Abstract
Estimating efficacy, safety and outcome of frameless image-guided robotic radiosurgery for the treatment of recurrent brain metastases after whole brain radiotherapy (WBRT). We performed a retrospective single-center analysis including patients with recurrent brain metastases after WBRT, who have been treated with single session radiosurgery, using the CyberKnife® Radiosurgery System (CKRS) (Accuray Inc., CA) between 2011 and 2016. The primary end point was local tumor control, whereas secondary end points were distant tumor control, treatment-related toxicity and overall survival. 36 patients with 140 recurrent brain metastases underwent 46 single session CKRS treatments. Twenty one patients had multiple brain metastases (58%). The mean interval between WBRT and CKRS accounted for 2 years (range 0.2–7 years). The median number of treated metastases per treatment session was five (range 1–12) with a tumor volume of 1.26 ccm (mean) and a median tumor dose of 18 Gy prescribed to the 70% isodose line. Two patients experienced local tumor recurrence within the 1st year after treatment and 13 patients (36%) developed novel brain metastases. Nine of these patients underwent additional one to three CKRS treatments. Eight patients (22.2%) showed treatment-related radiation reactions on MRI, three with clinical symptoms. Median overall survival was 19 months after CKRS. The actuarial 1-year local control rate was 94.2%. CKRS has proven to be locally effective and safe due to high local tumor control rates and low toxicity. Thus CKRS offers a reliable salvage treatment option for recurrent brain metastases after WBRT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Frameless image-guided robotic radiosurgery is a treatment concept for single-fraction irradiation and hypo-fractionated treatments of cerebral lesions, including brain metastasis not amenable to microsurgery. Its technical accuracy is comparable to that of frame-based systems, which, in addition to a higher patient comfort promotes an increasing application [1,2,3]. Brain metastases are with an incidence of 10–14 cases per 100,000 the most frequent type of brain malignancy and therefore represent a common indication of cranial radiosurgery, i.e. single-fractioned CyberKnife® Radiosurgery System (CKRS) [4,5,6]. The CKRS treatment of brain metastases is well established and scientifically evaluated [7, 8], including series, in which brain metastases represented at least 25% of all intracranial treatment indications. Its therapeutic profile is reflected by a high tumor control, low toxicity and the repeatability of the procedure for recurrent metastases [9]. Comparable to the results of retrospective studies reporting a local tumor control above 90% by using frame-based systems [10,11,12,13,14], CKRS was shown to be equally effective with respect to both, local tumor control and clinical outcome in brain metastases [15, 16]. CKRS is principally considered as an appropriate therapeutic alternative to surgery or, in selected patients as an adjacent treatment with postoperative radiosurgical boost to the resection cavity [17, 18]. However, it has not been recognized as an efficient and safe adjunct treatment for recurrent brain metastases after whole brain radiotherapy (WBRT), although it is a treatment, which is undertaken fairly commonly by now. Several studies addressed the question of defining possible salvage treatments after WBRT [14, 19,20,21], including only few publications, which report the use of CKRS in this specific context. Herein we describe a single-center experience with a retrospective evaluation of 36 patients treated for recurrent brain metastases after WBRT by single session CKRS.
Materials and methods
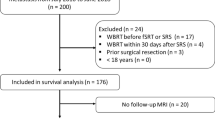
Between 2011 and 2015, 36 patients with 140 metastases of various histologies and completed WBRT underwent 46 single session radiosurgery procedures, using the CyberKnife® VSI Radiosurgery System (Accuray Inc., CA). All patients were prospectively filed in a customized digital database and admitted to radiosurgery treatment based on an interdisciplinary tumor board decision according to the following requirements:
-
Patient age between 18 and 80
-
Approved diagnosis of primary and histological assignment of brain metastases
-
Maximum tumor diameter ≤ 3 cm
-
Extracranial tumor stable or in remission, with or without systemic therapy
-
Exclusion of meningeal or ependymal tumor spread by thin sliced MRI and/or CSF examination
-
Previous WBRT
Additional criteria such as age, Karnofsky performance status (KPS) and quality of life were taken individually into account. The KPS was assessed in relation to both, general health condition and isolated neurological deficits. In general a KPS of 70 at the time-point of treatment was required. Nevertheless patients in good overall condition with a KPS < 70 due to an isolated neurological deficit were still amenable to CKRS treatment.
