled 6-month OSR of 75% (95% CI: 68-81%), 1-year overall survival rate (OSR) of 60% (95% CI: 51-69%), 18-month OSR of 48% (95% CI: 10-85%), 2-year OSR of 39% (95% CI: 19-58%), 1-year progression-free survival rate (PFSR) of 68% (95% CI: 39-98%), 2-year PFSR of 75% (95% CI: 58-91%), 6-month local control rate (LCR) of 93% (95% CI: 90-96%), and 12-month LCR of 86% (95% CI: 82-90%). Our meta-analysis findings confirm the efficacy of frameless radiosurgery in treating brain metastases. Using data from several trials, we were able to demonstrate stereotactic radiosurgery’s effectiveness as a therapy option for brain metastasis patients, demonstrating local control and reasonable overall survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most common type of brain tumor in adults is brain metastasis, accounting for 10–20% of all cancer patients and surpassing primary brain tumors by tenfold [1]. Lung, breast, melanoma, and kidney cancers account for most primary sites of brain metastases [2]. The symptoms include headaches, neurological deficits, and seizures. 40% of patients report headache as the first symptom, 15-20% have seizures, and 40% have localized neurological deficits such as hemianopsia, aphasia, and hemiparesis. Approximately 65% of patients suffer from cognitive impairment [3, 4].The number of brain metastases appears to have increased during the previous decade, regarding the utilization of magnetic resonance imaging (MRI), enhancement of therapeutic options for systematic disease, aging of the population, and the effectiveness of drugs that do not cross the blood-brain barrier [5,6,7]. The four main definitive therapies are whole-brain radiation therapy (WBRT), surgery, stereotactic radiosurgery (SRS), and medical therapy with chemotherapy, immunotherapy, or precision medicine approaches [8]. Young individuals with limited extracranial disease may benefit from surgical excision of a single brain metastasis, followed by radiosurgery for two to four metastases. The advantages of WBRT after surgery or radiosurgery remain uncertain. Thus, two therapies are available in patients with a favorable prognosis: WBRT after surgery or radiosurgery or observation with MRI follow-up [8].The use of SRS to treat brain metastases in patients has been on the rise. Multiple studies have shown its effectiveness when used alone or combined with WBRT [9,10,11,12]. Radiation therapy with SRS delivers a high dose of radiation to a specific area of an organ while limiting radiation to healthy tissue nearby [13]. An immobilizing head frame is used to immobilize the patient, and stereotactic coordinates target a specific area in the brain and enable precise immobilization and positioning accuracy of less than 1 mm during image capture and treatment [14]. There are several downsides to this intrusive technique, including discomfort and anxiety for the patient. The rigid head frame also requires the presence of a neurosurgeon during installation. Nevertheless, developments in computer engineering, radiologic technology, and radiological methods have offered the potential to transcend the constraints of traditional frames [15].In recent years, non-invasive frameless stereotactic systems have become preferred over traditional patient fixation methods. These frameless systems have shown positional accuracy within the 1–4 mm range, which may vary due to differences in patient fixation, positioning, and accuracy assessment methods [16, 17]. It is crucial to incorporate a small safety margin into the target volume to account for localization and set-up errors, which is essential for minimizing potential treatment-related complications of SRS. Furthermore, the volumes of normal brain tissue exposed to high radiation doses can indicate the development of brain radionecrosis. Studies suggest that brain radionecrosis can occur in up to 47% of treated lesions for brain volumes larger than ten cc receiving a dose of 12 Gy [18].In the current study, we aimed to explore the primary outcomes of frameless SRS, including overall survival (OS), progression-free survival (PFS), local control (LC), and radiological response, and secondary outcomes, including adverse radiation effects, further therapies, and radionecrosis for patients with brain metastases, which can assist neurosurgeons in treating these difficult patients.
Method
The study followed the recommended guidelines summarized in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [19].
Search strategy
An inclusive search was conducted thoroughly with relative keywords. Four online databases of PubMed/Medline, Scopus, Embase, and Web of Science (WOS) were surveyed until 2023 without any limitation. The search syntax included crucial keywords such as “brain”, “metastasis,”, “frameless”, and “radiosurgery”. For more features about the search methodology, please refer to the supplementary materials (Table S1).
Eligibility criteria
In this research, we utilized well-defined criteria for including or excluding to recruit the relevant studies. Moreover, we exploited the PICO structure as a systematic approach to guide our investigation. The inclusion criteria for this study were as follows:
-
1.
English studies.
-
2.
Studies conducted on human subjects with BM.
-
3.
Studies used frameless radiosurgery as treatment.
-
4.
Studies reported outcomes such as OS, PFS, and LC.
-
5.
Original articles, cross-sectional studies, cohorts, case-control, and clinical trials.
The exclusion criteria for this study were as follows:
-
1.
Non-English studies.
-
2.
In vivo and in vitro studies.
-
3.
Studies without BM confirmation or without employment of frameless radiosurgery as a treatment.
-
4.
Lack of outcome.
-
5.
Non-original articles such as Case reports/series, thesis, notes, conference abstracts, book chapters, letters, reviews, systematic reviews, and meta-analyses.
