Abstract
Purpose
Stereotactic Radiosurgery (SRS) is the primary treatment for patients with limited numbers of small brain metastases. Head fixation is usually performed with framed-based (FB) fixation; however, mask-based (MB) fixation has emerged as a less invasive alternative. A comparative meta-analysis between both approaches has not been performed.
Methods
Databases were searched until August 28th, 2023, to identify studies comparing MB and FB SRS in the treatment of brain metastases. Our outcomes of interest included local tumor control (LTC), radiation necrosis (RN), mortality, and treatment time (TT). Mean difference (MD), risk ratio (RR), and hazard ratio (HR) were used for statistical comparisons.
Results
From 295 articles initially identified, six studies (1 clinical trial) involving 509 patients were included. LTC revealed comparable RR at 6-months (RR = 0.95[95%CI = 0.89–1.01], p = 0.12) and a marginal benefit in FB SRS at 1-year (RR = 0.87[95%CI = 0.78–0.96], p = 0.005). However, in oligometastases exclusively treated with single-fraction SRS, LTC was similar among groups (RR = 0.92 [95%CI = 0.89–1.0], p = 0.30). Similarly, in patients with oligometastases treated with single-fraction SRS, RN (HR = 1.69; 95%CI = 0.72–3.97, p = 0.22), TT (MD = -29.64; 95%CI = -80.38–21.10, p = 0.25), and mortality were similar among groups (RR = 0.62; 95%CI = 0.22–1.76, p = 0.37).
Conclusion
Our findings suggest that FB and MB SRS, particularly oligometastases treated with single-fraction, are comparable in terms of LTC, RN, TT, and mortality. Further research is essential to draw definitive conclusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is estimated that 20% of patients with cancer will develop metastatic brain disease (MBD), the most common intracranial tumor in adults [1]. Treatment options for MBD include surgical resection [2], whole brain radiation therapy (WBRT) [3], and stereotactic radiosurgery (SRS) [4]. SRS is typically used to treat small metastatic tumors, especially when there are a limited number of lesions or the lesions are surgically-inaccessible [5]. Traditionally, head fixation for Gamma Knife SRS (GK-SRS) has relied on frame-based fixation (FB) techniques to ensure the accuracy of treatment delivery during the intervention. However, mask-based fixation (MB) has emerged as a less invasive alternative for patient care [6, 7].
Frequent concerns of FB SRS may be solved by MB SRS, which include anxiety, anticoagulation medication, intolerance to anesthetic agents, or multiple neurosurgical procedures [8]. MB SRS may achieve positioning accuracy by integrating cone-beam CT imaging, automatic image alignment, real-time adaptation, and motion tracking techniques [9]. However, while several double-arm studies have compared these two fixation approaches, the sample sizes are limited and their findings have been heterogeneous [6, 7, 10]. To the best of our knowledge, no previous meta-analysis has been attempted to address this knowledge gap.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines for this meta-analysis [11]. All steps were done in adherence with the Cochrane Handbook of Systematic Reviews and Meta-analysis of Interventions (version 6.3) [12]. The meta-analysis protocol was registered on February 21, 2024 on PROSPERO under ID: 516475.
Criteria of the included studies
We established strict inclusion criteria to identify observational cohort studies and a unique randomized controlled trial focusing on adult patients with MBD treated with SRS. The two study arms included patients treated with FB and MB SRS. Our outcomes of interest included local tumor control (LTC) at 6 months and 1 year, radiation necrosis (RN), mortality, and treatment time (TT).
To ensure the reliability of our findings, we eliminated studies that did not report at least one of the specified outcomes of interest. To decrease bias, we included studies with more than 10 participants. We excluded patients under 18 years, studies that did not compare MB and FB SRS, or reported at least one of our outcomes of interest. Considering the variations in SRS treatment regimens, we excluded studies that did not involve MBD, such as vestibular schwannomas, meningiomas, arteriovenous malformations, trigeminal neuralgia, or pituitary adenomas, for example. Additionally, case–control studies, cross-sectional studies, systematic reviews, meta-analyses, case reports, basic science research, conference abstracts, letters to the editor, and review articles were excluded.
