Abstract
This study aimed to evaluate, through histomorphometric analysis, the bone repair process in the tibia of rats treated with zoledronic acid and submitted to 808-nm low-level laser therapy (LLLT) by using arsenide aluminum gallium laser. For this purpose, 20 rats were used and distributed according to treatment: group 1—saline administration; group 2—treated with LLLT; group 3—treated with zoledronic acid; and group 4—treated with zoledronic acid and LLLT. The zoledronic acid was administered at a dose of 0.035 mg/kg every 2 weeks for 8 weeks. Subsequently, bone defects of 2 mm were prepared in the tibias of all groups. The bone defects in groups 2 and 4 were irradiated with LLLT in the immediate post-operative period. After 14 and 28 days of application, the animals were submitted and euthanized for histomorphometric analysis. The results were submitted to statistical analysis (α = 5%), and the intragroup comparison was performed using the t test. On the other hand, for intergroup comparison, the ANOVA test was performed, and to the groups presenting statistically significant difference, the Student-Newman-Keuls test was used. In intergroup comparison, group 1 (mean ± SD= 45.2 ± 18.56%) showed a lower bone formation compared with groups 2 (64.13 ± 3.51%) (p = 0.358) and 4 (15.2 ± 78.22%) (p = 0.049), at the 14-day period. Group 3 (20.99 ± 7.42%) also presented a lower amount of neoformed bone tissue, with statistically significant difference when compared with groups 1 (p = 0.002), 2, and 4 (p ≤ 0,001). After 28 days, group 1 presented a lower amount of neoformed bone tissue compared with the other groups, with p = 0.020. Thus, it was concluded that LLLT associated with zoledronic acid is effective for stimulating bone formation in surgically created defects in rats, at the periods studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone regeneration is a complex process which may include the interaction of biological phenomena. In this process, the action of a large amount of cells and proteins, as well as the active gene synthesis, is the main determinant for bone tissue restoration. However, critical defects resulting from trauma and severe bone pathologies can be a problem in craniofacial and orthopedic reconstructive surgeries, because they generate deficiency in bone repair process [1].
Nowadays, in addition to critical defects such as high impact trauma, there is another aggravating factor, that is the medications used for low bone mass or cancer metastasis to bone tissue. These drugs act by causing apoptosis in osteoclasts and retarding the bone restoring process after craniofacial reconstructive surgeries [2].
Among medications used, the bisphosphonates are an important therapeutic strategy commonly prescribed to prevent bone complications arising from benign and malignant bone diseases, due to their ability to reduce bone resorption and suppress bone remodeling, and are therefore classified as antiresorptive drugs [2,3,4,5,6,7]. Zoledronic acid is a third-generation drug with greater power of action than other bisphosphonates. Studies have shown that zoledronic acid is up to 100 times more effective than pamidronate and up to 10,000 times more effective than etidronate [2,3,4].
The main alternative currently used to enhance bone repair is laser [6, 8, 9]. The low-level laser therapy (LLLT) is used in dental practice in order to reduce pain, minimize inflammation, accelerate bone repair, and induce gingival fibroblasts proliferation [10].
Considering the influence of zoledronic acid in reducing bone resorption and of laser in stimulating bone formation, this study is justified for pioneering the evaluation of bone repair process in critical defects created in the tibia of rats treated with zoledronic acid associated with low-level laser therapy, through histological and histometric analysis.
Materials and methods
Animals
A total of 20 female rats (Rattus norvegicus albinus, Wistar) with approximately 450 g, from the biotherium of Sagrado Coração University (USC), were used. The animals were housed in polypropylene cages lined with autoclaved white pine shaving woods changed three times a week. During the entire trial period, the animals were kept in the biotherium at Sagrado Coração University (USC), Bauru, under controlled conditions of temperature (22 ± 2 °C) and 12 h of light/dark cycles, getting water and food without restriction. The project was approved by the Ethics Committee on Animal Research at USC, Bauru, with protocol number 006/13.