Study selection
The data attained from exploring each database was exported to the EndNote V.20 for a thorough screening process. Two independent reviewers managed the primary screening of initial records by removing duplicate articles from the study selection process. Then, the remaining studies underwent title/abstract screening, and the relevant studies went through a detailed evaluation, which was a full-text assessment. Afterward, studies that came to have the eligibility criteria were selected for data extraction and synthesis. In cases of a conflict, the disagreement was resolved by a third reviewer.
Data extraction
Two reviewers conducted the data extraction process to gather crucial information from the chosen studies. A third senior (MA.H) reviewer resolved the disagreements. The demographic characteristics of articles, characteristics of brain metastasis, SRS features, and outcomes were extracted. Also, pooled rates of OS, LC, and PFS were computed to represent the success of treatment. The complications of adverse radiation effects and radionecrosis were also investigated.
Data synthesis
To get the proper effect size, the Cochrane Handbook for Systematic Reviews of Interventions was used. The percentages of LC, OS, and PFS rates were pooled using a random effect model with a restricted maximum likelihood (REML) method. The Cochrane’s Q and I2 test was employed to assess heterogeneity. The heterogeneity was considered significant if I2 > 40% and Q test P-value < 0.001. We performed a subgroup analysis to account for potential moderators. Each study’s influence on the pooled estimates was determined using a sensitivity analysis with the leave-one-out meta-analysis. We examined to determine publication bias by funnel plot and ran regression-based Egger test. Statistical significance was considered as a p-value less than 0.05. All statistical analysis was done by STATA version 17.0 (Stata Corp, College Station, TX).
Quality assessment
The quality of the included studies was evaluated using the Newcastle-Ottawa Scale (NOS). The NOS quality evaluation has three sections: selection, comparability, and exposure/outcome. Studies were grouped according to their overall score: 1–3 for low-quality research, 4–6 for intermediate-quality research, and 7–9 for high-quality research (Table S2).
Results
Study selection
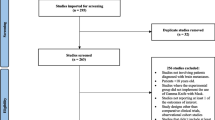
Our exploration of various databases yielded 499 studies, from which 117 were excluded due to duplication. After the initial screening, 382 studies underwent title/abstract screening, which excluded 329 studies. Eventually, 53 studies underwent a full-text screening. Eighteen studies were excluded from the full-text assessment due to the non-English studies (n = 2), wrong population (n = 5), wrong design (n = 7), and not reported outcome (n = 4). Ultimately, 35 studies were included for data extraction and synthesis. The details of the whole study selection process are summarized and depicted in Fig. 1.
Baseline characteristics
We extracted data from 35 eligible studies with 2253 cancer patients with single or multiple brain metastasis from cancer of any histology who received disparate prescription doses and fractions of SRS. Four clinical trials accompanied by 26 retrospectives and four prospective cohorts, published between 2005 and 2022, were enrolled for evaluation. Most of the studies took place in the US (18), followed by Germany (6) and Italy (3). The participants’ gender was reported in 31 studies (n = 2162), while 1066 (49.3%) and 1096 (50.7%) were male and female, respectively. The mean age of the patients in the studies ranged from 49.5 to 67 years. Table 1 depicts the characteristics of the enrolled studies.
Brain metastasis characteristics
Thirty-two studies demonstrated the primary site of brain metastasis in the enrolled patients (n = 2079), indicating 872 patients with lung cancer (41.9%), 353 with breast cancer (16.9%), 314 with melanoma (15.1%), 161 with gastrointestinal cancer (7.7%), 82 with renal cell carcinoma (RCC) (3.9%), 43 with adenocarcinoma (2%), 25 with gynecological cancer (1.2%), and 229 with other cancers (11%), as the most common primary sites. The mean number of brain metastasis in enrolled patients differed between 1 and 5; however, two studies reported an outranged mean number of 20 and 13 [35, 51]. Considering median tumor volume, 19 studies denoted a wide range from 0.11 to 19.53 cc. Eighteen studies reported the mean Karnofsky Performance Scale (KPS) Score of the patients, which ranged from 50 to 100%. The RPA classification was surveyed, and most of the patients had an RPA class II (0.69 [95%CI:0.61–0.78]), RPA class I (0.19 [95%CI:0.13–0.24]), and RPA class III (0.11 [95%CI:0.06–0.16]), retrospectively.The location of the tumor was also investigated, and analysis showed that the frontal lobe (0.23 [95%CI:0.17–0.29]) was the most common location followed by the parietal lobe (0.21 [95%CI:0.18–0.23]), cerebellar (0.18 [95%CI:0.13–0.22]), temporal (0.17 [95%CI:0.14–0.19]), occipital (0.12 [95%CI:0.09–0.15]), and brainstem (0.05 [95%CI:0.00-0.09]), retrospectively.
24 studies reported prior treatments, including WBRT (n = 371), chemotherapy (n = 262), surgery (n = 92), hormone therapy (n = 14), immunotherapy (n = 12), and targeted therapy (n = 8). In addition, we identified 30 patients who received WBRT following radiosurgery (Table 2).