Literature search strategy
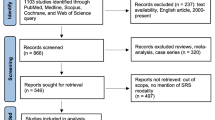
We conducted a comprehensive search of four electronic databases (PubMed, MEDLINE, EMBASE, and COCHRANE) from inception until August 28th, 2023. Keywords and free words were used to search for Radiosurgery, Stereotactic Radiosurgery, Gamma Knife Surgery, Frame-Based Fixation, and Mask-Based Fixation. The search strategy is represented in supplementary material 1.
We used Zotero software to remove duplicate references, screened each publication based on title and abstract, and subsequently performed a full-text review as a second step. Each manuscript was independently evaluated by two authors in a blinded manner; a third author resolved any conflicts. Additionally, we reviewed the bibliographic references of our included studies if they matched our eligibility criteria.
Data extraction
The following data were extracted using Excel spreadsheets: (1) Baseline characteristics of the examined population; (2) Summary of the features of the included studies, (3) Outcome measures, and (4) Domains subject to evaluation for quality assessment.
Assessing the risk of bias
We employed the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool to assess the potential risk of bias within the observational cohort studies [13]. Regarding risk of bias assessment for randomized controlled trials (RCT), we utilized the version 2 of the Cochrane risk-of-bias tool for randomized trials (ROB 2) from the Cochrane Handbook of Systematic Reviews of Interventions 6.3 [14, 15].
Outcome measures
A comprehensive assessment was conducted to compare the efficacy and safety of MB and FB SRS. The following outcomes were included for analysis:
-
Local Tumor Control (LTC): LTC evaluates the effectiveness of SRS in preventing the tumor recurrence at the SRS treatment site. LTC was achieved when stable disease or no progression was identified at postoperative MRI follow-up. If tumor progression was identified, we cataloged these as local treatment failures. The analyses were at 6 months, 1 year, and at last follow-up. LTC and treatment failures were assessed through univariate and multivariate analyses, comparing the risk ratio (RR) and hazard ratio (HR) among interventions.
-
Radiation Necrosis (RN): This variable evaluated the incidence rate of RN following SRS. RN was defined in accordance with standardized criteria [16]. Analysis of RN was performed through univariate and multivariate assessments RR and HR among groups.
-
Treatment in Time (TT): Average duration of the SRS session in minutes comparing MB versus FB fixation, we compared this variable by measuring mean difference (MD).
-
Mortality: Mortality was assessed by the incidence of death at the final follow-up after receiving SRS treatment for brain metastases. Univariate analysis was conducted using RR.
Statistical analysis
To evaluate dichotomous outcomes such as LTC, RN, and mortality we analyzed the pooled frequency of events among SRS study arms, calculating univariate RR. To mitigate potential confounders, we conducted pooled multivariate HR analyses with cox-regression after variable adjustment which included tumor size, treatment, location, and other significant variables in univariate analysis. TT, the only continuous variable, was assessed using MD across studies. We set a significance threshold of p-value < 0.05 with a 95% confidence interval (CI). Based on the identified studies, we conducted supplementary analyses on studies evaluating < 5 brain metastases and categorized them as oligometastases.
The analyzed variables in this study were derived on data reported in the original studies. To account for heterogeneity and facilitate the comparison between studies, we used a random-effects model using the DerSimonian-Laird method [17]. Forest plot graphics were used to represent the final results. All statistical analyses, including RR, MD, and HR calculations, were performed using RevMan V.5.4.1 software.
Assessment of heterogeneity
We utilized Cochran’s Q-Statistic to evaluate heterogeneity, with a significance level of p < 0.10. Then I2-statistic was employed to estimate the fraction of variance related to heterogeneity, with values more than 50% indicating strong heterogeneity [18].
Results
We identified 295 studies from our initial search,, among which six studies from three different countries met our inclusion criteria [6,7,8, 10, 19, 20]. These studies encompassed a total of 509 patients with MBD comparing FB to MB GK-SRS. The studies included one RCT [6], one non-randomized trial [8], and four observational cohort studies [7, 10, 19, 20]. The PRISMA flow diagram study selection process and the summary of included studies are detailed in Fig. 1 and Table 1.
