Abstract
The purpose of this study was to analyze histologically the effect of low-level laser therapy (LLLT) in combination with bisphosphonate on bone healing in surgically created critical size defects (CSD) in rat calvaria. One hundred Wistar female rats sham operated (sham) and ovariectomized (Ovx) were maintained untreated for 1 month to allow for the development of osteopenia in the Ovx animals. A CSD was made in the calvarium of each rat, and the animals were divided into five groups according to following treatments: (1) sham rats (control), (2) Ovx rats, (3) Ovx rats treated with LLLT, (4) Ovx rats treated with bisphosphonate, and (5) Ovx rats treated with bisphosphonate and LLLT. Groups 4 and 5 were irrigated with 1 ml of bisphosphonate, and groups 3 and 5 were submitted to LLLT (GaAlAs), 660 nm, 24 J, and 0.4285 W/cm2 on the CSD. Ten animals of each treatment were killed at 30 and 60 days. Histomorphometric assessments, using image analysis software, and histological analyses were performed. No defect was completely regenerated with the bone. Histometrically, it can be observed that groups 3 (37.49 ± 1.94%, 43.11 ± 2.39%) and 5 (35.05 ± 1.57%, 41.07 ± 1.89%) showed a significant bone neoformation when compared to groups 1 (16.81 ± 1.57%, 27.54 ± 1.49%), 2 (11.68 ± 0.98%, 22.51 ± 1.05%), and 4 (14.62 ± 1.70%, 25.67 ± 1.41%) in all experimental periods (P < 0.05). It was possible to conclude that the LLLT associated or not with bisphosphonate treatment was effective for stimulating bone formation in CSD in the calvaria of rats submitted to ovariectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estrogen deficiency, and specifically 17-estradiol deficiency, in postmenopausal women results in the development of osteoporosis [1], which is defined as an osteometabolic disorder of multifactorial etiology characterized by reduced bone mineral density and degradation of its microarchitecture, with a resultant increased weakness and a higher susceptibility to fractures. It is classified as primary postmenopausal osteoporosis (type I), senile (type II), and secondary [2]. The bone represents a rigid form of connective tissue subjected to continuous remodeling process, thus being a highly plastic tissue. The shape and density of the bone tissue are maintained throughout life by a balance between mechanical and physiological aspects [3]. On a normal adult skeleton, the amount of new bone deposited by osteoblasts corresponds exactly to that of osteoclastic bone resorption [4]. As a result of the hypoestrogenemia, which is a characteristic of the postmenopausal period, there is a reduction not only in the speed of osteoid deposit but also in the number of osteoblasts. Consequently, bone resorption increases in comparison with the deposit, reducing thus bone mass, especially on trabecular bone [5].
The need to develop prevention strategies for bone loss and obtain faster and denser bone regeneration has increased. Many treatments have been proposed, including estrogen replacement, physical activity programs, and bisphosphonate compounds [5].
As inhibitors of bone resorption, bisphosphonates are currently being widely used in the treatment of osteoporosis in postmenopausal women [6]. Alendronate may be the most widely prescribed bisphosphonate, which has been shown to prevent loss of bone mass by rapidly suppressing the osteoclastic resorption of bone [7], to preserve alveolar bone resorption in experimental periodontitis [8], and to enhance tendon-to-bone healing in vivo [9]. The dual suppression of both bone resorption and formation activities results in a substantial reduction in bone turnover [10] and may contribute to the pathogenesis of the oral lesions.
Another type of treatment is laser irradiation, a form of electromagnetic field that can elevate the structural stiffness of bone callus [11]. Laser light, particularly low-intensity laser therapy, may play a positive role in bone defect healing [12]. Some authors affirm that low-level laser (low-level laser therapy—LLLT) can accelerate bone formation by increasing both osteoblastic activity [13] and vascularization [14], provide collagen fibers organization [15], elevate ATP levels [16], activate the lymphatic system as well as the proliferation of epithelial cells and fibroblasts, and increase collagen synthesis by fibroblasts [17].