SRS characteristics
Different enrolled studies considered distinct modalities and technologies to conduct the treatment process with SRS. The most common technologies were linear accelerator (LINAC), Gamma Knife, CyberKnife, and C-arm LINAC; also, the included studies employed different techniques, such as intensity modulated SRS, volumetric intensity modulated radiosurgery, surface imaging-guided radiosurgery on Trilogy LINAC, Three-dimensional conformal radiotherapy, Volumetric modulated arc radiosurgery, single isocenter for multiple targets dynamic conformal arc, and single-isocenter non-coplanar. The median target volume, reported in 25 studies, ranged from 0.049 to 21.16 cm³. The median of marginal received dose in patients differed from 7 to 30 Gy (Gy) and mostly went through one fraction of SRS, with some cases of 2 to 5 fractions. SRS isodose line in 22 included studies was reported between 45% and 99%. Regarding radiation-related adverse events, radiation toxicity (n = 56), local failure (n = 23), headache (n = 24), fatigue (n = 15), seizure (n = 15), intracranial hemorrhage (n = 8), cerebral edema (n = 8), aggravation of pre-existing deficits (n = 4), nausea (n = 2), alopecia (n = 2), encephalitis (n = 1), and aphasia (n = 1) were the most frequent adverse events in 17 studies, respectively. Table 3 depicts the characteristics of SRS.
Meta-analysis outcomes
The meta-analysis outcomes are divided into survival rates, radiological responses, and complications. Table 4 represents the meta-analysis outcomes.
Survival outcomes
6-months OS rate
Twelve studies reported the 6-month OS rate. The studies demonstrated significant heterogeneity (I2 = 76.45%). Significant variability was verified (Q = 39.69, P-heterogeneity < 0.001), emphasizing the variety of the included research. Furthermore, the 6-month OS rate was 50–89%, and a pooled 6-month OS rate was 75% (95% CI: 68-81%) (Fig. 2).
Meta-regression revealed no significant association between number of brain metastasis (r: − 0.0070129, P-value: 0.312), tumor volume (r: − 0.0120412, P-value: 0.148), Isodense line (r: − 0.3902197, P-value: 0.441), median target volume (r: − 0.0020092, P-value: 0.854), prior WBRT (r: 0.1623343, P-value: 0.175), SRS margin dose (r: − 0.0061823, P-value: 0.442), and number of SRS fraction (r: − 0.0190764, P-value: 0.461).
6-months LC rate
The overall survival rates following therapy in 11 studies were analyzed. There was significant variability among the studies (I² = 67.52%). Significant heterogeneity was found between the studies (Q = 35.37, P-heterogeneity < 0.001), highlighting the variety of the included research. Additionally, the 6-month LC rate ranged between 88 and 99%, with a pooled 6-month LC rate of 93% (95% CI: 90-96%) (Fig. 3).
1-year OS rate
Eighteen studies were analyzed to determine the 1-year OS rates. There was significant heterogeneity among the studies (I2 = 93.21%, Q = 486.15, P-heterogeneity < 0.001). ). Moreover, Fig. 4 shows that the 1-year OS was between 35 and 95% and a pooled 1-year OS rate of 60% (95% CI: 51-69%).
1-year PFS rate
Three studies reported the 1-year PFS rate, which ranged between 43 and 94%. The results of the study exhibit significant heterogeneity (I2 = 97.66%). The heterogeneity test revealed substantial variations between the studies (Q = 117.00, P-heterogeneity < 0.001). The pooled 1-year PFS rate was 68% (95% CI: 39-98%) (Figure S36).
1-year LC rate
A total of 12 studies reported LC rates over 1-year ranging between 66 and 100%. The analysis indicates significant heterogeneity in the study results (I2 = 93.80%). The heterogeneity test revealed substantial variations amongst the studies (Q = 286.76, P-heterogeneity < 0.001), indicating that the rates that have been reported varied. Figure 5 shows that the pooled 1-year LC rate was 86% (95% CI: 82-90%).
18 months OS rate
We analyzed data from two studies that reported an 18-month OS rate. Significant heterogeneity was seen in the 18-month overall survival rates reported by the various studies (I2 = 93.07%, Q = 14.44, P-heterogeneity < 0.001). The 18-month OS was between 29% and 67%, and a pooled 18-month OS rate of 48% (95% CI: 10-85%) (Figure S37).
2-year OS rate
Five studies reported a 2-year OS rate ranging from 20 to 69%. There is a significant difference in the 2-year OS rates (I2 = 92.38%, Q = 48.09, P-heterogeneity < 0.001) between studies. The pooled 2-year OS rate was 39% (95% CI: 19-58%) (Figure S38).
2-year PFS rate
Three studies reported 2-year PFS rates, and significant heterogeneity among the studies was evident (I2 = 92.34%, Q = 24.99, P-heterogeneity < 0.001). The rate of 2-year PFS ranged from 59 to 88%, and the pooled 2-year PFS rate was 75% [95% CI: 58-91%] (Figure S39).
Radiological outcomes
The radiological outcomes, including complete response rate, partial response rate, progressive disease rate, and stable disease rate, were reported separately (Fig. 6).