Experimental design
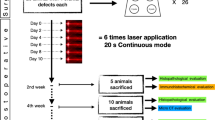
Animals were divided into 4 groups according to the treatment received after the creation of bone defects in tibia (n = 10): *group 1—saline administration (control) (sodium chloride 0.9%®, Darrow, Rio de Janeiro, RJ, Brazil); group 2—rats treated with LLLT (Photon Laser®, DMC, São Carlos, SP, Brazil); group 3—rats treated with zoledronic acid (Zometa®, Novartis Pharma AG, Basel, Switzerland); and group 4—rats treated with zoledronic acid and LLLT (Fig. 1).
The animals from groups 3 and 4 were submitted to intravenous administration of zoledronic acid (Zometa®, Novartis Pharma AG, Basel, Switzerland) at a dose of 0.035 mg/kg applied every 15 days for 8 weeks, continuing until the last experimental period. After 56 days from the beginning of bisphosphonate administration, the surgical procedure for creating bone defects was performed in animals’ tibia for all groups studied (Fig. 2) [11].
The rats from groups 1 and 2 received 0.9% saline intravenously (sodium chloride 0.9%®, Darrow, Rio de Janeiro, RJ, Brazil) to simulate the stress application of zoledronic acid (Zometa®, Novartis Pharma AG, Basel, Switzerland).
Surgical procedure
The animals were sedated by intramuscular injection of ketamine anesthetic 1% at a dose of 50 mg/kg (Francotar®, Virbac Ltda., São Paulo, SP, Brazil) associated with the sedative xylazine hydrochloride 2% at a dose of 5 mg/kg (Virbaxyl 2%®, Virbac Ltda., São Paulo, SP, Brazil), and the dosage was recommended by the manufacturer.
The trichotomy of the right and left tibias was performed as well as the antisepsis in the region to be incised with polyvinyl pyrrolidone Iodine degermant (PVP-I 10%®, Riodeine Degermante, Rioquímica, São Jose do Rio Preto, SP, Brazil), associated with the topic PVP-I (PVP-I topico®, Riodeine Degermante, Rioquímica, São Jose do Rio Preto, SP, Brazil). After antisepsis, animals received local anesthesia, by mepivacaine hydrochloride (0.3 mL/kg) (Scandicaine 2%® with 1:100,000 adrenaline, Septodont, Paris, France) to assist the hemostasis of surgical field.
With a blade number 15 (Feather Industries Ltda®, Tokyo, Japan) mounted on a scalpel number 3 (Hu-Friedy®, Leimen, Germany), an incision approximately 1 cm in length was made in the medial portion of the tibia until bone base, and then, soft tissue was divulsed and removed with the aid of periosteum retractors (Quinelato®, Rio Claro, São Paulo, Brazil), exposing the bone tissue.
Subsequently, the bone defect with 2 mm in diameter and 4 mm in depth was created in upper cortical tibias of the rats. The preparation of bone defect was performed through ostectomy using a spear milling cutter (Sistemas de Implantes [SIN]®, São Paulo, SP, Brazil) coupled to an electric motor (KaVo, Joinville, SC, Brazil) in constant irrigation with sodium chloride 0.9% (sodium chloride 0.9%®, Darrow, Rio de Janeiro, RJ, Brazil) during the whole preparation.
Immediately after milling, the animals in groups 2 and 4 were subjected to irradiation with arsenide aluminum gallium laser (ArAlGa) (Photon Laser®, DMC, Sao Carlos, SP, Brazil), with a wavelength of 808 nm, point size of 0.07 cm2, power of 0.03 W during 133 s per point, irradiance of 0.42 W/cm2, and energy of 4 J/point (57.14 J/cm2/point). Thus, the area received a total of 32 J of energy. The application was performed once in seven locations in the margin of bone defect, and also in the midpoint of bone defect, totaling eight points of application. In groups 1 and 3, there was no LLLT application [6].
