Abstract
The goal of this study was to analyze the role of cyclo-oxygenase-2 following bone repair in rats submitted to low-level laser therapy. A total of 48 rats underwent surgery to inflict bone defects in their tibias having been randomly distributed into two groups: negative control and laser exposed group, i.e., the animals were treated with low-level laser therapy by means of gallium arsenide laser at 16 J/cm2. The animals were killed after 48 h, 7 days, 14 days, or 21 days. The tibias were removed for morphological, morphometric, and immunohistochemistry analysis for cyclo-oxygenase-2. Statistical significant differences (P < 0.05) were observed in the quality of bone repair and quantity of formed bone between groups 14 days after surgery in the laser exposed group. In the same way, cyclo-oxygenase-2 immunoreactivity was more intense in bone cells for intermediate periods evaluated in this group. Taken together, such results suggest that low-level laser therapy is able to improve bone repair in the tibia of rats after 14 days of surgery as a result of an up-regulation for cyclo-oxygenase-2 expression in bone cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In past decades, laser has been indicated for several therapeutic purposes [1–4]. There are many laser types available in the global market, with different modes of action. Independent of its mode of presentation, a plethora of beneficial effects has been demonstrated for many in vitro and in vivo test systems, including antibacterial, antiviral, anti-tumor, cell differentiation, immuno-potentiating and repair activities [5–10]. Particularly, low-level laser therapy has been increasingly used to treat hard tissue injuries by promoting wound healing and reducing pain [11]. This is because this type of laser has been demonstrated as a noninvasive method for the stimulation of osteogenesis and to reduce the time of fracture consolidation through bioenergetic, bioelectrical, biochemical and biostimulatory effects on cells [12].
The tibia is the long bone most often fractured, and it is associated with a high incidence of nonunion and delayed healing [13]. With the aim of reducing the substantial incapacity associated with bone fracture and the high socioeconomic costs, a variety of interventions has been studied, including the use of low-level laser therapy [14]. However, few reports have been published with low-level laser therapy focusing on bone repair after mechanical damage in the tibias up to now [15].
Cyclo-oxygenase is the rate-limiting enzyme in the conversion of arachidonic acid to prostaglandins, and two isoforms, cyclo-oxygenase-1 and cyclo-oxygenase-2, have been identified. Cyclo-oxygenase-1 is constitutively expressed in many tissues and mediates the synthesis of prostaglandins required for normal physiological function. Cyclo-oxygenase-2 is normally undetectable in most tissues but is rapidly induced by pro-inflammatory or mitogenic stimuli [16]. Recently, cyclo-oxygenase-2 has been implicated in several different cellular mechanisms, such as angiogenesis, proliferation and the prevention of apoptosis [17]. To date, there are no reports that have investigated cyclo-oxygenase-2 expression during bone repair submitted to low laser therapy. This justifies this study and others as well.
Therefore, the aim of this study was to analyze the role of cyclo-oxygenase-2 following bone repair in rats submitted to low-level laser therapy. Certainly, such data will contribute to a better understanding of laser outcomes upon cellular systems during bone repair.
Material and methods
Animals and experimental design
All experimental protocols used in this study were approved by the Ethics Committee for Animal Research, Universidade do Sagrado Coração (USC), Bauru, SP, Brazil.
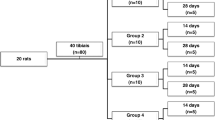
A total of 48 male rats (Rattus norvegicus albinus, Wistar), 8 weeks old and weighing approximately 250 g, were randomly distributed into two groups containing 24 animals each, with four different periods in which they were killed: (a) 2 days, (b) 7 days, (c) 14 days, and (d) 21 days. The groups were as follows: group 1 (control) a, b, c, d—negative control, animals not exposed to laser, and group 2 (laser-treated-group) a, b, c, d—animals treated with low-level laser. All control animals were submitted to the same procedures of handling as the laser-exposed animals were.
Surgical procedure
At the beginning of the experiment, all animals underwent surgery to produce bone defects in their tibias. General anesthesia was obtained by intramuscular administration of 1% ketamine (Francotar, Virbac Ltda., São Paulo, Brazil) associated with sedative, 2% xylazine hydrochloride (Virbaxyl 2%, Virbac Ltda.) in the recommended dose of 0.1 ml/100 g. A 1 cm incision was made to expose the tibia. A cavity in the upper metaphyseal region was prepared, 5 mm deep, in the upper metaphyseal region, and the bone marrow exposed. A carbon steel round bur under copious irrigation was used, and, after each procedure, the bur was discarded and a new one was mounted for the next animal.