In hard tissues, low-power laser irradiations significantly increased the number of viable osteocytes in the irradiated bone by a positive effect on bone matrix production in order to provide highly reactive and vital peri-implant bone tissue [18], and were also effective on the bone healing process in artificially created osseous cavities [19]. In vivo studies showed the biostimulatory effect of LLLT on bone regeneration [14, 20, 21]. It is photochemical in nature, with the energy probably being absorbed by intracellular chromophores and converted into metabolic energy, most likely involving the respiratory (cytochrome) chain [16]. Based on the individual effects of LLLT and bisphosphonate on the osteoblastic activity and trabecular bone volume, the purpose of this study was to analyze histologically the effect of the association of these two therapies on the bone healing of critical size defect (CSD) in rat calvaria.
Materials and methods
Animal care
One hundred female Wistar rats (2 months old and weighing 225 ± 25 g) were used in the study. The animals were kept in plastic cages with access to food and water ad libitum. Prior to surgical procedures, all animals were allowed to acclimatize to the laboratory environment for a period of 5 days. All protocols described below were approved by the Institutional Review Board of Araçatuba Dental School, São Paulo State University, Brazil (no. 22/08).
Study protocol
Surgical procedures
The Wistar female rats were submitted to either fictitious surgery (sham) or ovariectomy (Ovx). Ovx was performed via bilateral translumbar incisions under ketamine (0.04 ml/100 g) and xylazine (0.02 ml/100 g) anesthesia. The uterine tubes were ligated with suture (4.0 Catgut; Ethicon, São Paulo, Brazil), and after the removal of the ovaries, the incisions were closed (3.0 Catgut; Ethicon). After surgery, the animals were placed in cages; each cage had five animals. After 1 month, the animals were again anesthetized, and after the aseptic procedure, a semilunar incision was made at the scalp in the anterior region of the calvarium allowing for reflection of a full-thickness flap towards the posterior region of the skull. An 8-mm-diameter critical size defect was made with a trephine (3i Implant Innovations Inc., FL, USA) used in a low-speed handpiece under continuous sterile saline irrigation. The defect included a portion of the sagittal suture. Reference “L shape” marks were made 2 mm anterior and 2 mm posterior to the margins of the critical size defects, both of which were located on a longitudinal axis bisecting the surgical defect. The marks were made using a small tapered carbide fissure bur and then filled up with amalgam [22, 23]. Their purpose was to allow identification of the center line of the original defect during laboratory processing and also to be used as references to locate the original bone margins of the surgical defect during histometric analysis.
Groups
The animals were divided into five groups of 20 rats each, according to the following treatments performed on the CSD: (1) sham rats untreated (control), (2) Ovx rats untreated, (3) Ovx rats treated with LLLT, (4) Ovx rats treated with bisphosphonate (alendronate sodium 10−5 M), and (5) Ovx rats treated with bisphosphonate followed LLLT.
LLLT and bisphosphonate
The low-intensity laser (LLLT) used in this study was gallium–aluminum–arsenide (Bio Wave; Kondortech Equipment Ltd., São Carlos, São Paulo, Brazil) with wavelength of 660 nm and spot size of 0.07 cm2. After the displacement of the total retail and clothing of the surgical defect, the application of LLLT was performed once in eight points around the CSD, in contact with the bone tissue, and also in a central point of the CSD in the scanning procedure.
The treatment laser was emitted with power of 0.03 W during 133 s/point, irradiance of 0.42 W/cm2, and energy of 4 J/point (57.14 J/cm2/point). The area received a total energy of 32 J. The bisphosphonate (Endronax; Solvay Farma AS, Taboão da Serra, São Paulo, Brazil), containing alendronate sodium, microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, and magnesium stearate, was poured slowly into the CSD, using a syringe (1 ml) and an insulin needle (13 × 0.45 mm; Becton Dickinson Ind. Ltd, Curitiba, Paraná, Brazil) without bevel. Bisphosphonate solutions were prepared by dissolving the content of 10-mg bisphosphonate tablets into 10 ml distilled sterile saline. Any tablet filler particles were filtered away (Aphoticário pharmaceutical laboratories SA, Araçatuba, São Paulo, Brazil).