Twenty one of the patients (59%) had multiple cerebral metastases: 4 (11%) two lesions, 6 (17%) three lesions, 3 (8%) four lesions and 8 (22%) five lesions or more, respectively. For patients with multiple metastases, all tumors were treated in one treatment session (Fig. 1). Nine patients received additional treatments (6 two treatments, 2 three treatments and 1 patient four treatments) due to local or distant tumor recurrence. Time periods between initial WBRT and CKRS varied between the patients and were adapted individually with respect to time point of tumor recurrence, size of metastasis, KPS and prognosis. During WBRT patients received 30 Gray (Gy) over 2 weeks (10 × 3 Gy) or 40 Gy over 4 weeks (20 × 2 Gy). Indications for WBRT included brain metastases with or without previous neurosurgical resection as well as those not amenable to primary CKRS. Detailed patient and treatment characteristics are given in Table 1.
Single-session CKRS treatment plan of multiple recurrent brain metastases. 3D and multi-planar radiation planning images with beam directions and dose distribution based on the planning CT scan. The treatment plan illustrates a single-session CKRS intervention for simultaneous radiation of eight recurrent brain metastases in a patient with lung cancer
Treatment
CKRS was performed as an outpatient procedure in all patients. Prior to the treatment a thin sliced Gadolinium-enhanced MRI scan and a thin-cut CT scan were performed allowing 3D conformal treatment planning. The MRI additionally served as an initial baseline for MRI follow-up examinations. Patient motion during treatment was restricted by a customized thermoplastic mold and automatically corrected up to 0.1 mm in translations and 0.1° in rotations based on the detected information of the CK-specific image guidance system (6D skull tracking) in order to maintain optimal accuracy of the beam position [1]. The prescribed radiation dose was adjusted according to the radio-sensitivity/-resistance of the diagnosed tumor, size and volume of the metastasis and to the integrated volume of all metastases in case of multiple lesions, eloquent location and interval to previous irradiation.
Follow-up evaluation
The follow-up included clinical examination and MRI controls which were performed on a 3-month basis after CKRS in order to document local and distant tumor control. Local tumor recurrence was defined as a persistent radiographic increase of 25% or more in the size of a metastatic lesion. In cases where MRI could not discriminate between radiation reaction (pseudo progression) and tumor recurrence O-(2[18-F]-fluorethyl)-l-tyrosin (FET)-PET imaging was performed. Signs of radiation toxicity were scored morphologically according to the National Cancer Institute’s Common Toxicity Criteria version 2.0 [22]. Tumor volume calculations were based on the tumor margins on cross-sectional MRI studies before the beginning of treatment and at each follow-up. A complete resolution of the lesion after WBRT and radiosurgery was defined as complete remission, whereas a tumor volume reduction > 50% was rated as partial response. A tumor progression was defined as any tumor volume increase > 25% as compared to the “best” treatment response. Local tumor control was evaluated as “achieved” when complete resolution remained stable for at least two follow up visits. Any reappearance of a new lesion at the previously treated site was classified as a local tumor recurrence. Distant tumor recurrence was approved when a novel enhanced lesion occurred distant from the original metastasis site. Freedom from local recurrence/local tumor progression was defined by the time interval between the date of initial radiosurgical treatment and the date of diagnosis of local tumor recurrence/local tumor progression. Freedom from distant tumor recurrence was equally defined according to the above-mentioned intervals.