Laser therapy
A low-energy gallium arsenide laser, 735 nm in wavelength (DMC, Sao Carlos, Brazil), continuous wave, 3 mm laser beam diameter, at 16 J/cm2, with irradiation time of 1 min, was used in this experiment. Laser irradiation was initiated 24 h after the surgery and was performed, punctually, every 48 h for 15 days, or until the rat was killed. Laser irradiation was performed transcutaneously, at one point, above the lesion on the injured tibias.
Histopathological analysis
The animals were killed by the administration of an overdose of the anesthetic drug, in the experimental periods established, after 2 days, 7 days, 14 days, or 21 days, for histopathological and immunohistochemical analysis. The tibias were removed, fixed in 10% buffer formalin (Merck, Darmstadt, Germany) for 48 h and decalcified in 4% ethylene glycol tetra-acetic acid (EDTA) (Merck). Five-micrometer slices were obtained in semi-seriate fashion and stained with hematoxylin and eosin (H&E) (Merck).
Morphometry
For the morphometric analysis the regions of bone repair previously identified in the histopathological observation for each animal were measured in a blind fashion by one expert observer using an image analysis system (KS-300, Carl Zeiss, Germany) for Windows. The slices stained with H&E were observed. Three areas of the cortical region of the defect were selected, named C1 and C3, corresponding to the regions close to the wall defect, and C2, corresponding to the central region of the defect. The bone tissue presented in these regions was measured and the area registered at a magnification of ×20. After the registration, the areas were added, resulting in the total bone area of the defect. This analysis had been established in a previous study conduced by our group [18].
Immunohistochemistry
Paraffin was removed with xylene from serial sections of 4 μm and the sections were rehydrated in graded ethanol, then pretreated in a microwave with 0.01 M citric acid buffer (pH 6) for three cycles of 5 min each at 850 W for antigen retrieval. The material was pre-incubated with 0.3% hydrogen peroxide in phosphate-buffered saline (PBS) solution for 5 min for inactivation of endogenous peroxidase and then blocked with 5% normal goat serum in PBS solution for 10 min. The specimens were then incubated with anti-cyclo-oxygenase-2 polyclonal primary antibody (Santa Cruz Biotechnology, USA) at a concentration of 1:50. Incubation was carried out overnight at 4°C within the refrigerator. This was followed by two washes in PBS for 10 min. The sections were then incubated with biotin-conjugated secondary antibody anti-rabbit IgG (Vector Laboratories, Burlingame, CA, USA) at a concentration of 1:200 in PBS for 1 h. The sections were washed twice with PBS followed by the application of preformed avidin biotin complex conjugated to peroxidase (Vector Laboratories) for 45 min. The bound complexes were visualized by the application of a 0.05% solution of 3-3′-diaminobenzidine solution and counterstained with Harris hematoxylin. For control studies of the antibodies, the serial sections were treated with rabbit IgG (Vector Laboratories) at a concentration of 1:200 in place of the primary antibody. Additionally, internal positive controls were performed with each staining batch.
Data analysis
Sections stained for immunohistochemistry were examined under an optical microscope for the percentages of immuno-positive bone cells. A total of 8–10 fields per slice for each animal at ×400 magnification were evaluated by systematic sampling. These values were used as labeling indices (%).
Statistical methods
The values obtained from morphometric analysis were submitted to the Kruskal–Wallis statistical test and Dunn’s post-hoc analysis if a significant effect was detected. Sigma Stat program (Jandel Scientific, Chicago, USA), version 2.0 for Windows was used.
Results
General findings
Neither postoperative complications nor behavioral changes were observed. The rats returned rapidly to their normal diet and showed no loss of weight during the experimentation (data not shown). None of the animals died during the experiment.