The soft tissues were then repositioned and sutured to achieve primary closure (4-0 Silk; Ethicon, São Paulo, São Paulo, Brazil). Each animal received an intramuscular injection of 24,000 IU benzathine penicillin G (Pentabiótico, Veterinário Pequeno Porte; Fort Dodge Saúde Animal Ltd., Campinas, São Paulo, Brazil) postoperatively.
Tissue processing
Ten animals from each group were euthanized at 30 or 60 days postoperatively. The area of the original surgical defect and the surrounding tissues were removed in block. The blocks were fixed in 10% neutral formalin, rinsed with water, and then decalcified in 18% EDTA solution. After an initial decalcification, each specimen was divided longitudinally into two blocks, exactly along the center line of the original surgical defect, using the long axis of both L marks as references. Cross-sections were then obtained using the short axis of each L mark as references. Each specimen then measured 12 mm in length along the longitudinal axis running through the center of the defect, allowing for identification of the original surgical defect margins during both histologic and histometric evaluations. After additional decalcification, they were processed and embedded in paraffin. Serial sections, 6 μm thick, were cut in a longitudinal direction starting at the center of the original surgical defect. The sections were stained with hematoxylin and eosin for analysis under light microscopy.
Histomorphometric analysis
Before the analysis, criteria were established in order to conduct a more objective evaluation of the acute (neutrophils) and chronic (macrophages, lymphocytes, and plasma cells) inflammatory infiltrates. The following criteria were used to describe the inflammatory infiltrate in each field using a light microscope with a 40 Â objective: (a) light, 1–100 cells; (b) moderate, 100–250 cells; and (c) intense, more than 250 cells [23]. Four histological sections, representing the center of the original surgical defect, were selected for the histologic and histometric analyses in order to increase the reliability of the data used in the statistical analysis. The histologic and histometric analyses were performed by an examiner blinded with respect to the treatment rendered. The images of the histologic sections were captured by a digital camera coupled to a light microscope with an original magnification of ×32. The digital images were saved on a computer. A composite digital image was then created by combining three smaller images because it was not possible to capture the entire defect in one image at the level of magnification used. The composite image was created based on anatomic reference structures (e.g. blood vessels and bone trabeculae) within each of the histologic sections. The Imagelab 2000 software (Diracon Bio Informática Ltd., Vargem Grande do Sul, São Paulo, Brazil) was used for the histomorphometric analysis. The following criteria, based in part on the work of Melo et al. [24], were used to standardize the histomorphometric analysis of the digital images:
-
(a)
The total area to be analyzed corresponded to the entire area of the original surgical defect. This area was determined by first identifying the external and internal surfaces of the original calvarium at the right and left margins of the surgical defect, and then connecting them with lines drawn following their respective curvatures. Considering the total length of the histologic specimen, a distance of 2 mm was measured from the right and left edges of the specimen towards the center in order to determine the margins of the original surgical defect. The newly formed bone area was delineated within the confines of the total area.
-
(b)
The total area was measured in square millimeters and was considered to represent 100% of the area to be analyzed. The newly formed bone area was also measured in square millimeters and calculated as a percentage of the total area.
Statistical analysis
The values of newly formed bone area for each animal were represented by the mean percentage of the four histologic sections. These percentage data were transformed into arccosine for the statistical analysis. The significance of the differences between groups in relation to the newly formed bone area was determined by an analysis of variance, followed by a post hoc Tukey test when the analysis of variance suggested a significant difference between groups (P < 0.05).
