Abstract
A new low-level laser therapy (LLLT) protocol is proposed and compared to another previously studied, in animal models, aiming to establish a more practical LLLT protocol. Protocol 1, the same used in other works and based on the clinical LLLT protocol for bone regeneration, consists of punctual transcutaneous applications in the defect region with fluence of 16 J/cm2 every 48 h for 15 days. Protocol 2, proposed in this work, consists of three sessions: the first application directly on the defect site with fluency of 3.7 J/cm2, during the surgical procedure, followed by two transcutaneous applications, 48 and 120 h postoperatively. The Thera Lase® (λ = 830 nm) was used, and the dosimetry of the first application of protocol 2 was calculated based on in vitro studies. Forty-five male rats were used, in which critical-size bone defects with 8 mm of diameter were surgically created in calvaria. The animals were randomly divided into three groups of 15 animals, named group 1 (protocol 1), group 2 (protocol 2), and control, which was not submitted to laser treatment. After 7, 15, and 45 days, five animals of each group were euthanized, and the pieces of calvarial bone were collected for microscopic and immunohistochemistry for vascular endothelial growth factor (VEGF), osteocalcin (OC), and osteopontin (OP) analysis. Histomorphometry showed that newly formed bone of 15-day samples from group 2 is higher than the control group (p < 0.05, ANOVA, Tukey). At 7 days, in the central part of the defect, VEGF expression was the same for all groups, OC was higher for protocol 2, and OP for protocol 1. The results suggest LLLT using the protocol 2 hastened the bone healing process in the early periods after surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Improvement in the bone regeneration process is a subject of great interest because it is closely related to several treatments such as orthopedic and dental surgeries, orthodontic movement, and bone integration of dental implants [1]. In this context, the low-level laser therapy (LLLT) has been successfully used, mainly due to its anti-inflammatory action and ability to speed up the healing [2–4]. The effect produced by the LLLT is characterized by the principle of biomodulation, which is the use of native raw material produced by our body to produce changes in tissue, which, in turn, contributes to the recovery of several pathological conditions [5]. The laser radiation absorbed by cytochromes of the mitochondria triggers a cascade of events resulting in increased ATP production and thus provides better cell proliferation conditions, stimulating the tissue renewal process [6, 7].
Pretel et al. [3] studied the effects of LLLT on bone repair in rats through defects created in the mandible. They observed an advanced tissue response in the animals treated by LLLT in relation to the non-treated group and attributed this result to the modulation of the initial inflammatory response, promoting new bone matrix formation. More recently, the association between LLLT and biomaterials and other regenerative procedures has been investigated. Pinheiro et al. [8] studied the influence of LED phototherapy in repair of bone defects treated by mineral trioxide aggregate, bone morphogenetic proteins, and guided bone regeneration. They observed less inflammation and increase of both collagen and bone deposition when LED light was used alone or in association with these biomaterials. Ribeiro et al. [9] studied calcitonin associated to LLLT in bone repair and concluded that this combination improved the bone repair. Omasa et al. [10] verified the enhancement of mini-implant stability when LLLT is used. This result was associated to the increase of BMP-2 gene expression in surrounding cells.
In experimental tooth movement, Kawasaki et al. [11] determined that LLLT can accelerate the tooth movement and increase the alveolar bone remodeling. Ninomiya et al. [12] related that the laser irradiation promotes the activity of a higher number of osteoblasts and a decrease in the number of osteoclasts, suggesting that the laser irradiation contributes to bone regeneration due to decrease in the number of osteoclasts. These results were reinforced by Kim et al. [13] and Fujita et al. [14] who studied the effects of laser irradiation on the osteoclastogenesis process in induced tooth movement. The effect of the combination of anti-inflammatory drugs and low-intensity laser therapy on bone repair was studied by Matsumoto et al. [15] in an animal model. The authors demonstrated that this therapy optimizes the bone repair process in rats treated with anti-inflammatory drugs.
Most studies reported in the literature on laser therapy for bone regeneration consists of several sessions of application for a long period, similar to protocols established for clinical applications. For instance, Matsumoto et al. [15] and Ribeiro et al. [16] held transcutaneous applications every 48 h throughout the experimental period, which ranged from 7 to 21 days, using infrared laser (λ = 735 nm and a fluence of 16 J/cm2). Kim et al. [13] and Fujita et al. [14] made daily applications for 7 days, using fluences of 9.6 J/cm2 and λ = 808 nm (infrared) and 54 J/cm2 and λ = 810 nm (infrared), respectively.