Histopathological analysis
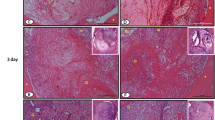
Regarding the control group, all the defects were filled by blood clot after 48 h (Fig. 1). On the seventh day, the central region of the defect was filled by granulation tissue (Fig. 2a). In one of the specimens, a small focus of calcifying hyaline cartilage was noticed. After 14 days, irregular and highly cellularized woven bone was seen (Fig. 3a). Most of the specimens showed osteogenic activity also in the medular region, due to the presence of bone fragments resulting from bone perforation. Within 21 days, regular bone trabeculas were seen, covered by osteoblastic cells (Fig. 4). The repaired bone was thinner than the wall defects.
In the group treated with low-level laser, blood clot filled the bone defects after 48 h. On day 7, granulation tissue and woven bone could be seen. In the periphery, however, new bone formation could be seen coming from the bony walls (Fig. 2b). In the same way, bone tissue becoming mature was noticed after 14 days (Fig. 3b). On the 21st day, well-defined trabeculas underwent remodeling, resulting in the formation of a bone bridge thinner than the original bony walls.
Morphometry
The area of newly formed bone was examined using the slices stained with H&E. The results showed statistically significant differences (P < 0.05) between groups after 14 days. Such findings are shown in Table 1.
Immunohistochemistry
Cyclo-oxygenase-2 expression was detected predominantly in the cytoplasm. Sections stained for immunohistochemistry were examined under an optical microscope for the percentages of immunopositive bone cells.
After 2 days of surgery, cyclo-oxygenase-2 immunoreactivity could be seen in the central region of the lesion in rats of the control group. A similar pattern occurred in the group treated with low-level laser (Fig. 5). After 7 days, cyclo-oxygenase-2 immunoexpression could be seen in the granulation tissue in the control group, whereas cyclo-oxygenase-2 was positively detected in the surrounding bone tissue in the rats exposed to laser (Fig. 6b). This was more evident on the 14th day, either in the control group (Fig. 6a) or in the laser-exposed group (Fig. 6b). Twenty-one days after the surgery, the control group and the group treated with laser showed cyclo-oxygenase-2 expression in some cells of the bone marrow (Fig. 7). All morphometric findings are also summarized in Fig. 8.
Discussion
It has been postulated that low-level laser has therapeutic efficacy on various clinical conditions [9]. Taking into consideration that some inflammatory mediators, such as prostaglandin and arachidonic acid products, play important roles during bone repair, the aim of this study was to evaluate the role of cyclo-oxygenase-2 during low-energy laser on bone repair. To the best of our knowledge, the approach has not been addressed so far. For this, we chose an experimental test system, since animal models have commonly been the basis for studying the relationship between mechanical trauma and therapies [12]. Histopathological, morphometric and immunohistochemical analyses were used in this setting to measure the quantity of formed bone, since we aimed to evaluate the putative up-regulation of osteogenesis induced by laser after mechanical trauma simulating surgical procedures in clinical practice.
After 48 h, our results demonstrated that low-level laser therapy failed to exert any activity, as depicted by similar histopathological patterns between laser-exposed and non-exposed tibias. Although more bone formation was detected in the laser-exposed group under subjective histopathological analysis after 7 days, this finding was not proven statistically, because no statistically significant differences were noticed in the morphometric analysis. Taken as a whole, therefore, we assumed that laser therapy was not able to accelerate bone repair immediately after mechanical trauma in this trial.
Conversely, low-level laser exposure resulted in significant induction of bone formation 14 days after the beginning of the therapy. This was seen in the histopathological and morphometric analyses. Therefore, the laser energy level used appears to have stimulated an increase in the healing of the bone when compared with the healing seen in the tibia controls. Our data are fully in line with others [19, 20]. It is important to stress that this positive effect is transient, since no positive results were maintained 21 days after surgery. Overall, several authors have postulated that low-power laser can stimulate bone cell proliferation and alkaline phosphatase (ALP) activity, which reflects osteoblastic activity [21]. Nevertheless, it seems that multiple doses, rather than the intensity of laser irradiation, are more effective for bone formation [22]. This emphasizes our positive results, since laser was applied every 48 h after surgery. In a study by Ozawa et al. [23], however, laser irradiation beyond 14 days failed to cause stimulation. The authors concluded that the stimulatory action of laser irradiation occurs during the proliferative and earlier stages of differentiation of immature precursors but does not occur during later stages. It is important to keep in mind that it is sometimes difficult to compare studies about the action of low-level laser on bone because the dosimetric parameters, experimental models and duration of treatments are very distinct. Further studies are necessary to elucidate this issue.