Clinical follow-up included assessment of neurologic functions and toxic side effects. The overall systemic functional status was evaluated according to the Karnofsky performance score (KPS) and its prerequisites [23]. If the KPS remained unchanged or was better after treatment, it was referred to a stabilized, improved clinical status, respectively. Otherwise, the status was considered as deteriorated. Adverse radiation-induced effects were defined as acute when occurring within the first 90 days and as late when occurring afterwards. Both were assessed according to the central nervous system toxicity criteria listed in the Radiation Therapy Oncology Group (RTOG) Late Radiation Morbidity Scoring Criteria [24]. The cause of death was determined from in- or external medical records as well as supplementary phone calls to general practitioners. The cause of death was documented according to the study protocol of Patchell et al., distinguishing between death due to cerebral progression and systemic death in the context of the underlying disease [25].
Outcome measurement
The primary outcome measure was local tumor control. Secondary outcome measures were recurrence of distant brain metastases, treatment-related toxicity and overall survival.
Statistical methods
Reference point of the study was the date of radiosurgical treatment. Length of overall survival, freedom from local and distant tumor recurrences were estimated with the Kaplan–Meier method using the software IBM SPSS statistics [26].
Results
Follow-up information was available for all 36 patients who underwent 46 CKRS interventions for a total of 140 metastases. The average number of follow-up visits was 1.85 (range 1–9) per patient. A gender distribution of 9 males and 27 females was observed. The median age was 53 years (28–71 years) and the median follow-up period 42 months (3–81 months; mean 24.8 months). All patients received prior WBRT of 30–40 Gy. The median interval between completed WBRT and initiation of CKSR was 3.6 years (0.2–7 years). In addition 33 of 36 patients received chemotherapy, most of them with cisplatin. At the time point of CKRS all patients showed novel distant brain metastases throughout the radiological follow-up. The initial diagnosis of brain metastasis was based on radiological findings and in half of the cases additionally on histopathological results. Primary tumors were referred to breast carcinoma (17 patients), non-small cell lung cancer (11 patients), colorectal cancer (2 patients), malignant melanoma (2 patients), and gastrointestinal, pharyngeal and endocrine tumors (1 patient each). In one patient the histology was not specified and related to CUP. 18 of the patients underwent no neurosurgical intervention at all, 14 patients underwent surgery once and 4 of the patients underwent repeated neurosurgical interventions before WBRT. Neurological symptoms such as headache, seizures or focal neurological deficits were present in four patients (11%) after WBRT and prior to CKRS. The median KPS score was 80 before (range 50–100) and 70 after treatment (range 50–100) due to deterioration in nine and amelioration in two patients, respectively. All tumor locations in the brain were treated, including basal ganglia, pre-central and central cortex as well as brainstem lesions.
CKRS treatment parameters
The median dose prescription to the tumor margin was 18 Gy (median, range 7–20 Gy) prescribed to the 70% isodose line. The minimum and maximum median tumor doses were 17 Gy (range 7.5–22.4 Gy) and 25.7 Gy (range 20.1–30 Gy), respectively. The median number of beams was 196 (range 52–505), applied to a median tumor volume of 1.32 ccm (mean; median 0.46; range 0.02–16.31 ccm) per metastases. Notably, few patients received a simultaneous treatment of multiple metastases within the same treatment session, thus a mean total tumor volume of 4.02 ccm (median 1.19; range 0.07–74.99 ccm) was treated per patient and session. The median duration of treatment was 56 min (range 23–130 min).