Complete response rate
Seven studies reported a complete response rate ranging from 11–37%with a pooled rate of 26% [95%CI:20-33%]. Moreover, significant heterogeneity was determined between studies (I2:82.24%, P-heterogeneity < 0.001, Q:34.05 (Fig. 6).
Partial response rate
Seven studies determined that the partial response rate varied between 15% and 71%. The pooled rate of partial response rate was 38% [95%CI:24-51%]. The heterogeneity among the studies was remarkably determined by I2:95.59%, Q:132.91, and P-heterogeneity < 0.001 (Fig. 6).
Stable disease rate
The stable disease rate was reported in seven studies. The stable disease rate varied between 5 and 48% with a pooled rate of 29% [95%CI:17-41%]. The heterogeneity was high between the studies, with an I2:94.91%, Q:118.03, and P-heterogeneity < 0.001 (Fig. 6).
Progressive disease rate
Eight studies reported progressive disease rates ranging from 3 to 47%. The pooled rate of progressive disease was 12% [95%CI:2-22%]. I2:97.70%, Q:88.20, and P-heterogeneity48 determined significant overall heterogeneity (Fig. 6).
Adverse radiation effect
The results from nine studies indicated symptomatic adverse radiation effects ranging between 0.01 and 0.27, with a pooled rate of 8% [95%CI:3-13%]. Adverse asymptomatic radiation effects were found in three studies, ranging between 2% and 14%, with a pooled rate of 9% [95%CI:1-16%]. Interestingly, a test of group difference revealed no significant difference between the pooled rate of symptomatic and asymptomatic adverse radiation effect (P-value:0.82) (Figure S40).
Radionecrosis
One significant side effect of radiotherapy is radiation necrosis. According to several studies, the pooled rate of grade 2 radiation necrosis was found to be 8% [95%CI: -2-18%], while the rate of grade 3 radiation necrosis was reported in three studies with a pooled rate of 3% [95%CI:0-5%]. Additionally, three studies reported grade > 1 radiation necrosis with a pooled rate of 14% [95%CI: -1-29%]. The pooled grade > 2 radiation necrosis rate was determined in three studies, and the result was 4% [95%CI:0-8%]. The pooled rate of radiation necrosis in grades 1 or 2 and 2 or 3 was found to be 15% [95%CI:3-27%] and 9% [95%CI: 2-17%], respectively. Overall, regardless of grade, the total pooled rate of radiation necrosis was 9% [95%CI: 4-14%] (Figure S41).
Further therapy
Fourteen studies have reported the need for further treatment using WBRT ranging from 0.06 to 0.36 and a combined rate of 16% [95%CI:12-21%]. Nine studies included surgery as a further treatment, with rates ranging from 0.02 to 0.48 and a combined rate of 6% [95%CI: 0-12%]. Salvage SRS was necessary according to 12 studies, with rates varying from 2 to 50% and a combined rate of 15% [95%CI:7-22%]. Further therapies beyond SRS, surgery, and WBRT were reported in two studies, with rates ranging from 5 to 13%. The combined rate of such treatments was 5% [95%CI:0-11%]. However, four studies reported no need for further treatment, with rates ranging from 2 to 7% and a combined rate of 3% [95%CI:0-7%].
Sensitivity analysis
A sensitivity analysis was performed for each of the pooled estimates to assess the robustness of our outcomes; thus, we went over the analysis many times, with one excluded study each time. As a result of excluding each study, the rerun analysis indicated robust results for 6-months OS (p-value < 0.05 for each study), 6-months LC (p-value < 0.05 for each study), 1-year OS (p-value < 0.05 for each study), 1-year PFS (p-value < 0.05 for each study), 1-year LC (p-value < 0.05 for each study), 18 months OS (p-value < 0.05 for each study), 2-year OS (p-value < 0.05 for each study), 2-year PFS (p-value < 0.05 for each study), complete response rate (p-value < 0.05 for each study), stable disease rate (p-value < 0.05 for each study), and partial response rate (p-value < 0.05 for each study). However, no robust outcome was determined for the progressive disease rate.
Publication bias
Egger’s regression asymmetry test was conducted to evaluate the publication bias that suggested no significant publication bias concerning complete response rate (p = 0.3656), partial response rate (p = 0.9929), stable disease rate (p = 0.2728), progressive disease rate (p = 0.3084), 2-Year OS rate (p = 0.12), 1-Year PFS rate (p = 0.42), and 2-Year PFS rate (p = 0.12); however, there was significant bias regarding 6-Months OS rate (p = 0.00), 1-Year OS rate (p = 0.01), 6-months LC rate (p = 0.04), and 12-months LC rate (p = 0.00). In the cases of considerable publication bias, the trim and fill method was employed to achieve more symmetrical funnel plots and determine the influence of missing studies on estimated effect sizes. Using the trim and fill method, we generated an updated pooled estimate for 6-Months OS rate, 1-Year OS rate, and 6-month LC rate, which were 78% (95% CI: 75-81%), 84% (95% CI: 82-86%), and 96% (95% CI: 94-97%), respectively.