Fukuhara et al. [17] conducted an extensive study using osteoblast culture of rat calvaria and determined that the fluence of 3.75 J/cm2 is “optimal” because it caused a greater formation of bone nodules, compared with lower (1.25 J/cm2) and higher (6.25 J/cm2) fluences. Additionally, Ozawa et al. [18] demonstrated that the laser therapy is effective when used in the initial stages, i.e., its efficacy is greater in the cell proliferation phase, in relation to the bone matrix maturation and mineralization phases. More recently, Barbosa et al. [19] showed that laser therapy, using the infrared spectrum, is more efficient than the red spectrum.
Thus, based on the findings of Fukuhara et al. [17], this present work studied the bone regeneration obtained by a protocol with dosimetry of 3.7 J/cm2 directly applied on the bone defect during the surgical procedure, followed by two transcutaneous applications with a fluence of 16 J/cm2 in comparison to the protocol already reported in literature, consisting of punctual transcutaneous applications in the defect region with fluence of 16 J/cm2 every 48 h for 15 days [15].
Material and methods
A total of 45 male rats (Rattus norvegicus, albinus, Wistar), weighing an average of 200 g, a Thera Lase® (DMC Equipamentos Ltda, São Carlos, Brazil), optical microscope Nikon H550, and Image Pro-Plus software were used.
Surgical procedure
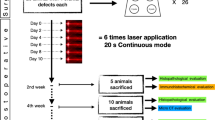
The present study was approved by the Ethical Committee from Universidade Sagrado Coração–USC, Bauru, São Paulo State, Brazil, and was conducted according to recommendations set forth by the National Institute of Health (NIH) [20]. A total of 45 male Wistar rats, weighing an average of 200 g, were used. The animals were kept in a plastic cage in an experimental animal room and were fed with a standard laboratory diet and water. A full-thickness bone defect with 8-mm diameter was surgically created in the skull (Fig. 1). Preoperatively, general anesthetic was intramuscularly induced in animals with xylazine chlorhydrate (20 mg/kg, Anasedan, Vetbrands, Brazil) and ketamine (100 mg/kg, Dopalen, Vetbrands, Brazil) for body weight. The dorsal part of the cranium was shaved and aseptically prepared for surgery. A linear incision of 2 cm long was made on the skin at the median sagittal line. The musculature and the periosteum were reflected, exposing the parietal bone, and the defect was created by means of a trephine bur operating with low rotation under irrigation with sterile physiological solution (0.9 % NaCl).
Group 1 (N = 15) was treated with protocol 1 that consisted of transcutaneous applications in four points equidistant from each other around the defect, applied at a distance of 1 mm from the edge, with a fluence of 16 J/cm2 (power 50 mW, exposure time of 9 s). Applications were made every 48 h starting 24 h after surgery, extending for 15 days.
Group 2 (N = 15) was treated with protocol 2 that consisted of three applications: the first during surgery, directly into the defect, before closing the skin, in four points as described in protocol 1, with a fluence of 3.7 J/cm2 (power 50 mW, exposure time of 3 s) followed by two transcutaneous applications 48 and 96 h after surgery, with fluence of 16 J/cm2.
The control group (C) (N = 15) was not treated; however, it was handled the same way as the treated groups.
The soft tissues and skin incisions were closed with 4-0 silk-interrupted sutures.
After 7, 15, and 45 days postoperatively, five animals from each group were euthanized with a lethal dose of anesthetic associated to relaxant, and the specimens containing the bone defect were collected and prepared for microscopic analysis.
Microscopic analysis
The bone samples were kept 48 h in phosphate buffered formalin (Merck, 10 %) and decalcified using the ethylenediaminetetraacetic acid (EDTA) method and processed by routine microscopy. Serial 6-μm-thick sections were cut in the central region of the defect and stained with hematoxylin/eosin and Masson trichromic. The specimens were analyzed with a Nikon H550L optical microscope. Other sections were selected for immunohistochemical analysis.
Immunohistochemistry assay
The immunohistochemistry reactions were performed in order to identify the endothelial vascular growth factor (VEGF), osteopontin (OP), and osteocalcin (OC), respectively, in blood vessels and in repairing bone. Anti-VEGF, anti-OC, and anti-OP produced in goats (Santa Cruz Biotechnology, Santa Cruz, CA, USA, goat polyclonal) were the primary antibodies. The secondary antibody used in reactions was the biotinylated rabbit anti-goat (Pierce Biotechnology). Avidin and biotin complex (Vector Laboratories) was used in order to amplify the reaction signal, and the chromogen chosen was the diaminobenzidine (Dako). At the end of the immunohistochemistry reaction, the slices were counterstained with Harris hematoxylin. All samples were accompanied by a negative control.