Cyclo-oxygenase is a key enzyme in the conversion of arachidonic acid to prostanoids. The expression of isoform cyclo-oxygenase-2 is relevant to many pathological processes, including inflammation, tissue repair and, ultimately, to carcinogenesis [24]. When the expressivity of cyclo-oxygenase-2 was investigated, our results showed that laser therapy promotes an up-regulation for this inflammatory mediator. In the period of 48 h, the immunohistochemistry of cyclo-oxygenase-2 confirmed the main activity of the enzyme in the central region of the defect in both experimental groups and also in the control group. This situation was not maintained after 7 days, when immunoreactivity was more intense in the granulation tissue and newly formed bone cells of the laser-exposed group than in the control group. In the following periods, i.e., 14 days and 21 days, immuno-expression of cyclo-oxygenase-2 was observed in the bone marrow, with a more pronounced effect in the laser-exposed group. Since low-level laser therapy is able to increase the formation of new capillaries through the release of growth factors such as vascular endothelial growth factor (VEGF) [25], stimulate DNA and RNA synthesis in the cell nucleus and, consequently, increase cell proliferation and differentiation [26], we believe that the immuno-expression of cyclo-oxygenase-2 found in bone tissue could also be helpful to bone repair. In 1997, Sato et al. [27] suggested that cyclo-oxygenase-2 could be involved in the early stage of osteogenesis, probably associated with the maturation of osteoblasts. Furthermore, Zhang et al. [28] affirmed that cyclo-oxygenase-2 enzyme acts on osteoblastogenesis, regulating osteoblastic differentiation genes such as Cbfa-1 and osterix. More recently, it has been postulated that bone cells are able to produce cyclo-oxygenase-2 after mechanical trauma [29], being, therefore, important to bone formation [30]. Our results are in agreement with those of these previous studies.
In summary, our results support the notion that low-level laser therapy is able to improve bone repair in the tibia of rats after 14 days of surgery as a result of an up-regulation for cyclo-oxygenase-2 expression. However, this suggestion should be verified by further investigation.
References
Hammond A (2004) Rehabilitation in rheumatoid arthritis: a critical review. Musculoskelet Care 2:135–151
Cetiner S, Kahraman SA, Yucetas S (2006) Evaluation of low-level laser therapy in the treatment of temporomandibular disorders. Photomed Laser Surg 24:637–641
Faria Amorim JC, Sousa GR, Silveira Lde B, Prates RA, Pinotti M, Ribeiro MS (2006) Clinical study of the gingiva healing after gingivectomy and low-level laser therapy. Photomed Laser Surg 24:588–594
Landthaler M, Hohenleutner U (2006) Laser therapy of vascular lesions. Photodermatol Photoimmunol Photomed 22:324–332
Turner J, Jode L (1999) Low level laser therapy. Prima Books, Stockholm
Kipshidge N, Nikolaychik V, Keelan MH (2001) Low-power helium:neon laser irradiation enhances production of vascular endothelial growth factor and promotes growth of endothelial cells in vitro. Lasers Surg Med 28:355–364
Chen WR, Liu H, Ritchey JW, Bartels KE, Lucroy MD, Nordquist RE (2002) Effect of different components of laser immunotherapy in treatment of metastatic tumors in rats. Cancer Res 62:4295–4299
Dube A, Bansal H, Gupta PK (2003) Modulation of macrophage structure and function by low level He-Ne laser irradiation. Photochem Photobiol Sci 2:851–855
Lan CC, Wu CS, Chiou MH, Hsieh PC, Yu HS (2006) Low-energy helium-neon laser induces locomotion of the immature melanoblasts and promotes melanogenesis of the more differentiated melanoblasts: recapitulation of vitiligo repigmentation in vitro. J Invest Dermatol 126:2119–2126
Bayat M, Vasheghani MM, Razavi N, Taheri S, Rakhshan M (2005) Effect of low-level laser therapy on the healing of second-degree burns in rats: a histological and microbiological study. J Photochem Photobiol B 78:171–177
Nissan J, Assif D, Gross MD, Yaffe A, Binderman I (2006) Effect of low intensity laser irradiation on surgically created bony defects in rats. J Oral Rehabil 33:619–924