Survival and treatment response
At the time point of the last follow-up 17 patients (47.2%) had died, 14 patients (39%) due to progressive systematic disease, one (2.8%) from progressive central nervous system disease (meningeosis carcinomatosa). For two patients the cause of death was unknown. The 6, 12, 18, and 24-month actuarial survival rates were 88.9% (95% CI 39.2–52.8), 75.0% (95% CI 27.6–45.5), 66.7% (95% CI 21.8–40.1), and 69.1% (95% CI 18.6–36.2), respectively. The overall median survival rate was 19 months (Fig. 2). Local recurrences were observed in two of the patients within the first 6 months after treatment corresponding to a local tumor control rate at 6 and 12 months of 94.2% (95% CI 46.0–55.5), (Fig. 3). Distant novel brain metastases were observed within the 1st year after treatment in nine patients within the 1st year and in another two patients within the 2nd year after treatment. Accordingly the 6 and 12-month actuarial distant tumor control rates in the brain were 83.3% (95% CI 32.8–50.6) and 75% (95% CI 23.2–44.4), whereas the 18-, respectively 24-month distant tumor control rate was 72.2% (95% CI 19.3–41.6) (Suppl. Fig. 1). CKRS re-treatment was performed in nine patients (25%) for new, distant metastases, including six patients (17%) that required two additional treatments each, two patients 3 (5.6%) and one patient 4 (2.8%) additional treatments, respectively.
Overall survival after WBRT followed by CKRS. Kaplan–Meier analysis with survival function applied for 36 patients who underwent single-session CKRS for recurrent brain metastases after WBRT. The median overall survival after CKRS accounted for 19 months, whereas the 6, 12, 18, and 24-month actuarial survival rates were 88.9, 75.0, 66.7, and 69.1%, respectively
Local tumor control after CKRS. Local tumor control was calculated for 140 metastases treated by CKRS after WBRT using the Kaplan–Meier estimation method. The graph displays a local tumor control rate of 94.2% at 6, 12, 18 and 24 months after CKRS. Two patients developed probable local recurrences within the first 6 months after treatment
Side effects and complications
The overall morbidity after CKRS treatment was 22.8% (8 patients). Among these eight patients, four experienced aggravation of preexisting neurological deficits, e.g. partial hemiparesis. However, in one of these patients worsening of neurologic function was related to progressing radiation necrosis at the contralateral hemisphere and not to CKRS treatment. Other two patients (5.6%) experienced abnormal fatigue, one patient deterioration due to systemic tumor progression and one patient was observed with diminished level of consciousness related to meningeosis carcinomatosa. Adverse radiation effects, as diagnosed on MR, occurred in eight patients (22.2%). Of these patients 3 (8.3%) were found to be symptomatic, one patient experienced deterioration of a pre-existing hemiparesis due to perifocal edema and received additional steroid treatment. Another two patients suffered from focal alopecia. Asymptomatic radiation reaction was observed in five patients (14%). None of the patients showed radiation necrosis, nor required surgical decompression of space-occupying radio-necrotic lesions. Conclusively treatment-related side effects or symptoms had occurred in five patients (14%). There was no association between complications and tumor volume, lesion number or CKRS frequency. A summary of post-treatment complications is given in Table 2.
Discussion
CKRS as a salvage therapy for recurrent brain metastases after WBRT
Robotically guided CKRS was shown to be efficient and safe in selected patients with brain metastases [15, 27] and represents an attractive and convenient treatment option because of its low risk and minimal invasiveness [9]. It has been shown to be beneficial when applied on its own or in combination with other treatment modalities [11, 28,29,30], providing a high local tumor control and repeatability of treatments for both, local or distant recurrences [3, 9, 31]. Additionally, single or multiple metastases can be treated in a single session even on an outpatient basis, which offers a high patient comfort. However, patients with recurrent brain metastases, who underwent WBRT before CKRS might bare a higher risk of local failure or side effects. Scientific evidence in this specific subgroup of patients is scarce [19, 21, 32], thus investigations confirming CKRS, as feasible, safe and efficient salvage therapy for recurrent brain metastases are needed and addressed herein. We report on a patient series where CKRS was applied after WBRT for recurrent brain metastases of various histologies. All patients were prospectively analyzed and selected by an interdisciplinary tumor board for CKRS treatment. Our objective was to assess the therapeutic impact of CKRS after WBRT using the same selection criteria as those used for patients recently treated with frame-based techniques after WBRT [4, 12, 14, 20, 33,34,35].