Results
Qualitative histologic analysis
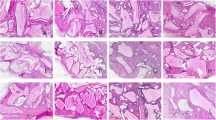
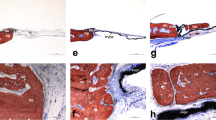
In groups 1, 2, and 4 at 30 days, almost all of the surgical defects were filled up with connective tissue with collagen fibers parallel to the wound surface and a moderate number of fibroblasts. Newly formed bone surrounded by a small number of osteoblasts was restricted to areas close to the borders of the surgical defect (Fig. 1). At 60 days, most specimens presented similar bone formation when compared to the 30-day specimens (Fig. 2), whereas a few showed increased bone formation. The connective tissue presented a moderate number of fibroblasts and many collagen fibers. Light acute and chronic inflammatory infiltrates were present throughout the surgical defect. In a few specimens, areas with an intense chronic inflammatory infiltrate were noted. In groups 1, 2, and 4, a light and predominantly chronic inflammatory infiltrate was dispersed throughout the defect at 60 days.
a Panoramic view of the defect in group 2—30 days (hematoxylin and eosin stained; original magnification ×5). b Connective tissue thinner than the original calvarium, with collagen fibers parallel to the wound surface and a moderate number of fibroblasts (hematoxylin and eosin stained; original magnification ×40). c Newly formed bone surrounded by a small number of osteoblasts restricted to areas close to the borders of the surgical defect (hematoxylin and eosin stained; original magnification ×40)
In groups 3 and 5 at 30 days, most specimens presented a greater amount of newly formed bone close to the borders of the surgical defect when compared to the specimens of groups 1, 2, and 4 (Fig. 3). The newly formed bone was surrounded by a small number of osteoblasts. The connective tissue presented a moderate number of fibroblasts and numerous collagen fibers, which were thicker and more organized than the ones observed in the specimens of groups 1, 2, and 4. At 60 days, all specimens presented newly formed bone, surrounded by a small number of osteoblasts, extending towards the center of the defect. In three specimens, the newly formed bone covered almost the entire length of the surgical defect. However, it was thinner than that of the original calvarium (Fig. 4). The connective tissue showed similar characteristics to those observed in the 30-day specimens. Light acute and chronic inflammatory infiltrates were observed in most specimens, while a few demonstrated an intense chronic inflammatory infiltrate that consisted of lymphocytes, plasma cells, and histiocytes. In groups 3 and 5, a light and predominantly chronic inflammatory infiltrate was dispersed throughout the defect at 60 days.
Histometric and statistical analyses
The histometric data are showed in Table 1. In the intergroup analysis, all groups revealed a greater bone neoformation when compared to group 2 at 30 (11.68 ± 0.98%) and 60 (22.51 ± 1.05%) days, respectively (P < 0.05). Furthermore, it can be observed that groups 3 (37.49 ± 1.94%, 43.11 ± 2.39%) and 5 (35.05 ± 1.57%, 41.07 ± 1.89%) showed a significant bone neoformation compared to groups 2 (11.68 ± 0.98%, 22.51 ± 1.05%) and 4 (14.62 ± 1.70%, 25.67 ± 1.41%) in all experimental periods, respectively. In the intragroup analysis, statistical analysis of histometric data showed a greater bone neoformation at 60 days when compared to the period of 30 days in all groups (P < 0.05).
Discussion
This study evaluated histologically the effects of LLLT and bisphosphonate on the bone healing of CSD in rat calvaria. Estrogen deficiency, and in particular 17-estradiol deficiency in postmenopausal women, induces a decreased osteoblastic activity while at the same time the activity of osteoclasts increases, which leads to a disturbed bone remodeling process and the development of osteoporosis [1]. Disorders in the osseous system similar to those observed in postmenopausal women may be obtained in an experimental model of sexually mature female rats as a result of bilateral ovariectomy. Such animal model of osteopenia has been used by many researchers to assess changes in the osseous system induced by estrogen deficiency and to examine the effects of drugs on those changes [25]. In the present study, previously reported models of bilateral ovariectomy were reproduced [26, 27], which demonstrated that estrogen deficiency 30 days after performing bilateral ovariectomy caused a statistically significant increase not only in the body weight but also in the thymus mass of the examined female rats as well as a statistically significant decrease in the uterus mass.