da Silva RV, Camilli JA (2006) Repair of bone defects treated with autogenous bone graft and low-power laser. J Craniofac Surg 17:297–301
Heckman JD, Sarasohn-Kahn J (1997) The economics of treating tibia fractures. The cost of delayed unions. Bull Hosp Joint Dis 56:63–72
Nicola RA, Jorgetti V, Rigau J, Pacheco MT, dos Reis LM, Zângaro RA (2003) Effect of low-power GaAlAs laser (660 nm) on bone structure and cell activity: an experimental animal study. Lasers Med Sci 18:89–94
Lirani-Galvao AP, Jorgetti V, da Silva OL (2006) Comparative study of how low-level laser therapy and low-intensity pulsed ultrasound affect bone repair in rats. Photomed Laser Surg 24:735–740
Kargman S, Charleson S, Cartwright M, Frank J, Riendeau D, Mancini J, Evans J, O’Neill G (1996) Characterization of prostaglandin G/H synthase 1 and 2 in rat, dog, monkey, and human gastrointestinal tracts. Gastroenterology 111:445–454
Dempke W, Rie C, Grothey A, Schmoll HJ (2001) Cyclooxygenase-2: a novel target for cancer chemotherapy? J Cancer Res Clin Oncol 127:411–417
Miranda SR, Filho HN, Marques Padovan LE, Ribeiro DA, Nicolielo D, Matsumoto MA (2006) Use of platelet-rich plasma under autogenous onlay bone grafts. Clin Oral Implant Res 17:694–699
Gerbi ME, Pinheiro AL, Marzola C, Limeira Júnior Fde A, Ramalho LM, Ponzi EA, Soares AO, Carvalho LC, Lima HV, Gonçalves TO (2005) Assessment of bone repair associated with the use of organic bovine bone and membrane irradiated at 830 nm. Photomed Laser Surg 23:382–388
Khadra M, Kasem N, Haanaes HR, Ellingsen JE, Lyngstadaas SP (2004) Enhancement of bone formation in rat calvarial bone defects using low-level laser therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 97:693–700
Coombe AR, Ho CT, Darendeliler MA, Hunter N, Philips JR, Chapple CC, Yum LW (2001) The effects of low level laser irradiation on osteoblastic cells. Clin Orthod Res 4:3–14
Khadra M (2005) The effect of low level laser irradiation on implant-tissue interaction. In vivo and in vitro studies. Swed Dent J Suppl 172:1–63
Ozawa Y, Shimizu N, Kariya G, Abiko Y (1998) Low-energy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone 22:347–354
Shibata M, Kodani I, Osaki M, Araki K, Adachi H, Ryoke K, Ito H (2005) Cyclo-oxygenase-1 and -2 expression in human oral mucosa, dysplasias and squamous cell carcinomas and their pathological significance. Oral Oncol 41:304–312
Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN (1998) Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93:705–716
Wang W, Bergh A, Damber JE (2007) Increased expression of CCAAT/enhancer-binding protein beta in proliferative inflammatory atrophy of the prostate: relation with the expression of COX-2, the androgen receptor, and presence of focal chronic inflammation. Prostate 67:1238–1246
Sato Y, Arai N, Negishi A, Ohya K (1997) Expression of cyclooxygenase genes and involvement of endogenous prostaglandin during osteogenesis in the rat tibial bone marrow cavity. J Med Dent Sci 44:81–92
Zhang X, Schwarz EM, Young DA, Puzas E, Rosier RN, O’Keefe RJ (2002) Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest 109:1405–1415
Li J, Burr DB, Turner CH (2002) Suppression of prostaglandin synthesis with NS-398 has different effects on endocortical and periosteal bone formation induced by mechanical loading. Calcif Tissue Int 70:320–329
Forwood MR (1996) Inducible cyclo-oxygenase (COX-2) mediates the induction of bone formation by mechanical loading in vivo. J Bone Miner Res 11:1688–1693
Acknowledgments
The authors are grateful to Maira Cristina Rondina Couto for histology assistance and Wilson Aparecido Orcini for morphometry assistance. R V Ferino is a recipient of a Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) fellowship [Programa Institucional de Bolsas de Iniciação Científica (PIBIC)].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsumoto, M.A., Ferino, R.V., Monteleone, G.F. et al. Low-level laser therapy modulates cyclo-oxygenase-2 expression during bone repair in rats. Lasers Med Sci 24, 195–201 (2009). https://doi.org/10.1007/s10103-008-0544-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-008-0544-4