The soft tissues were carefully repositioned and sutured in plans, using absorbable wire (polyglactin 910—Vycril 4.0®, Ethicon, Johnson Prod, Sao Jose dos Campos, SP, Brazil) with continuous stitches and monofilament wire (Nylon 5.0®, Ethicon, Johnson, Sao Jose dos Campos, SP, Brazil) in the deep plan, and interrupted stitches in the most external points, obtaining the primary wound closure. After suturing, the area antisepsis was performed again with topic polyvinyl pyrrolidone iodine (PVP-I topico®, Riodeine, Rioquímica, Sao Jose do Rio Preto, SP, Brazil).
Post-operatively, the animals received intramuscular administration of Pentabiotic (0.1 mL/kg) (Fort Dodge Saúde Animal Ltda®, Campinas, SP, Brazil) with one dose in the immediate post-operative period and sodium dipyrone (1 mg/kg/day) (Ariston Indústrias Químicas e Farmacêuticas Ltda®, Sao Paulo, SP, Brazil), totaling 3 doses. No food or movement restriction was imposed to the animals, which were kept in individual cages throughout the experiment.
After 14 and 28 days of surgical bone defect creation, the animals were euthanized by anesthetic overdose of ketamine 1% (Francotar®, Virbac Ltda., São Paulo, SP, Brazil), intraperitoneally, for specimens’ removal.
Histological processing
Tibia samples containing the bone defect region were removed and immersed in 10% buffered neutral formalin (Bio-Optica Milano S.p.a., Milano, Italy) for 48 h. Then, they were submitted to decalcification with 5% ethylenediaminetetraacetic acid (EDTA) (Titriplex® III, Merck, Darmstadt, Germany) for 3 months. After decalcification, the parts were washed in running water for 24 h, dehydrated, diaphanized, and embedded in paraffin (Histosec®, Merck, Darmstadt, Germany). From the paraffin blocks containing tibia portions, longitudinal sections of 5 and 6 μm in thickness were obtained. Serial sections of 6 μm in the longitudinal direction were stained with hematoxylin and eosin (HE) and were later used for the morphological analysis of bone repair process occurred in the different groups studied.
Morphological analysis
For morphological evaluation, some aspects were considered: the pattern of inflammatory infiltrate, neoformation, maturation, remodeling, and bone viability. The images were obtained using a 1.3-megapixel resolution image capture camera (Leica DFC 300FX, Leica Microsystems, Heerbrugg, Switzerland) coupled to a visible light microscope and a computer (Leica Aristoplan Microsystems, Leitz, Bensheim, Germany).
Histometric analysis
For histometric analysis, individualization and delimitation of the defect area corresponding to the cortical region of tibia were performed, and this area was called total area (TA). Within the total area (TA), the delimitation of areas corresponding to neoformed bone was carried out, and neoformed bone area (NBA) was obtained. Always using the resources of ImageJ “software,” the calculation of total area (TA) previously defined was held, transferring the measured values for a “calculation sheet,” with the value of total area (TA) considered 100% of the total area analyzed. Next, the calculation of neoformed bone area (NBA), previously delimited, was also performed by transferring the measured values to another “calculation sheet.” The value of NBA was calculated as a percentage of TA, as follows:
The NBA values of each animal were used for calculating the means and standard deviations of each group.
The results were submitted to the normality test of Kolmogorov-Smirnov and found the data distributed normally. O power analysis was applied and for α = 5%, the power of the study was almost 1. The intragroup comparison in different periods was performed using the t test (α = 5%). On the other hand, for intergroup comparison, the one-way ANOVA test (α = 5%) was performed, and to the groups presenting statistically significant difference, the Student-Newman-Keuls test (α = 5%) was used.
Results
No complications were observed (allergy, fracture, or infection) during the post-operative period in any animal.