In the analysis, we included studies that compared two groups of patients with MBD, the study arms included MB SRS and FB SRS, all treatments were delivered using GK-SRS. While the majority of patients received single-fraction SRS, some patients with high-tumor volume received hypofractionated SRS. Only three studies treated oligometastases exclusively with single-fraction SRS at doses of 22–24 Gy. We conducted supplementary analyses on studies evaluating oligometastases using single-fraction SRS in order to mitigate confounders. The range of age was 19–90 years of age. The total number of lesions reported in observational cohorts were 1,243 metastases [7, 8, 10, 19, 20]. The median number of metastases in the RCT was 2.31 (range 1–7) in the FB SRS and 2.22 (range 1–6) for the MB SRS [6]. Most of the included lesions were small metastases with mean volumes ranging from 0.3–2.18 ml. Notably, considering only Begley et al. had baseline tumor volume differences, we performed supplemental analyses to only include matching tumor volumes. All included studies focused on patients with brain metastasis, with one study specifically targeting metastases located in the brainstem only. The most common primary cancers included lung, breast, melanoma, gastrointestinal, and genitourinary tumors. Four studies included patients who had previously received WBRT for MBD. Comfortability was only reported in one study significantly favoring MB SRS. Baseline demographic characteristics are described in Table 2.
Local tumor control
Univariate analysis of LTC at 6 months revealed a non-significant RR of 0.95 favoring FB SRS (95%CI = 0.89–1.01, p = 0.12); the pooled studies were homogeneous (I2 = 13%, p = 0.33). At 1-year, LTC revealed a significant RR of 0.87 favoring FB SRS (95%CI = 0.78–0.96, p = 0.03), studies were heterogeneous (I2 = 63%, p = 0.03). This is illustrated in Fig. 2. However, as observed in Supplementary Fig. 1, oligometastases exclusively treated with single-fraction SRS exhibited similar LTC among groups (RR = 0.92 [95%CI = 0.89–1.0], p = 0.30). Multivariate local failure analysis in the overall cohort showed a non-significant HR of 0.98 (95%CI = 0.34–2.82, p = 0.97), with study heterogeneity (I2 = 68%, p = 0.04); multivariate local failure is depicted in Supplementary Fig. 2.
Radiation necrosis
Univariate RN analysis showed a non-significant RR of 0.76 favoring FB SRS (95%CI = 0.44–1.30, p = 0.32), with homogeneous studies (I2 = 0%, p = 0.63). Univariate RN RR analysis is represented in Fig. 3. Multivariate analysis revealed a non-significant RN HR of 1.69 in favor of MB SRS was shown (95%CI = 0.72–3.97, p = 0.22), studies were homogeneous (I2 = 0%, p = 0.63). Multivariate HR analysis of RN is shown in Supplementary Fig. 3. Similarly, oligometastases exclusively treated with single-fraction SRS yielded similar RN among groups (RR = 2.00 [95%CI = 0.33–12.02], p = 0.45).
Treatment time
TT analysis in single-fraction SRS revealed showed a non-significant MD of -29.64 min (95%CI = -80.38–21.10, p = 0.25). The included studies were homogeneous (I2 = 0%, p = 0.89). TT analysis can be found in Supplementary Fig. 4.
Mortality
Mortality univariate analysis in single-fraction SRS revealed a non-significant RR of 0.62 (95%CI = 0.22–1.76, p = 0.37). The included studies had low heterogeneity (I2 = 26%, p = 0.25). Mortality RR analysis can be found in Supplementary Fig. 5.
Risk of bias
We utilized the ROB2 tool for the only RCT, which adhered to an intention-to-treat protocol and was deemed to have a "Low risk of bias" [6]. This assessment was based on its randomization process, comprehensive follow-up, and data completeness. The remaining five observational cohort studies exhibited different bias levels in accordance to the ROBINS-I. Three were categorized as "Low risk of bias," attributed to the adequate follow-up time, proper analyses, and the mitigation of confounders through multivariate analysis and propensity score matching [7, 8, 19]. The other two cohort studies were catalogued as "Moderate risk of bias" due to insufficient adjustment for confounders [10, 20]. These evaluations are detailed in Supplementary Fig. 6. Further analysis produced a symmetric funnel plot for our primary outcome of interest, indicating no evidence of publication bias.