Treatment efficacy
The local tumor control rate that was achieved in our patient cohort was relatively high with 94.2%, at 6, 12, 18 and 24 months, respectively as only two patients showed suspicious local tumor relapse within the first 6 months after CKRS treatment. These rates are positively comparable to other recent reports using both, frameless and frame-based techniques [4, 14, 20]. Chao et al., using Gamma Knife RS analyzed the data of 111 patients, who underwent SRS for a total of 243 brain metastases of various histologies after WBRT. The local control rate at 1 year was 68 and 59% at 2 years, respectively. The distant tumor control rate was 86% at 1 year, and 51% at 2 years [14]. Noel et al. [20] treated 54 patients presenting with 97 recurrent metastases after WBRT with frame-based stereotactic radiotherapy. 1- and 2-year local control rates were 91.3 and 84% and 1- and 2-year brain control rates were 65 and 57%, respectively. These results go in line with our findings as well as with the previously confirmed hypothesis of Kondziolka et al. [33] that radiosurgery plus WBRT would provide improved local brain tumor control over WBRT alone in patients with two to four brain metastases. He reported a local failure rate of 8% at 1 year in patients who had boost radiosurgery after WBRT. Gwak et al. [19], used the CyberKnife System in 100 recurrent brain metastases after WBRT (46 patients). The local tumor control rate after 1 year was 64%, the distant tumor control rate 57%, respectively. Demographic data such as age, KPS and number of intracerebral metastases did not differ relevantly from our findings. Our median overall survival (19 months), is somewhat higher compared to overall survival rates reported by other authors after SRS either with or without WBRT [4, 36,37,38,39]. Although our patient selection criteria did not vary from those of the above-mentioned studies, we presume that these favorable results are certainly related to a stringent, interdisciplinary selection process for patients, considered suitable for CKRS treatment. Nevertheless, even if the role of CKRS in this context as an alternative to repetitive radiotherapy seems to be favorable, it requires further prospective evaluation especially with regard to preselected patient subgroups and extended treatment indications.
Treatment safety
CKRS treatment was performed for all patients with local or new distant brain metastases and a stable systemic tumor status, good quality of life at the time point of treatment, respectively. Treatment planning and execution was comparable for all patients, including those with multiple metastases. The presence of multiple metastases was not found to have a prognostic impact in our patient selection, thus tumor control was independent from the number of initially treated metastases. However, CKRS re-treatment was required in nine patients (25%) for new, distant metastases, including six, two and one patient that required additional two, three and four treatments, respectively. These recurrences were observed in six patients (17%) diagnosed with breast cancer, 2 (5.5%) with lung cancer and 1 (2.8) with a colorectal carcinoma, respectively. The additional treatments were equally well tolerated what underlines the low risk profile of CKRS. Only four patients experienced neurologic symptoms of pre-existing deficits after CKRS and two patients developed new symptoms such as abnormal fatigue within the first 6 months after CKRS treatment. These patients received additional steroid treatment just as seven other patients due to progressing edema. Asymptomatic radiation reactions on follow-up imaging were observed in 5 (13.8%) whereas symptomatic reactions in three patients (8.3%). Two of them suffered from alopecia and one from worsening hemiparesis. Patients neither died of radiation-induced complications nor required surgery for space-occupying radio-necrosis. The overall complication rate in our cohort appears equivalent to that of other studies using SRS after WBRT [4, 14]. However, Gwak et al. [19] found a considerable incidence of clinically significant radiation toxicity (21–22%) including radiation necrosis related to tumor volume and cumulative dose. Although our study does not bring these effects to bear and confirms safety in this specific treatment concept, we emphasize that such need to be followed carefully among upcoming studies in order to generate reliable guidelines for the application of CKRS after WBRT.