Morphological analysis
At 14 days, in all specimens of group 1, there is remaining bone, an increased number of fibroblasts, connective tissue, and fine bone trabeculae with osteoblasts. In group 2, there is neoformed trabecular bone with osteoblasts surrounded by organized connective tissue with the presence of blood vessels. In group 3, the bone tissue does not have osteoblasts and is surrounded by connective tissue. In group 4, the defect presents organized connective tissue surrounding a mature bone, but this bone has osteoblasts, making it more viable. At 28 days, in all groups, the surgical cavity is completely filled by trabecular bone developed (Figs. 3 and 4).
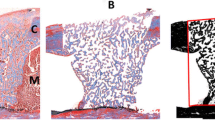
Photomicrographs of sections stained with hematoxylin and eosin in post-operative periods of 14 days: a Group 1, b group 2, c group 3, and d group 4; and 28 days: e group 1, f group 2, g group 3, and h group 4. Note the presence of remaining bone (RB), connective tissue (CT), neoformed bone (NB), and mature bone (M). Original magnification of × 400
Histometric analysis
The histometric analysis was performed within and between groups, obtaining the following results.
In group 1, when comparing the periods, there was an increase of bone tissue over time, with an average and standard deviation of 55.6 ± 18.56% for 14 days and 85.36 ± 8.34% for 28 days, presenting a statistically significant difference (p = 0.019).
In group 2, neoformed bone tissue was observed in larger quantities at the final period, with an average of 64.13 ± 3.51% and 97.3 ± 2.16% for 14 and 28 days, respectively. The statistical analysis confirmed a statistically significant difference when comparing the periods studied, with p ≤ 0.001.
In group 3, neoformed bone tissue was also observed in larger quantities at the final period, with an average of 20.99 ± 7.42% and 98.86 ± 1.95% for 14 and 28 days, respectively, with a statistical significant difference (p ≤ 0.001).
Similarly to previous groups, group 4 showed a tendency to increase the amount of bone tissue over time, with 78.22 ± 15.2% and 96.76 ± 2.84% for 14 and 28 days, respectively, although there was no significant difference between the periods (p = 0.095).
In intergroup comparison, at the 14-day period, group 1 showed a tendency to lower bone formation compared with group 2 (p = 0.358), with no significant difference. Group 1 behaved similarly, with less new bone formation compared with group 4, but in turn, with a significant difference (p = 0.049), unlike what was noted in relation to group 3, where group 1 showed an increased bone formation (p = 0.002). Group 3 also showed a lower amount of neoformed bone tissue, with a statistically significant difference compared with groups 2 and 4 (p ≤ 0.001). At the 28-day period, group 1 presented a lower amount of neoformed bone tissue in comparison with the other groups (p = 0.020) (Table 1 and Fig. 5).
Graphic representation of means and standard deviation of the histometric related to bone growth area in percentage, at different periods (days). The results show that there is a significant intragroup difference. In the intergroup evaluation, the results show a significant difference—14 days: a ≠ A and b ≠ B; and 28 days: c ≠ C. ANOVA and the Student-Newman-Keuls test (p < 0.05)
Discussion
The bone remodeling process is continuous and progressive; however, in the presence of critical defects, this remodeling is deficient, making the reconstruction a challenge [6, 12], which becomes greater when the patient is using bisphosphonate.
Some medications, such as bisphosphonates, are known to decrease bone turnover and damage the bone defect repair [5, 9, 11,12,13,14,15,16,17,18,19]. On the other hand, the LLLT has the potential to promote the repair of bone defects [6,7,8,9,10, 20, 21]. Nevertheless, there is no study in literature that evaluates the action of zoledronic acid associated with laser therapy in bone repair process. Therefore, the aim of this study was to evaluate, through histological and histometric analysis, the influence of zoledronic acid application, the most potent bisphosphonate, associated or not with low-level laser therapy, in the repair process of critical bone defects created in tibias of rats.