In the intergroup evaluation, the histometric results of the present study showed that all groups revealed a greater bone neoformation when compared to group 2 at 30 and 60 days, respectively. This result can be explained by the action of gonadal hormones, particularly estrogen, on growth factor production and cytokine expression in osteoblasts and osteoclasts in vitro, which may be important for regulating bone remodeling. Some of these growth factors are essential to stimulate angiogenesis [28]. The osteoblastic cells under estrogenic stimulation produce transforming growth factor and the insulin-like growth factors. Transforming growth factor, which is a potent mitogen in osteoblastic cells, has been reported to inhibit osteoclast recruitment and resorption [28].
The literature recommends several treatments for osteoporosis. Most of the drugs used in the treatment of osteoporosis act mainly to impede the progression of the disease [1], because most of these treatments increase both the synthesis of bone matrix and bone resorption [5]. Alendronate sodium is part of the group of bisphosphonates that have been used to correct the continuous loss of bone mass due to postmenopausal osteoporosis. Bisphosphonates are known to inhibit bone resorption and have been currently used as therapeutic agents for bone diseases such as osteoporosis, Paget’s disease, and cancer-related hypercalcemia [27]. It is assumed that bisphosphonates mainly act through cellular mechanisms (e.g., inactivation of osteoclasts) other than the physicochemical mechanism of crystal dissolution, although the precise mechanisms of action remain to be elucidated [29]. Although prolonged use of oral bisphosphonates has not increased skeletal fragility or impaired fracture healing in osteoporotic patients, a loss of forearm bone density has been described after intravenous pamidronate therapy in patients with Paget’s disease [29].
The administration of bisphosphonates has been reported to have an inhibitory effect on root resorption in experimental orthodontic tooth movements [30], beneficial effects on the cementum healing, as well as a reduction in the loss of root mass after reimplantation of dried dog teeth [31]. It is clinically important to prevent the occurrence of root resorption because this generally worsens the prognosis of a reimplanted tooth [32]. Reports in the literature suggest that this drug enlarges the trabecular bone volume due to increased osteoblastic activity, bone mineralization [33], histomorphometric parameters [27], and enhancement of mechanical properties of the bone in ovariectomized rats [34].
Despite these factors, group 3 showed a relative increase in bone neoformation in the present study, but not a statistically significant difference when compared to group 1 at 30 and 60 days, respectively. The local dose of alendronate used in this study may have led to an enhanced inflammatory reaction in the bone, which could have impaired the healing process [9]. Additional factors, such as drug concentration, period of maintenance of the drug within the CSD, time for biological response, mode, time, and frequency of drug application may have influenced the results obtained in the present study.
LLLT has shown to have various biostimulating effects, such as promotion of wound healing [35] and enhancement of both fibroblastic [36] and chondral proliferation [37]. LLLT has also been reported to increase collagen synthesis [15], anti-inflammatory activity [38], and nerve regeneration [39]. Studies have demonstrated the photobiological and photochemical effects of low-intensity lasers, showing that low-energy irradiation of injured tissues improves wound healing by accelerated epithelization, high degree of vascularization, increased collagen synthesis, and stimulation of fibroblast activity [40].
LLLT can stimulate bone cellular proliferation [41] and ALP activity, which reflects osteoblastic activity [42]. It can also increase intracellular calcium level [43], but its mechanism of action in bone is poorly understood.
Biomodulation is an area of controversy as there are conflicting results that have been reported. However, studies have suggested a positive effect of LLLT on bone healing either in vivo [44] or in vitro [45], but did not find any effect of LLLT on the repair of soft or mineralized tissues [11]. These inconsistent reports may be attributed to the wide variety of laser setups and types used in different studies.