Discussion
SRS, with or without WBRT, should be considered as the first-line therapeutic option for BM [55]. Compared to the typical frame-based SRS, non-invasive, frameless SRS improves patient comfort and minimizes anxiety. Frameless SRS also simplifies providing fractionated radiation, which may be effective when BMs are big, irregularly shaped, or located near essential tissues [56,57,58,59,60]. Kondziolka et al. observed a 9% discomfort rate during frame installation despite utilizing sedatives [61]. Furthermore, using SRS without WBRT resulted in fewer adverse effects, such as cognitive impairment [62]. Andrews et al. [9] reported no differences in outcomes between Gamma Knife and LINACs for BMs, whether SRS was used with or without WBRT. The effectiveness of frameless-based SRS for extracranial malignancies has been extensively demonstrated [63].Based on the findings of this meta-analysis, it is evident that frameless radiosurgery leads to improved OS, PFS, and LC rates in patients with BMs. Our study has determined the pooled estimated survival rates at six months, one year, 18 months, and two years for OS and PFS, as well as the six-month and 12-month LC rates. The results demonstrate that a significant proportion of patients experienced improved survival following frameless radiosurgery, with a pooled 6-month OS rate of 75% and a 1-year OS rate of 60%. Moreover, evidence suggests that frameless radiosurgery may offer long-term survival benefits, with estimated OS rates of 48% at 18 months and 39% at two years. Additionally, the PFS rate estimates indicate positive outcomes, with estimated 1-year and 2-year PFS rates of 68% and 75%, respectively, signifying that a substantial number of patients were able to avoid disease progression. Notably, the pooled 6-month and 12-month LC rate estimates showed significant improvement at 93% and 86%, respectively, highlighting the effectiveness of frameless radiosurgery in controlling local tumors.The feasibility and toxicity of CyberKnife Frameless SRS (CK-SRS) were examined in a study. The remaining five patients had a median follow-up length of 19 months. The entire cohort had a median survival duration of 12 months after CK-SRS. The two-year rates for LC, CSS, and OS were 26%, 26%, and 22%, respectively. Symptoms improved or remained stable following CK-SRS, except for one patient who reported greater pain. The treatment was well tolerated, with just one case of Grade 2 and 3 mucositis [64].An analysis of LINAC-based frameless SRS techniques for BM patients was conducted by Ibrahim et al. [65]. Overall, the median survival and time were 8.7 and 5.3 months, respectively. LR as a first event was 25% and 38% after one and two years, respectively, while distant brain recurrence as a first event was 18% and 21%. 31% of patients died before experiencing a brain event. A study examined the outcome and prognostic characteristics of LINAC-based frameless SRS in BM from malignant melanoma. The median follow-up period was seven months, while the median OS was nine months. The 6-, 12-, and 24-month OS rates were 71%, 39%, and 25%, respectively. The median intracerebral control period was 5.3 months, with 6- and 12-month intracerebral PFS rates of 48% and 38%, respectively. The most prevalent clinical adverse effect was headache. The most prevalent radiological result during follow-up was localized edema in the SRS high-dose location [66].Lee et al. [67] studied the effectiveness of VMAT in sequential or simultaneous integrated tumor boost in WBRT for patients with poor prognosis and four or more BMs. The follow-up period spanned 0.3 to 16.5 months. The OS at six and twelve months was 66.7% and 41.7%, respectively. The local PFS at six and twelve months was 100% and 62.5%. In research by Nichol et al. [68], 60 patients with one to 10 BMs who received fractionated therapies were evaluated. At 30.5 months of follow-up, the median survival was 10.1 months, the rates of complete and partial brain response rates were 56%, and LC was 88%. Zhexi et al. [69] investigated the outcomes of frame-based and frameless LINAC SRS. The average follow-up time was 13.2-year s. The total obliteration rate was similar (Frame-based 82.5% vs. Frameless 80.0%) and did not change significantly over time (log-rank p = 0.536). Both frameless and frame-based LINAC SRS are equally successful in obliterating intracranial arteriovenous malformations. In research by Lau et al., single-isocenter frameless VMAT was administered in 15 patients, with a median dosage of 20 Gy in three BMs. The median follow-up period was 7.1 months. At one year, local and regional control was obtained in 81.5% and 60% of cases, respectively, with an OS of 39%; there was no treatment-related toxicity of grade 3 or above. There was no evident link between the dosage administered to normal brain tissue and the level of toxicity [70].An experiment examined LC, brain-distant progression (BDP), toxicity, and OS in BM patients treated with hypofractionated stereotactic radiotherapy (HSRT). In 1.2 years after therapy, the median LC rate was 30 months (96.96%), the median BDP rate was 24 months (12.24%), and the median OS rate was 14 months (69.33%). KPS and managed extracranial disease were linked with a considerable survival advantage [71]. In a study of 98 patients with BMs, Kim et al. discovered that HSRT patients had equal LC and OS rates and a decreased risk of toxicity compared to those treated with SRS. This was even though HSRT was utilized on big lesions in difficult places [72].Buss et al. [73] evaluated the LC of BM treated with single-fraction SRS using frameless or frame-based immobilization. The median follow-up duration for frameless SRS was 10.5 months, while framed SRS took 7 months. Patients treated with frameless SRS had greater neurological symptoms before treatment and were more likely to get a tyrosine kinase inhibitor concurrently or within 4 weeks of treatment. The frameless SRS group exhibited a larger average metastatic volume than the frame-based SRS group, although the difference was insignificant. At one year, LC in BM treated with frameless SRS was 92%, compared to 86% for framed SRS. OS was comparable between groups (p = 0.46).