Numerous studies report low-level laser therapy is able to contribute to the acceleration of bone repair process. However, there is no consensus in literature regarding the low-level laser application protocol (Table 2) [6, 21,22,23,24,25]. The laser therapy is dependent on the wavelength, dose, density, duration of irradiation, and frequency of the treatment [8]. Several studies report that the application of a laser with wavelengths ranging from 630 to 890 nm resulted in a higher expression of osteocalcin, osteopontin, and collagen type I [6, 8, 20,21,22, 24, 25]. In the present study, selected 808-nm wavelengths positively influence bone repair. Another parameter that influences the effect of the low-level laser therapy (ArAlGa) is the application period, in the current study, corroborating with the research of Garcia and colleagues [6], in which the laser therapy was applied in a single session in the immediate post-operative period. This protocol was proposed mainly to reduce the patient discomfort, since it is a single application.
The results found in study in relation to the groups treated or not with laser therapy showed that at 14 days, group 2 had a tendency to increased bone formation in comparison with group 1. When comparing groups 3 and 4, at the same period, group 4 showed an increased bone formation. After 28 days, group 2 exhibited higher bone density than group 1, different from what occurred between groups 3 and 4, which showed similar values. These results corroborate with others described in literature [6, 21,22,23,24,25]. The laser action stimulates the differentiation of undifferentiated mesenchymal cells into osteoblasts, cell proliferation, and increased formation of bone matrix and, therefore, accelerates the metabolism of bone repair process [22].
In addition to LLLT, another factor that influences bone remodeling is the use of bisphosphonates, which is represented by the zoledronate in this study. Zoledronic acid is indicated for inhibiting bone resorption, improving bone density, as treatment for Paget’s disease, malignant hypercalcemia, bone metastases, and osteoporosis. This medication presents a high half-life in bone tissue due to their chemical properties, which allows exerting its action for months or years [5].
The bone repair process using zoledronic acid has been evaluated through clinical [14, 26], histological [2, 5, 14, 16,17,18], and imaginologic [16, 26] behavior, through immunohistochemistry [15], mechanical tests by locking and retention, and even fatigue fractures [4, 16].
Nevertheless, there is still no consensus in literature regarding the way and periods of zoledronic acid application. This research followed the application model of Hokugo et al. [11] and Yu et al. [12], in which zoledronic acid injections were applied intravenously, interspersed weekly, until the final euthanasia period.
This study compared animals submitted to treatment with or without zoledronic acid, and it was noted that at the 14-day period, group 1 showed a higher bone tissue formation compared with group 3. At the 28-day period, the results were reversed; that is, the bone quantity was lower in group 1. It is suggested that these results occur due to the action of bisphosphonates in reducing angiogenesis in the initial period of repair process and as the effects on blood vessels are temporary, bone tissue increases in the final period [27,28,29], as was found in our study, corroborating with Garcia et al. [6] and Cardemil et al. [5], both of which also relate increased bone density in groups subjected to zoledronate application for longer periods.
The only research evaluating the application of bisphosphonates associated with LLLT has concluded that laser is effective to accelerate the repair associated or not with bisphosphonates [6]. However, this study differs from ours, since the bone type is different (calvaria), the bisphosphonate used is alendronate administered orally, and an ovariectomy is carried out, simulating osteoporosis.
In the present study, the tibia was elected for presenting similar characteristics to the anterior region of human mandible, including type of ossification and bone quality. The bisphosphonate zoledronic acid administered intravenously was chosen for being a high action potential bisphosphonate, which after applied leads to lower serum levels of TRAP over from bone resorption. Thus, this drug reduces bone resorption and keeps bone density stable [5].
This research did not choose for simulating osteoporosis because the drug has additional indications such as to bone metastases and others aforementioned. Furthermore, a short period of 14 days was chosen, with the intention of evaluating the acute onset of repair process, clinically correlating with the long half-life of the drug.
In intragroup analysis, data showed higher bone formation at 28 days in comparison with 14 days for all groups. Regarding intergroup comparisons, a decreased bone formation was noted in group 3 at the initial period, with a statistically significant difference in relation to the other groups. In the same period, the group 1 was less effective in repairing bone defects in comparison with group 4. At 28 days, the groups 2, 3, and 4 had the defect completely filled with bone tissue, while the group 1 presented a lower amount of bone.