In the analysis of the histometric evaluation results, it was possible to observe that groups 3 and 5 showed a significant bone neoformation compared to groups 2 and 4 in all experimental periods, respectively. The present results are in agreement with another study in the literature, which showed that laser therapy associated with bisphosphonate treatment reversed the vertebral osteopenia caused by the ovariectomy [46].
The true mechanism that leads to a positive effect of laser light on different tissues is not fully understood, and this aspect makes the comparative analysis of the present results difficult because of the diversity of techniques, methods, and experimental models, as well as treatment protocols reported [47]. Several hypotheses have been suggested for this mechanism.
One report [13] suggested that laser energy stimulates porphyrins and cytochromes to increase cellular activity, increasing thus the concentration of ATP and ALP, as well as the release of Ca. A similar study [16] has suggested that the magnitude of the biomodulating effect depends on the physiologic status of the cell at the time of irradiation. This may explain why the biomodulating effect is not always detectable. Another study [42] showed that the stimulant effect of laser light occurs during the initial phase of proliferation and initial differentiation of undifferentiated cells; this does not occur during more advanced stages.
In the intragroup analysis, statistical analysis of histometric data showed a greater bone neoformation at 60 days when compared to the period of 30 days in all groups. These results differ from Takagi and Urist [48] to create CSDs in rat calvarias who observed that in some of the groups studied, there was no significant difference in bone formation between the periods.
Within the limits of this study, it can be concluded that the laser therapy associated or not with bisphosphonate treatment was effective for stimulating a bone formation in CSD in the calvaria of ovariectomized rat. However, despite the effects of the association of these therapies in the bone tissue, further studies are necessary in order to verify its potential use in postmenopausal osteoporosis.
References
Mangolas SC (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115–137
Gali JC (2001) Osteoporose. Acta Ortop Bras 9:53–62
Consensus Development Conference on Osteoporosis (1993) Am J Med 95A:5S–16S
Friedlander AH (2002) The physiology, medical management and oral implications of menopause. J Am Dent Assoc 133:73–81
Botell M (2001) Osteoporosis en la menopausia, prevención y estratégias terapêuticas atuales. Rev Cub Obst Ginecol 27:199–204
Park-Wyllie LY, Mamdani MM, Juurlink DN, Hawker GA, Gunraj N, Austin PC, Whelan DB, Weiler PJ, Laupacis A (2011) Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 305:783–789
Manolagas SC (2010) From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev 31:266–300
Menezes AM, Rocha FA, Chaves HV, Carvalho CB, Ribeiro RA, Brito GAJ (2005) Effect of sodium alendronate on alveolar bone resorption in experimental periodontitis in rats. Periodontol 2000 76:1901–1909
Thomopoulos S, Matsuzaki H, Zaegel M, Gelberman RH, Silva MJ (2007) Alendronate prevents bone loss and improves tendon-to-bone repair strength in a canine model. J Orthop Res 25:473–479
Grey A, Bolland MJ, Wattie D, Horne A, Gamble G, Reid IR (2009) The antiresorptive effects of a single dose of zoledronate persist for two years: a randomized, placebo-controlled trial in osteopenic postmenopausal women. J Clin Endocrinol Metab 94:538–544
Luger EJ, Rochkind S, Wollman Y, Kogan G, Dekel S (1998) Effect of low power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers Surg Med 22:97–102