Discussion
The present meta-analysis evaluated treatment outcomes in patients with MBD treated with GK-SRS, comparing the head fixation methods of FB and MB in a population of 509 patients. Univariate analysis revealed similar LTC rates at 6 months, while 1-year LTC analysis favored FB SRS over MB SRS. Univariate and multivariate RN rates showed no differences among groups. However, pooled LTC, RN, TT, and mortality in patients with oligometastases treated exclusively with single-fraction SRS was similar among both fixation methods. Comfortability was only reported in one study which favored MB SRS. These results suggest similar efficacy for MB and FB SRS, particularly for single-fraction SRS of oligometastases in MBD. It is crucial to emphasize that these conclusions are primarily representative of observational cohort studies, rather than RCTs. Our patient population primarily included patients with MBD with low tumor burden, hence, our conclusions may not generalize to MBD patients with larger tumor volumes or high tumor burden requiring longer treatment times.
Multiple RCT have shown that SRS is an effective therapy in patients with MBD [21,22,23,24], however, these results are exclusively derived from the use of FB SRS. In our study, 6-month LTC and multivariable failure rates were comparable between study arms. Although we identified a significant advantage in 1-year LTC outcomes with the FB SRS, this LTC advantage was no longer significant after adjusting for patients with oligometastases treated exclusively with single-fraction SRS. We hypothesize that this marginal benefit could be attributed to confounders among studies, this statement is further supported by the results of the only RCT. Overall, our study indicates that, after accounting for potential confounders, LTC is comparable between FB and FB SRS in oligometastases treated with single-fraction SRS, which included data from the only available RCT.
Yet, given the superior precision and stability of the FB method described in the literature, this significant difference in LTC at 12 months warrants further exploration in larger patient cohorts [25]. FB SRS uses a rigid, invasive frame to achieve high precision with margins typically less than 1 mm, minimizing patient movement and enhancing targeting accuracy [26, 27]. In contrast, MB SRS, requires larger margins, often ranging from 1 to 3 mm, which allow for greater potential movement [26, 27]. Although we primarily evaluated patients with oligometastases, multi-institutional observational evidence suggests that SRS is also effective in patients with multiple brain metastases [5]. However, the impact of the use of MB SRS for patients with multiple metastases have yet to be defined. Our ability to assess mortality and survivorship was constrained, with only two studies providing mortality data, and the analysis of overall survival was inconsistent, limiting our capacity for pooled analyses [6, 7, 19]. Future studies should focus on examining both overall survival and progression-free survival across SRS fixation methods, rather than crude mortality rates.
RN is the most common complication following SRS, commonly occuring from months to years after treatment [22, 28, 29], with an incidence of 5–15% [16, 30,31,32,33]. In our analysis four studies included patients who had previously undergone WBRT, a known risk factor for RN [34]. There were no differences in RN rates among fixation approaches, even in studies evaluating single-fraction SRS. This suggests that MB SRS may offer a comparable safety profile to FB SRS, despite the requirement for a target margin and providing less conformal treatments [35, 36].
Regarding the remaining secondary outcomes treatment durations were similar between approaches, however, one study highlighted an increased requirement for breaks in FB SRS, emphasizing the downsides of FB SRS’s invasiveness [7]. Additionally, the analyzed measure was delivery time, which does not account for the time needed for frame fixation and subsequent wound care. Increased treatment times have previously been associated with greater intracranial displacement during frameless SRS [37]. These factors should be considered when selecting the treatment approach, as these may possibly impact LTC and RN rates.
Only one study examined patient comfortability, this study reported higher satisfaction with MB SRS [6, 25], consistent with similar studies on SRS [25]. The importance of patient comfort in clinical decision-making cannot be underestimated, as it may significantly impact treatment adherence, patient satisfaction, and the psychological well-being of cancer patients. Repeated treatments for patients with progression can be challenging, particularly when using FB fixation. MB fixation may be an excellent alternative option in those cases [6, 25].
Overall, our findings indicate that FB and MB are comparable fixation methods in terms of LTC, RN, TT, and mortality, particularly for patients with oligometastases treated with single-fraction SRS. While both approaches appear to provide similar outcomes, potentially supporting a transition towards frameless-SRS, larger RCTs are necessary to draw definitive conclusions. Discussions with patients about treatment options for MBD should involve a detailed review of the advantages and disadvantages of each method, with a focus on prioritizing patient comfort and quality of life. Future research should aim to address the gaps identified in this meta-analysis by including patients with larger metastatic brain disease, examining quality of life outcomes, investigating cases with multiple brain metastases, assessing long-term effects, conducting subgroup analyses based on tumor types, and carrying out prospective, randomized studies comparing FB and MB SRS.