Limitations
It is important to note that there is considerable heterogeneity across the studies we have analyzed. Differences in patient demographics, treatment methods, and outcome measures can all contribute to this variability, impacting our findings’ overall reliability and generalizability. Despite employing a random-effects model to address heterogeneity, residual variability among studies may still impact the strength of our conclusions. Additionally, the potential for publication bias is a significant limitation. Despite our use of sensitivity analysis and the trim and fill technique to mitigate publication bias, excluding unpublished or negative studies could introduce bias into our pooled estimates, potentially compromising the integrity and dependability of our findings. Furthermore, variations in the quality and methodology of the included studies may influence the overall reliability of our findings and impact their internal validity.
Conclusion
The meta-analysis presents compelling evidence supporting the effectiveness of frameless radiosurgery in improving the 6-month, 1-year, and 2-year overall survival rates, as well as progression-free survival and local control rates in patients with brain metastases. These results indicate that frameless stereotactic radiosurgery has a positive impact on both survival and local disease control. However, due to significant variability among the studies, it is essential for future research to focus on standardizing treatment protocols and outcome measures to draw more definitive conclusions. Furthermore, our study highlights the importance of conducting prospective trials to confirm these findings and potentially influence clinical guidelines. Despite some limitations, such as publication bias and study variations, this meta-analysis provides a foundation for a potential shift in treatment approaches. It underscores the need to integrate advanced radiosurgical techniques in the management of brain metastases, which could lead to improved patient outcomes and should be considered in clinical practice.
Data availability
No datasets were generated or analysed during the current study.
References
Stewart BW, Kleihues P (2003) World cancer report, vol 57. IARC press Lyon
Delattre JY et al (1988) Distribution of brain metastases. Arch Neurol 45(7):741–744
Mehta MP et al (2003) Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol 21(13):2529–2536
Kaal ECA, Niël CGJH, Vecht CJ (2005) Therapeutic management of brain metastasis. Lancet Neurol 4(5):289–298
Bendell JC et al (2003) Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 97(12):2972–2977
Crivellari D et al (2001) High incidence of central nervous system involvement in patients with metastatic or locally advanced breast cancer treated with epirubicin and docetaxel. Ann Oncol 12(3):353–356
Soffietti R et al (2020) Management of brain metastases according to molecular subtypes. Nat Reviews Neurol 16(10):557–574
Lin X, DeAngelis LM (2015) Treatment of Brain metastases. J Clin Oncol 33(30):3475–3484
Andrews DW et al (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363(9422):1665–1672
Manon R et al (2005) Phase II trial of radiosurgery for one to three newly diagnosed brain metastases from renal cell carcinoma, melanoma, and sarcoma: an Eastern Cooperative Oncology Group study (E 6397). J Clin Oncol 23(34):8870–8876
Kocher M et al (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol 29(2):134–141
Tos SM et al (2024) Stereotactic radiosurgery for intracranial cavernous malformations of the deep-seated locations: systematic review and meta-analysis. Neurosurg Rev 47(1):186
Mazeron JJ et al [History of radiosurgery] Cancer radiotherapie: journal de la Societe francaise de radiotherapie oncologique, 2012. 16 Suppl: pp. S2-4
Leksell L (1951) The stereotaxic method and radiosurgery of the brain. Acta Chir Scand 102(4):316–319
Nath SK et al (2010) Optically-guided frameless linac-based radiosurgery for brain metastases: clinical experience. J Neurooncol 97(1):67–72
Solberg TD et al (2008) Quality assurance of immobilization and target localization systems for frameless stereotactic cranial and extracranial hypofractionated radiotherapy. Int J Radiat Oncol Biol Phys 71(1 Suppl):S131–S135
Salter BJ et al (2001) The TALON removable head frame system for stereotactic radiosurgery/radiotherapy: measurement of the repositioning accuracy. Int J Radiat Oncol Biol Phys 51(2):555–562
Minniti G et al (2011) Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol 6:48
Page MJ et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Nath SK et al (2010) Single-isocenter frameless intensity-modulated stereotactic radiosurgery for simultaneous treatment of multiple brain metastases: clinical experience. Int J Radiation Oncology* Biology* Phys 78(1):91–97
Kraft J et al (2021) Distance to Isocenter is not associated with an increased risk for local failure in LINAC-based single-isocenter SRS or SRT for multiple brain metastases. Radiother Oncol 159:168–175