Limitations
The current analysis refers to patients with recurrent brain metastases after WBRT. Additional inclusion criteria for patients having access to CKRS after WBRT were age, KPS and quality of life at the time point of treatment. Another precondition for CKRS treatment were a controlled extracranial disease or its remission, and exclusion of meningeal or ependymal tumor spread. Of course, a stringent pre-selected subpopulation leads to some bias. Even if our results have full validity based on reliable scientific methods and reporting, outcome interpretation may be adjusted to the fact of subgrouping and compared to other results with precaution.
The moderate number of patients might be a drawback, which is overcome by the homogeneity of single-center data and the representative number of 140 treated metastases. The retrospective type of analysis with scarce follow-up data for some of the patients is another limitation that needs to be anticipated in order to generate appropriate (long-term) follow-up results for all of the patients.
Interestingly pathological results, especially in terms of molecular features of each tumor entity have not been matched to responsiveness and recurrence of brain metastases after CKRS so far.
Conclusions
Single-session, frameless, image-guided robotic CKRS has been proven to be a safe and effective treatment for recurrent brain metastases after WBRT in selected patients. CKRS resulted in good local tumor control (1-year-rate 94.2%) and thus seems to have a positive influence on overall survival. CKRS can be administered after WBRT for both, one-time and repetitive treatment of single or multiple metastases with a low risk for neurologic deficits or adverse radiation effects. However, outcome was particularly favorable in a pre-selected patient cohort with a constant KPS ≥ 70. In summary frameless CKRS for recurrent brain metastases after WBRT is a valuable salvage option, which should be investigated within further prospective trials and become more readily reported in the literature.
References
Chang SD, Main W, Martin DP, Gibbs IC, Heilbrun MP (2003) An analysis of the accuracy of the CyberKnife: a robotic frameless stereotactic radiosurgical system. Neurosurgery 52:140–146 (discussion 146–147)
Fu D, Kuduvalli G (2008) A fast, accurate, and automatic 2D-3D image registration for image-guided cranial radiosurgery. Med Phy 35:2180–2194. https://doi.org/10.1118/1.2903431
Adler JR Jr, Murphy MJ, Chang SD, Hancock SL (1999) Image-guided robotic radiosurgery. Neurosurgery 44:1299–1306 (discussion 1306–1297)
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ Jr (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665–1672. https://doi.org/10.1016/s0140-6736(04)16250-8
Lang FF, Sawaya R (1998) Surgical treatment of metastatic brain tumors. Semin Surg Oncol 14:53–63
Stelzer KJ (2013) Epidemiology and prognosis of brain metastases. Surg Neurol Int 4:S192–S202. https://doi.org/10.4103/2152-7806.111296
Nishizaki T, Saito K, Jimi Y, Harada N, Kajiwara K, Nomura S, Ishihara H, Yoshikawa K, Yoneda H, Suzuki M, Gibbs IC (2006) The role of CyberKnife radiosurgery/radiotherapy for brain metastases of multiple or large-size tumors. Minim Invasive Neurosurg 49:203–209. https://doi.org/10.1055/s-2006-947998
Wang ZZ, Yuan ZY, Zhang WC, You JQ, Wang P (2009) Brain metastasis treated with CyberKnife. Chin Med J 122:1847–1850
Wowra B, Muacevic A, Tonn JC (2012) CyberKnife radiosurgery for brain metastases. Prog Neurol Surg 25:201–209. https://doi.org/10.1159/000331193
Flickinger JC, Kondziolka D, Lunsford LD, Coffey RJ, Goodman ML, Shaw EG, Hudgins WR, Weiner R, Harsh GR, Sneed PK et al (1994) A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys 28:797–802