Guzzardella GA, Fini M, Torricelli P, Giavaresi G, Giardino R (2002) Laser stimulation on bone defect healing: an in vitro study. Lasers Med Sci 17:216–220
Freitas IGF, Baranauskas V, Cruz-Höfling MA (2000) Laser effects on osteogenesis. Appl Surf Sci 154–155:548–554
Trelles MA, Mayayo E (1987) Bone fracture consolidates faster with low-power laser. Lasers Surg Med 7:36–45
Garavello-Freitas I, Baranauskas V, Joazeiro PP, Padovani CR, Dal Pai-Silva M, Da Cruz-Höfling MA (2003) Low-power laser irradiation improves histomorphometrical parameters and bone matrix organization during tibia wound healing in rats. J Photochem Photobiol 70:81–89
Karu T, Pyatibrat L, Kalendo G (1995) Irradiation with He–Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol 27:219–223
Alghamdi KM, Kumar A, Moussa NA (2011) Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci 27(1):237–249
Dortbudak O, Haas R, Mailath-Pokorny G (2002) Effect of low power laser irradiation on bony implant sites. Clin Oral Implants Res 13:288–292
Nissan J, Assif D, Gross MD, Yaffe A, Binderman I (2006) Effect of low intensity laser irradiation on surgically created bony defects in rats. J Oral Rehabil 33:619–924
Lunger EJ, Rochkind S, Wollman Y, Kogan G, Dekel S (1998) Effect of low power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers Surg Med 22:97–102
Barushka O, Yaakobi T, Oron U (1995) Effect of low-energy laser (He–Ne) irradiation. Bone 16:47–55
Bosch C, Melsen B, Vargervik K (1998) Importance of the critical-size bone defect in testing bone-regenerating materials. J Craniofac Surg 9:310–316
Furlaneto FA, Nagata MJ, Fucini SE, Deliberador TM, Okamoto T, Messora MR (2007) Bone healing in critical-size defects treated with bioactive glass/calcium sulfate: a histologic and histometric study in rat calvaria. Clin Oral Implants Res 18:311–318
Melo LG, Nagata MJ, Bosco AF, Ribeiro LL, Leite CM (2005) Bone healing in surgically created defects treated with either bioactive glass particles, a calcium sulfate barrier, or a combination of both materials. A histological and histometric study in rat tibias. Clin Oral Implants Res 16:683–691
Frost HM, Jee WSS (1992) On the rat model of human osteopenias and osteoporosis. Bone Miner 18:227–236
Pytlik M, Janiec W, Misiarz-Myrta M, Gubata I (2003) Effects of simvastatin on the development of osteopenia caused by ovariectomy in rats. Pol J Pharmacol 55:63–71
Pytlik M, Kaczmarczyk-Sedlak I, Sliwiński L, Janiec W, Rymkiewicz I (2004) Effect of concurrent administration of alendronate sodium and retinol on development of changes in histomorphometric parameters of bones induced by ovariectomy in rats. Pol J Pharmacol 56:571–579
Houde N, Chamoux E, Bisson M, Roux S (2009) Transforming growth factor-beta1 (TGF-beta1) induces human osteoclast apoptosis by up-regulating Bim. J Biol Chem 284:23397–23404
Gutteridge DH, Retallack RW, Ward LC, Price RI, Stewart GO, Stuckey BG, Prince RL, Kent GN, Bhagat CI, Thompson RI, Nicholson GC (2003) Bone density changes in Paget’s disease 2 years after iv pamidronate: profound, sustained increases in pagetic bone with severity-related loss in forearm nonpagetic cortical bone. Bone 32:56–61
Igarashi K, Adachi H, Mitani H, Shinoda H (1996) Inhibitory effect of the topical administration of a bisphosphonate (risendronate) on root resorption incident to orthodontic tooth movements in rats. J Dent Res 75:1644–1649
Levin L, Bryson EC, Caplan D, Trope M (2001) Effect of topical alendronate on root resorption of dried replanted dog teeth. Dent Traumatol 17:120–126
Berggreen E, Sae-Lim V, Bletsa A, Heyeraas KJ (2001) Effect of denervation on healing after tooth replantation in the ferret. Acta Odontol Scand 59:379–385