Mayo CS et al (2010) Initial experience with volumetric IMRT (RapidArc) for intracranial stereotactic radiosurgery. Int J Radiation Oncology* Biology* Phys 78(5):1457–1466
Bilger A et al (2017) Local control and overall survival after frameless radiosurgery: a single center experience. Clin Translational Radiation Oncol 7:55–61
Minniti G et al (2011) Frameless linac-based stereotactic radiosurgery (SRS) for brain metastases: analysis of patient repositioning using a mask fixation system and clinical outcomes. Radiat Oncol 6:1–6
Pham N-LL et al (2014) Frameless, real-time, surface imaging-guided radiosurgery: update on clinical outcomes for brain metastases. Translational Cancer Res, 3(4)
Liepa Z et al (2012) Initial experience with using frameless image-guided radiosurgery for the treatment of brain metastases. Experimental Oncology
Munshi A et al (2019) Dose fall-off patterns with volumetric modulated arc therapy and three-dimensional conformal radiotherapy including the organ at risk effect. Experience of linear accelerator-based frameless radiosurgery from a single institution. Cancer/Radiothérapie 23(2):138–146
De Potter B et al (2013) Hypofractionated frameless stereotactic intensity-modulated radiotherapy with whole brain radiotherapy for the treatment of 1–3 brain metastases. Neurol Sci 34:647–653
Cleary RK et al (2017) Postoperative fractionated stereotactic radiosurgery to the tumor bed for surgically resected brain metastases. Cureus, 9(5)
Bossart E et al (2021) Assessment of single isocenter linear accelerator radiosurgery for metastases and base of skull lesions. Physica Med 81:1–8
Han EY et al (2019) Dosimetric comparison of fractionated radiosurgery plans using frameless Gamma Knife ICON and CyberKnife systems with linear accelerator–based radiosurgery plans for multiple large brain metastases. J Neurosurg 132(5):1473–1479
Hanna SA et al (2019) Frameless image-guided radiosurgery for multiple brain metastasis using VMAT: a review and an institutional experience. Front Oncol 9:703
Minniti G et al (2020) Initial experience with single-isocenter radiosurgery to target multiple brain metastases using an automated treatment planning software: clinical outcomes and optimal target volume margins strategy. Adv Radiation Oncol 5(5):856–864
Vulpe H et al (2020) Frameless stereotactic radiosurgery on the gamma knife icon: early experience from 100 patients. Neurosurgery 86(4):509–516
Pan H et al (2012) Frameless, real-time, surface imaging-guided radiosurgery: clinical outcomes for brain metastases. Neurosurgery 71(4):844–852
Park HR et al (2019) Frameless fractionated gamma knife radiosurgery with ICON™ for large metastatic brain tumors. J Korean Med Sci, 34(8)
Breneman JC et al (2009) Frameless image-guided intracranial stereotactic radiosurgery: clinical outcomes for brain metastases. Int J Radiation Oncology* Biology* Phys 74(3):702–706
Lohkamp L-N et al (2018) Efficacy, safety and outcome of frameless image-guided robotic radiosurgery for brain metastases after whole brain radiotherapy. J Neurooncol 138:73–81
Eder MM et al (2022) Single-isocenter stereotactic radiosurgery for multiple brain metastases: impact of patient misalignments on target coverage in non-coplanar treatments. Z Med Phys 32(3):296–311
Wegner RE et al (2021) Single fraction Frameless Stereotactic Radiosurgery on the Gamma Knife icon for patients with brain metastases: time to abandon the Frame? Adv Radiation Oncol 6(5):100736
Furuse M et al (2008) Frameless stereotactic radiosurgery with a bite-plate: our experience with brain metastases. min-Minimally Invasive Neurosurg 51(06):333–335
Bennion NR et al (2016) A comparison of clinical and radiologic outcomes between frame-based and frameless stereotactic radiosurgery for brain metastases. Practical Radiation Oncol 6(6):e283–e290
Chen JC et al (2009) Control of brain metastases using frameless image-guided radiosurgery. NeuroSurg Focus 27(6):E6
Kasper E et al (2017) Stereotactic radiosurgery for brain metastasis from gynecological malignancies. Oncol Lett 13(3):1525–1528
Kelly PJ et al (2012) Stereotactic irradiation of the postoperative resection cavity for brain metastasis: a frameless linear accelerator-based case series and review of the technique. Int J Radiation Oncology* Biology* Phys 82(1):95–101
Kamath R et al (2005) Initial clinical experience with frameless radiosurgery for patients with intracranial metastases. Int J Radiation Oncology* Biology* Phys 61(5):1467–1472
Prabhu RS et al (2013) Clinical outcomes for a novel 6 degrees of freedom image guided localization method for frameless radiosurgery for intracranial brain metastases. J Neurooncol 113:93–99
Brömme J et al (2013) Adjuvant therapy after resection of brain metastases. Frameless image-guided LINAC-based radiosurgery and stereotactic hypofractionated radiotherapy. Strahlenther Onkol 189(9):765–770