Hasegawa T, Kondziolka D, Flickinger JC, Germanwala A, Lunsford LD (2003) Brain metastases treated with radiosurgery alone: an alternative to whole brain radiotherapy? Neurosurgery 52:1318–1326 (discussion 1326)
Joseph J, Adler JR, Cox RS, Hancock SL (1996) Linear accelerator-based stereotaxic radiosurgery for brain metastases: the influence of number of lesions on survival. J Clin Oncol 14:1085–1092. https://doi.org/10.1200/jco.1996.14.4.1085
Muacevic A, Wowra B, Siefert A, Tonn JC, Steiger HJ, Kreth FW (2008) Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neuro-Oncol 87:299–307. https://doi.org/10.1007/s11060-007-9510-4
Chao ST, Barnett GH, Vogelbaum MA, Angelov L, Weil RJ, Neyman G, Reuther AM, Suh JH (2008) Salvage stereotactic radiosurgery effectively treats recurrences from whole-brain radiation therapy. Cancer 113:2198–2204. https://doi.org/10.1002/cncr.23821
Breneman JC, Steinmetz R, Smith A, Lamba M, Warnick RE (2009) Frameless image-guided intracranial stereotactic radiosurgery: clinical outcomes for brain metastases. Int J Radiat Oncol Biol Phys 74:702–706. https://doi.org/10.1016/j.ijrobp.2008.11.015
Tamari K, Suzuki O, Hashimoto N, Kagawa N, Fujiwara M, Sumida I, Seo Y, Isohashi F, Yoshioka Y, Yoshimine T, Ogawa K (2015) Treatment outcomes using CyberKnife for brain metastases from lung cancer. J Radiat Res 56:151–158. https://doi.org/10.1093/jrr/rru092
Soltys SG, Adler JR, Lipani JD, Jackson PS, Choi CY, Puataweepong P, White S, Gibbs IC, Chang SD (2008) Stereotactic radiosurgery of the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys 70:187–193. https://doi.org/10.1016/j.ijrobp.2007.06.068
Choi CY, Chang SD, Gibbs IC, Adler JR, Harsh GR, Lieberson RE, Soltys SG (2012) Stereotactic radiosurgery of the postoperative resection cavity for brain metastases: prospective evaluation of target margin on tumor control. Int J Radiat Oncol Biol Phys 84:336–342. https://doi.org/10.1016/j.ijrobp.2011.12.009
Gwak HS, Yoo HJ, Youn SM, Lee DH, Kim MS, Rhee CH (2009) Radiosurgery for recurrent brain metastases after whole-brain radiotherapy: factors affecting radiation-induced neurological dysfunction. J Korean Neurosurg Soc 45:275–283. https://doi.org/10.3340/jkns.2009.45.5.275
Noel G, Proudhom MA, Valery CA, Cornu P, Boisserie G, Hasboun D, Simon JM, Feuvret L, Duffau H, Tep B, Delattre JY, Marsault C, Philippon J, Fohanno D, Baillet F, Mazeron JJ (2001) Radiosurgery for re-irradiation of brain metastasis: results in 54 patients. Radiother Oncol 60:61–67
Olson AC, Wegner RE, Rwigema JC, Heron DE, Burton SA, Mintz AH (2012) Clinical outcomes of reirradiation of brain metastases from small cell lung cancer with CyberKnife stereotactic radiosurgery. J Cancer Res Ther 8:411–416. https://doi.org/10.4103/0973-1482.103522
Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K, Gunderson L, McCormick B, Morrisintegral M, Rich T, Shipley W, Curran W (2000) Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phy 47:13–47
Schag CC, Heinrich RL, Ganz PA (1984) Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 2:187–193. https://doi.org/10.1200/jco.1984.2.3.187
Radiation Therapy Oncology Group (2009) RTOG/EORTC late radiationmorbidity scoring schema. http://www.rtog.org/members/ toxicity/late.html
Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322:494–500. https://doi.org/10.1056/nejm199002223220802
Kaplan ELMP. (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:475–481
Shimamoto S, Inoue T, Shiomi H, Sumida I, Yamada Y, Tanaka E, Inoue T (2002) CyberKnife stereotactic irradiation for metastatic brain tumors. Radiat Med 20:299–304