Limitations
To the best of our knowledge, this study represents the first meta-analysis to comparatively assess MB and FB SRS in patients with MBD. While we believe this study adds a valuable contribution to the existing research, this study is subject to several limitations that future research should aim to address. Firstly, our analysis is based on observational studies rather than clinical trials, highlighting the critical need for future clinical trials in this area. Additionally, although we attempted to reduce heterogeneity by conducting subgroup analyses based on timing, supplementary analyses on homogenous tumor volumes, applied strict inclusion criteria, and performing multivariable analyses, the need for more homogeneous studies are critical. Such studies would improve the generalizability of our findings by examining brain metastases from the same primary cancer, homogeneous tumor volumes, lesion numbers, and similar radiation regimens, as some of these patients received prior radiotherapy. Furthermore, the definitions of tumor progression and radiation necrosis, as well as the specifics regarding margins, accuracy, and target delineation for fixation approaches, were not uniformly described. An analysis of quality of life could not be conducted, as this variable was not examined in any of the studies, an important aspect that warrants attention in a demographic with, unfortunately, a dismal prognosis. Multidisciplinary boards, including radiation oncologists and neurosurgical staff, commonly evaluate complex cases with MBD. However, variations in dose selection and postoperative care across these teams could introduce confounders that may skew outcomes in favor of one treatment approach over another. Future research should also include analyses across different age groups to provide a more comprehensive understanding of the interventions’ impacts.
Conclusion
The current meta-analysis, involving 509 patients with MBD treated with SRS, compared the efficacy of FB and MB head fixation methods. Our findings suggest that FB and MB SRS, particularly oligometastases treated with single-fraction SRS, are comparable in terms of LTC, RN, TT, and mortality. Further research is essential to draw definitive conclusions.
Data availability
No datasets were generated or analysed during the current study.
Code availability
Not applicable.
References
Achrol AS, Rennert RC, Anders C et al (2019) Brain metastases. Nat Rev Dis Primers 5(1):5
Sawaya R, Hammoud M, Schoppa D et al (1998) Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 42(5):1044–1055; discussion 1055-6
Noordijk EM, Vecht CJ, Haaxma-Reiche H et al (1994) The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys 29(4):711–717
Mahajan A, Ahmed S, McAleer MF et al (2017) Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 18(8):1040–1048
Yamamoto M, Serizawa T, Shuto T et al (2014) Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 15(4):387–395
Régis J, Merly L, Balossier A et al (2022) Mask-Based versus Frame-Based Gamma Knife ICON Radiosurgery in Brain Metastases: A Prospective Randomized Trial. Stereotact Funct Neurosurg 100(2):86–94
Wegner RE, Horne ZD, Liang Y et al (2021) Single fraction frameless stereotactic radiosurgery on the Gamma Knife icon for patients with brain metastases: time to abandon the frame? Adv Radiat Oncol 6(5):100736
Grimm MA, Köppen U, Stieler F et al (2020) Prospective assessment of mask versus frame fixation during Gamma Knife treatment for brain metastases. Radiother Oncol 147:195–199
Carminucci A, Nie K, Weiner J, Hargreaves E, Danish SF (2018) Assessment of motion error for frame-based and noninvasive mask-based fixation using the Leksell Gamma Knife Icon radiosurgery system. J Neurosurg 129(Suppl1):133–139
Begley SL, Goenka A, Schulder M (2023) Brainstem metastases treated with stereotactic radiosurgery: masked versus framed immobilization. World Neurosurg 175:e1158–e1165
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol 74(9):790–799
Higgins JPT, Thomas J, Chandler J et al (2019) Cochrane handbook for systematic reviews of interventions. John Wiley & Sons
Sterne JA, Hernán MA, Reeves BC et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Int Coach Psychol Rev. Published online July 28, 2010. https://doi.org/10.1002/9780470712184
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898