Samanci Y et al (2021) Hypofractionated frameless gamma knife radiosurgery for large metastatic brain tumors. Clin Exp Metastasis 38:31–46
Muacevic A et al (2010) Feasibility, safety, and outcome of frameless image-guided robotic radiosurgery for brain metastases. J Neurooncol 97:267–274
Minniti G et al (2020) Neurological outcome and memory performance in patients with 10 or more brain metastases treated with frameless linear accelerator (LINAC)-based stereotactic radiosurgery. J Neurooncol 148:47–55
Nath SK et al (2010) Optically-guided frameless linac-based radiosurgery for brain metastases: clinical experience. J Neurooncol 97:67–72
Lau SK et al (2015) Single-isocenter frameless volumetric modulated arc radiosurgery for multiple intracranial metastases. Neurosurgery 77(2):233
Mok S et al (2017) Frameless stereotactic radiosurgery for brain metastases: a review of outcomes and prognostic scores evaluation. Hong Kong Med J
Kimmell KT et al (2015) Comparative effectiveness analysis of Treatment options for single brain metastasis. World Neurosurg 84(5):1316–1332
Breneman JC et al (2009) Frameless image-guided intracranial stereotactic radiosurgery: clinical outcomes for brain metastases. Int J Radiat Oncol Biol Phys 74(3):702–706
Stafford SL et al (2003) A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 55(5):1177–1181
Minniti G et al (2016) Single-Fraction Versus Multifraction (3 × 9 Gy) Stereotactic Radiosurgery for Large (> 2 cm) Brain Metastases: A Comparative Analysis of Local Control and Risk of Radiation-Induced Brain Necrosis. Int J Radiat Oncol Biol Phys 95(4):1142–1148
Higuchi Y et al (2009) Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys 74(5):1543–1548
Sakamoto T et al (1998) Audiological assessment before and after fractionated stereotactic irradiation for vestibular schwannoma. Radiother Oncol 49(2):185–190
Kondziolka D et al (2016) Quality of the patient experience during radiosurgery: measurement toward improvement. Stereotact Funct Neurosurg 94(3):134–139
Zindler JD et al (2017) Whole brain radiotherapy versus stereotactic radiosurgery for 4–10 brain metastases: a phase III randomised multicentre trial. BMC Cancer 17(1):500
Ma L et al (2017) Emerging technologies in stereotactic body radiotherapy. Chin Clin Oncol 6(Suppl 2):S12
Voynov G et al (2006) Frameless Stereotactic Radiosurgery for Recurrent Head and Neck Carcinoma. Technol Cancer Res Treat 5(5):529–535
Ibrahim A et al (2021) Frameless Stereotactic Radiosurgery with Linear Accelerator (LINAC)-Based technology for Brain metastases: outcomes analysis in 141 patients. Cureus 13(6):e15475
Hauswald H et al (2015) Linear accelerator-based stereotactic radiosurgery in 140 brain metastases from malignant melanoma. BMC Cancer 15:537
Lee SH et al (2012) Clinical application of RapidArc volumetric modulated arc therapy as a component in whole brain radiation therapy for poor prognostic, four or more multiple brain metastases. Radiat Oncol J 30(2):53–61
Nichol A et al (2016) Volumetric radiosurgery for 1 to 10 brain metastases: a Multicenter, Single-Arm, phase 2 study. Int J Radiat Oncol Biol Phys 94(2):312–321
He Z et al (2023) Frameless versus frame-based stereotactic radiosurgery for intracranial arteriovenous malformations: a propensity-matched analysis. Clin Translational Radiation Oncol 41:100642
Lau SK et al (2015) Single-isocenter Frameless Volumetric Modulated Arc Radiosurgery for multiple intracranial metastases. Neurosurgery, 77(2): p. 233 – 40; discussion 240.
Navarria P et al (2016) Hypo-fractionated stereotactic radiotherapy alone using volumetric modulated arc therapy for patients with single, large brain metastases unsuitable for surgical resection. Radiat Oncol 11(1):76
Kim YJ et al (2011) Single-dose versus fractionated stereotactic radiotherapy for brain metastases. Int J Radiat Oncol Biol Phys 81(2):483–489
Buss EJ et al (2020) Single-fraction stereotactic radiosurgery outcomes for Brain metastases with Frameless Gamma Knife ICON Radiosurgery: an update. Int J Radiat Oncol Biol Phys 108(3):e676
Funding
There is no funding source with authors to declare.
Author information
Authors and Affiliations
Contributions
Term: M.H, M.MConceptualization: M.H, Methodology: M.H, M.MInvestigation: Y.Gh, A.N, P.M, S.E, M.AWriting - Original Draft: M.M, Y.Gh, A.N, P.M, S.E, M.A, A.A, MZ.NWriting - Review & Editing: M.H, M.M, B.H, J.PSupervision: M.H, B.H, J.SProject administration: M.H, B.H, J.S.
Corresponding authors
Ethics declarations
Ethical approval
The study is deemed exempt from receiving ethical approval.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Habibi, M.A., Mirjnani, M.S., Ghazizadeh, Y. et al. Frameless stereotactic radiosurgery for brain metastasis: a systematic review and meta-analysis. Neurosurg Rev 47, 423 (2024). https://doi.org/10.1007/s10143-024-02666-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-024-02666-9