Hara W, Tran P, Li G, Su Z, Puataweepong P, Adler JR Jr, Soltys SG, Chang SD, Gibbs IC (2009) Cyberknife for brain metastases of malignant melanoma and renal cell carcinoma. Neurosurgery 64:A26–A32. https://doi.org/10.1227/01.neu.0000339118.55334.ea
Wang CC, Floyd SR, Chang CH, Warnke PC, Chio CC, Kasper EM, Mahadevan A, Wong ET, Chen CC (2012) CyberKnife hypofractionated stereotactic radiosurgery (HSRS) of resection cavity after excision of large cerebral metastasis: efficacy and safety of an 800 cGy × 3 daily fractions regimen. J Neuro-oncol 106:601–610. https://doi.org/10.1007/s11060-011-0697-z
Pontoriero A, Conti A, Iati G, Mondello S, Aiello D, Rifatto C, Risoleti E, Mazzei M, Tomasello F, Pergolizzi S, De Renzis C (2016) Prognostic factors in patients treated with stereotactic image-guided robotic radiosurgery for brain metastases: a single-center retrospective analysis of 223 patients. Neurosurg Rev 39:495–504. https://doi.org/10.1007/s10143-016-0718-7
Wowra B, Muacevic A, Tonn JC (2009) Quality of radiosurgery for single brain metastases with respect to treatment technology: a matched-pair analysis. J Neuro-oncol 94:69–77. https://doi.org/10.1007/s11060-009-9802-y
Ippen FM, Mahadevan A, Wong ET, Uhlmann EJ, Sengupta S, Kasper EM (2015) Stereotactic radiosurgery for renal cancer brain metastasis: prognostic factors and the role of whole-brain radiation and surgical resection. J Oncol. https://doi.org/10.1155/2015/636918
Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC (1999) Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 45:427–434
Loeffler JS, Kooy HM, Wen PY, Fine HA, Cheng CW, Mannarino EG, Tsai JS, Alexander E 3rd (1990) The treatment of recurrent brain metastases with stereotactic radiosurgery. J Clin Oncol 8:576–582 https://doi.org/10.1200/jco.1990.8.4.576
Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N (2000) Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 47:291–298
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295:2483–2491. https://doi.org/10.1001/jama.295.21.2483
Sperduto PW, Shanley R, Luo X, Andrews D, Werner-Wasik M, Valicenti R, Bahary JP, Souhami L, Won M, Mehta M (2014) Secondary analysis of RTOG 9508, a phase 3 randomized trial of whole-brain radiation therapy versus WBRT plus stereotactic radiosurgery in patients with 1–3 brain metastases; poststratified by the graded prognostic assessment (GPA). Int J Radiat Oncol Biol Phys 90:526–531. https://doi.org/10.1016/j.ijrobp.2014.07.002
Sneed PK, Suh JH, Goetsch SJ, Sanghavi SN, Chappell R, Buatti JM, Regine WF, Weltman E, King VJ, Breneman JC, Sperduto PW, Mehta MP (2002) A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys 53:519–526
Chen JC, Petrovich Z, Giannotta SL, Yu C, Apuzzo ML (2000) Radiosurgical salvage therapy for patients presenting with recurrence of metastatic disease to the brain. Neurosurgery 46:860–866 (discussion 866–867)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2018_2771_MOESM1_ESM.docx
Suppl. Fig. 1 Distant tumor control after CKRS. Kaplan–Meier analysis used for estimating distant tumor control, revealing a 6 and 12-month actuarial control rate of 83.3% and 75%. At 18 and 24 months after CKRS treatment the distant tumor control rate remained unchanged, i.e. 72.2%.
Supplementary material 1 (DOCX 62 KB)
Rights and permissions
About this article
Cite this article
Lohkamp, LN., Vajkoczy, P., Budach, V. et al. Efficacy, safety and outcome of frameless image-guided robotic radiosurgery for brain metastases after whole brain radiotherapy. J Neurooncol 138, 73–81 (2018). https://doi.org/10.1007/s11060-018-2771-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2771-2