Ali FS, Arevalo O, Zorofchian S et al (2019) Cerebral radiation necrosis: Incidence, pathogenesis, diagnostic challenges, and future opportunities. Curr Oncol Rep 21(8):66
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Kutuk T, Kotecha R, Tolakanahalli R et al (2022) Zero setup margin mask versus frame immobilization during Gamma Knife IconTM stereotactic radiosurgery for brain metastases. Cancers. 14(14):3392. https://doi.org/10.3390/cancers14143392
Bennion NR, Malouff T, Verma V et al (2016) A comparison of clinical and radiologic outcomes between frame-based and frameless stereotactic radiosurgery for brain metastases. Pract Radiat Oncol 6(6):e283–e290
Vogelbaum MA, Brown PD, Messersmith H et al (2022) Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol 40(5):492–516
Kocher M, Soffietti R, Abacioglu U et al (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol 29(2):134–141
Andrews DW, Scott CB, Sperduto PW et al (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363(9422):1665–1672
Aoyama H, Shirato H, Tago M et al (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295(21):2483–2491
Pavlica M, Dawley T, Goenka A, Schulder M (2021) Frame-based and mask-based stereotactic radiosurgery: the patient experience, compared. Stereotact Funct Neurosurg 99(3):241–249
Kienzler JC, Tenn S, Chivukula S et al (2022) Linear accelerator–based radiosurgery for trigeminal neuralgia: comparative outcomes of frame-based and mask-based techniques. J Neurosurg 137(1):217–226
Jhaveri J, Chowdhary M, Zhang X et al (2019) Does size matter? Investigating the optimal planning target volume margin for postoperative stereotactic radiosurgery to resected brain metastases. J Neurosurg 130(3):797–803
Trifiletti DM, Lee CC, Kano H et al (2016) Stereotactic radiosurgery for brainstem metastases: an international cooperative study to define response and toxicity. Int J Radiat Oncol Biol Phys 96(2):280–288
Miller JA, Bennett EE, Xiao R et al (2016) Association between radiation necrosis and tumor biology after stereotactic radiosurgery for brain metastasis. Int J Radiat Oncol Biol Phys 96(5):1060–1069
Mathieu D, Kondziolka D, Flickinger JC et al (2008) Tumor bed radiosurgery after resection of cerebral metastases. Neurosurgery. 62(4):817–823; discussion 823-4
Do L, Pezner R, Radany E, Liu A, Staud C, Badie B (2009) Resection followed by stereotactic radiosurgery to resection cavity for intracranial metastases. Int J Radiat Oncol Biol Phys 73(2):486–491
Jensen CA, Chan MD, McCoy TP et al (2011) Cavity-directed radiosurgery as adjuvant therapy after resection of a brain metastasis. J Neurosurg 114(6):1585–1591
Brennan C, Yang TJ, Hilden P et al (2014) A phase 2 trial of stereotactic radiosurgery boost after surgical resection for brain metastases. Int J Radiat Oncol Biol Phys 88(1):130–136
Sneed PK, Mendez J, Vemer-van den Hoek JGM et al (2015) Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg. 123(2):373–386
Bush A, Vallow L, Ruiz-Garcia H et al (2021) Mask-based immobilization in Gamma Knife stereotactic radiosurgery. J Clin Neurosci 83:37–42
Romano KD, Trifiletti DM, Garda A et al (2017) Choosing a prescription isodose in stereotactic radiosurgery for brain metastases: Implications for local control. World Neurosurg 98:761-767.e1
Seneviratne DS, Vallow LA, Hadley A et al (2020) Intracranial motion during frameless Gamma-Knife stereotactic radiosurgery. J Radiosurg SBRT 6(4):277–285
Acknowledgments
Partial results of the present study were presented at the American Association of Neurological Surgeons’ Annual Meeting 2024, in May 2024, in Chicago, USA.
Funding
The authors received no financial support for the authorship and/or publication of this article.
Author information
Authors and Affiliations
Contributions
Study design: Data collection: Data analysis: Manuscript draft writing: Manuscript editing: Manuscript approval: all authors.
Corresponding author
Ethics declarations
Ethics declaration
This study was exempted from the institutional ethics review board.
Consent to participate and publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pichardo-Rojas, P.S., Vázquez-Alva, D., Alvarez-Castro, J.A. et al. Comparative effectiveness of frame-based and mask-based Gamma Knife stereotactic radiosurgery in brain metastases: A 509 patient meta-analysis. J Neurooncol (2024). https://doi.org/10.1007/s11060-024-04738-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11060-024-04738-8