Based on the methodology studied, it was possible to conclude that LLLT associated with zoledronic acid is effective to stimulate bone formation in surgically created defects in rats, at the periods studied.
References
Cheng A, Daly CG, Logan RM, Stein B, Goss AN (2009) Alveolar bone and the bisphosphonates. Aust Dent J 54:S51–S61
Yamashita J, Koi K, Yang DY, McCauley LK (2011) Effect of zoledronate on oral wound healing in rats. Clin Cancer Res 17:1405–1414
Borromeo GL, Tsao CE, Darby IB, Ebeling PR (2011) A review of the clinical implications of bisphosphonates in dentistry. Aust Dent J 56:2–9
Palacio EP, Müller SS, Sardenberg T, Mizobuchi RR, Galbiatti JA, Durigan A Jr, Savarese A, Ortolan EV (2012) Detecting early biomechanical effects of zoledronic acid on femurs of osteoporotic female rats. J Osteoporos 2012:162802
Cardemil C, Omar OM, Norlindh B, Wexell CL, Thomsen P (2013) The effects of a systemic single dose of zoledronic acid on post-implantation bone remodelling and inflammation in an ovariectomised rat model. Biomaterials 34:1546–1561
Garcia VG, Conceição JM, Fernandes LA, Almeida JM, Nagata MJH, Bosco AF, Theodoro LH (2013) Effects of LLLT in combination with bisphosphonate on bone healing in critical size defects: a histological and histometric study in rat calvaria. Lasers Med Sci 28:407–414
Wynn RL (2005) Bisphosphonates, hypercalcemia of malignancy, and osteonecrosis of the jaw. Gen Dent 53:392–395
Ebrahimi T, Moslemi N, Rokn AR, Heidari M, Nokhbatolfoghahaie H, Fekrazad R (2012) The influence of low-intensity laser therapy on bone healing. J Dent 9:238–248
Jakse N, Payer M, Tangl S, Berghold A, Kirmeier R, Lorenzoni M (2007) Influence of low- level laser treatment on bone regeneration and osseointegration of dental implants following sinus augmentation. An experimental study on sheep. Clin Oral Implants Res 18:517–524
Nascimento SB, Cardoso CA, Ribeiro TP, Almeida JD, Albertini R, Munin E, Arissawa EA (2010) Effect of low level laser therapy and calcitonin on bone repair in castiated rats: a densitometric study. Photomed Laser Surg 28:45–49
Hokugo A, Christensen R, Chung EM, Sung EC, Felsenfeld AL, Sayre JW, Garrett N, Adams JS, Nishimura I (2010) Increased prevalence of bisphosphonate-related osteonecrosis of the jaw with vitamin D deficiency in rats. J Bone Miner Res 25:1337–1349
Yu YY, Lieu S, Hu D, Miclau T, Colnot C (2012) Site specific effects of zoledronic acid during tibial and mandibular fracture repair. PLoS One 7:e31771–e31780
Botell M (2001) Osteoporosis en la menopausia, prevención y estratégias terapêuticas atuales. Rev Cuba Obstet Ginecol 27:199–204
Amanat N, McDonald M, Godfrey C, Bilston L, Little D (2007) Optimal timing of a single dose of zoledronic acid to increase strength in rat fracture repair. J Bone Miner Res 22:867–876
Camacho AF, López JP, Vicente HA (2013) Short-term effect of zoledronic acid upon fracture resistance of the mandibular condyle and femoral head in an animal model. Med Oral Patol Oral Cir Bucal 18:e421–e426
Back DA, Pauly S, Rommel L, Hass NP, Schmidmaier G, Wildemann B, Greiner SH (2012) Effect of local zoledronate on implant osseointegration in a rat model. BMC Musculoskelet Disord 13:42–50
Yaman F, Agaçayak S, Atilgan S, Benliday E, Ucan MC, Erol B, Kaya B, Gunay A, Guven S (2012) Effects of systemic zoledronic acid administration on osseointegration of hydroxyapatite-coated and resorbable blast material surface implants in rabbit models. Int J Oral Maxillofac Implants 27:1443–1447