Bohic S, Rey C, Legrand A, Sfihi H, Rohanizadeh R, Martel C, Barbier A, Daculsi G (2000) Characterization of the trabecular rat bone mineral: effect of ovariectomy and bisphosphonate treatment. Bone 26:341–348
Sliwiński L, Janiec W, Pytlik M, Folwarczna J, Kaczmarczyk-Sedlak I, Pytlik W, Cegieła U, Nowińska B (2004) Effect of administration of alendronate sodium and retinol on the mechanical properties of the femur in ovariectomized rats. Pol J Pharmacol 56:817–824
Kana JS, Hutschenreiter G, Haina D, Waidelich W (1981) Effect of low-power density laser radiation on healing of open skin wound in rats. Arch Surg 116:293–296
Boulton M, Marshall J (1986) He–Ne laser stimulation of human fibroblast proliferation and attachment in vitro. Lasers Life Sci 1:123–134
Schultz RJ, Krishnamurthy S, Thelmo W, Rodriguez JE, Harvey G (1985) Effects of varying intensities of laser energy on articular cartilage. Lasers Surg Med 5:557–588
Honmura A, Yanase M, Obata J, Haruki E (1992) Therapeutic effect of Ga-Al-As diode laser irradiation on experimentally induced inflammation in rats. Lasers Surg Med 12:441–449
Anders JJ, Borke RC, Woolery SK, Van de Merwe WP (1993) Low power laser irradiation alters the rate of regeneration of the rat facial nerve. Lasers Surg Med 13:72–82
Dortbudak O, Hass R, Pokorny G (2000) Biostimulation of bone marrow cells with a diode soft laser. Clin Oral Implants Res 11:540–545
Ueda Y, Shimizu N (2003) Effects of pulse frequency of low-level laser therapy (LLLT) on bone nodule formation in rat calvarial cells. J Clin Laser Med Surg 21:271–277
Ozawa Y, Shimizu N, Kariya G, Abiko Y (1998) Low-energy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone 22:347–354
Coombe AR, Ho CTG, Darendeliler MA, Hunter N, Philips JR, Chapple CC, Yum LW (2001) The effects of low-level laser irradiation on osteoblastic cells. Clin Orthop Res 4:3–14
Silva Júnior AN, Pinheiro AL, Oliveira MG, Weismann R, Ramalho LM, Nicolau RA (2002) Computerized morphometric assessment of the effect of low-level laser therapy on bone repair: an experimental animal study. J Clin Laser Med Surg 20:83–87
Kipshidze N, Nikolaychic V, Keelan MH, Shankar LR, Khanna A, Kornowski R, Leon M, Moses J (2001) Low power helium: neon laser irradiation enhances production of vascular endothelial growth factor and promotes growth of endothelial cells in vitro. Lasers Surg Med 28:355–364
Diniz JS, Nicolau RA, de Melo ON, do Carmo Magalhães F, de Oliveira Pereira RD, Serakides R (2009) Effect of low-power gallium-aluminum-arsenium laser therapy (830 nm) in combination with bisphosphonate treatment on osteopenic bone structure: an experimental animal study. Lasers Med Sci 24:347–352
Pyczek M, Sopala M, Dabrowski Z (1994) Effect of low energy laser power on the bone marrow of the rat. Folia Biol 42:151–156
Takagi K, Urist MR (1982) The reaction of the dura to bone morphogenetic protein (BMP) in repair of skull defects. Ann Surg 196:100–109
Acknowledgments
Juliana Mendonça da Conceição received a scholarship from the São Paulo State Foundation for Research (FAPESP: 2007/55072-5). This paper is attributed to the Department of Periodontology, Araçatuba Dental School, São Paulo State University (UNESP) Araçatuba, São Paulo, Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garcia, V.G., da Conceição, J.M., Fernandes, L.A. et al. Effects of LLLT in combination with bisphosphonate on bone healing in critical size defects: a histological and histometric study in rat calvaria. Lasers Med Sci 28, 407–414 (2013). https://doi.org/10.1007/s10103-012-1068-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1068-5