Okamoto Y, Hirota M, Monden Y, Murata S, Koyama C, Mitsudo K, Iwai T, Ishikawa Y, Tohnai I (2013) High-dose zoledronic acid narrows the periodontal space in rats. Int J Oral Maxillofac Surg 42:627–631
Çankaya M, Senel ÇF, Duman KM, Muci E, Dayisoylu EH, Balaban F (2013) The effects of chronic zoledronate usage on the jaw and long bones evaluated using RANKL and osteoprotegerin levels in an animal model. Int J Oral Maxillofac Surg 42:1134–1139
Ribeiro DA, Matsumoto MA (2009) Low-level laser therapy improves bone repair in rats treated with anti-inflammatory drugs. J Oral Rehabil 35:925–933
Matsumoto MA, Ferino RV, Monteleone GF, Ribeiro DA (2009) Low-level laser therapy modulates cycloxygenase-2 expression during bone repair in rats. Laser Med Sci 24:195–201
Fáravo-Pípi E, Ribeiro DA, Ribeiro JU, Bossini P, Oliveira P, Parizotto NA, Tim C, Araújo HSSA, Renno ACM (2011) Low-level laser therapy induces differential expression of osteogenic genes during bone repair in rats. Photomed Laser Surg 29:311–317
Fernandes KR, Ribeiro DA, Rodrigues NC, Tim C, Santos AA, Parizotto NA, Araujo HS, Driusso P, Rennó ACM (2013) Effects of low-level laser therapy on the expression. Of osteogenic genes related in the initial stages of bone defects in rats. J Biomed Opt 18:038002
Barbosa D, Villaverde AGJB, Arisawa EAL, Souza RA (2014) Laser therapy in bone repair in rats: analysis of bone optical density. Acta Ortop Bras 22:71–74
Marques L, Holgado LA, Francischone LA, Ximenez JPB, Okamoto R, Kinoshita (2015) A new LLLT protocol to speed up the bone healing process—histometric and immunohistochemical analysis in rat calvarial bone defect. Lasers Med Sci 30:1225–1230
Sener I, Bereket C, Kosker H, Turer A, Tek M, Kaplan S (2013) The effects of zoledronic acid on mandibular fracture healing in an osteoporotic model: a stereological study. J Craniofac Surg 24:1221Y1224
Hikita H, Miyazawa K, Tabuchi M, Kimura M, Goto S (2009) Bisphosphonate administration prior to tooth extraction delays initial healing of the extraction socket in rats. J Bone Miner Metab 27:663–672
Aguirre JI, Altman MK, Vanegas SM, Franz SE, Bassit AC, Wronski TJ (2010) Effects of alendronate on bone healing after tooth extraction in rats. Oral Dis 16:674–685
Kobayashi Y, Hiraga T, Ueda A, Wang L, Matsumoto-Nakano M, Hata K, Yatani H, Yoneda T (2010) Zoledronic acid delays wound healing of the tooth extraction socket, inhibits oral epithelial cell migration, and promotes proliferation and adhesion to hydroxyapatite of oral bacteria, without causing osteonecrosis of the jaw, in mice. J Bone Miner Metab 28:165–175
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: PLS, LMS, JLG, BLCI, LMLR; performed the experiments: VCB, EJG, JLG, PLS; analyzed the data: WO, BLCI, LMLR, PLS; contributed reagents/materials/analysis tools: WO, VCB, EJG, LMS; wrote the paper: PLS, LMS, JLG.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (attached supplementary materials—protocol number 006/13).
Informed consent
The authors declare that it was not necessary to have informed consent since the research was not conducted in humans.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Buchignani, V.C., Germano, E.J., dos Santos, L.M. et al. Effect of low-level laser therapy and zoledronic acid on bone repair process. Lasers Med Sci 34, 1081–1088 (2019). https://doi.org/10.1007/s10103-019-02810-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-019-02810-8