The evaluation of results was made using scores ranging from 1 to 4, semiquantitatively, taking into account the protein expression as absent, slight, moderate, and intense. These values were used to map the biomolecular events present in the bone regeneration. The analysis was performed in a standardized way, through photomicrographs near the margins of the defect (two pictures) and in the central region (one picture) using a Nikon H550L optical microscope with × 400 magnification.
Results
General findings
Postoperative complications or changes in animal behavior were not observed. In addition, healing of the surgical site occurred normally, and no animals were lost during the experiment.
Microscopic analysis
Seven days
At 7 days, the first bone healing events were noted in all specimens, with defects mainly filled by blood clots and granulation tissue.
In group C (control) at 7 days (Fig. 2a), the defects were filled by highly vascularized connective tissue (CT), in the organization stage. Granulation tissue (GT) was also observed in the majority of the central region of the defect. Some specimens showed discrete areas of osteogenesis in the edges of the defect near native bone (NB).
Specimens submitted to protocol 1 (Fig. 2b) show fibrous connective tissue highly vascularized (CT). Areas with little mononuclear cell infiltration adjacent to granulation tissue (GT) were noted. Slight presence of primary bone tissue (PB) in the edges of the surgical defect was noted near native bone (NB).
Group 2 (Fig. 2c), submitted to protocol 2, showed a similar pattern to other groups in this period, demonstrating that laser application directly on the site of the defect with this dosimetry did not lead to tissue necrosis or other undesirable effects. Areas of osteogenesis adjacent to the edges of the defect, with primary bone (PB), highly vascularized connective tissue (CT) as well as regions with granulation tissue (GT) were noted.
Fifteen days
At 15 days, the similarity between the control and group 1 was noted (Fig. 2d, e), with areas of fibrous connective tissue (CT) and inflammatory infiltration in the areas of granulation tissue (GT). Areas with primary bone development (PB) are present in the edges of the critical defect.
In samples of group 2 (Fig. 2f) (protocol 2), it is possible to observe that the defects are filled with fibrous connective tissue with mild inflammatory infiltrate. Osteogenic areas, larger than control and group 1, were observed along the wall of the surgical defect (Fig. 2f).
Forty-five days
The defects in group C (Fig. 2g) (control) present filled by a thin layer of fibrous connective tissue. Mild and diffuse inflammatory infiltrates were observed. The osteogenic activity in the edge of bone defect, and also the presence of primary bone (PB), located near the wall of the defect was noted. Similar patterns were observed in group 1 (Fig. 2h) and group 2 (Fig. 2i), but in group 2, short primary bone trabeculae (PB) were noted in the defect region.
Histomorphometry
At 7 days, the defects were mostly filled by blood clots and connective tissue. Thus, the histomorphometry of bone tissue cannot be performed. Figure 3a and b shows the histomorphometry of newly formed bone and connective tissue, according to the groups for the periods of 15 and 45 days. Statistical tests were performed, and the comparison between results of control and group 2, at 15 days, were statistically different according to the ANOVA followed by Tukey test (p < 0.05).
Immunohistochemistry
All groups presented positive immunolabeling for VEGF, OP, and OC (Fig. 4). At 7 days, in the central part of defect, group 2 (protocol 2) showed higher expression of OC (score 2) while the others were scored as 1 (Fig. 4i). OP was higher for group 1 (protocol 1) (score 2), the others were scored as 1 (Fig. 4e), and VEGF expression was the same for all groups (score 1). For the other periods, expressions were similar among the groups.
Immunolabeling of central part of defect at 7 days. The first column corresponds to control group, the second column to group 1, and the last column to group 2. a–c VEGF (control, group 1 and group 2, respectively), d–f osteopontin, showing higher expression for group 1, and g–i for osteopontin, showing higher expression for group 2
Discussion
The use of LLLT for improvement of bone regeneration has been investigated in animal models as well as in vitro experiments with cell culture, proving its positive effect.
There is a large variety of LLLT protocols. Most applications involve use daily or on alternate days for a period of 2 weeks [8, 15]. In an attempt to reduce the number of LLLT sessions, a protocol with only three applications is presented, in which, the first being directly into the defect site, with dosimetry based on the findings of Fukuhara et al. [17]. Although the temperature was not monitored during the experiments, as this protocol was applied directly into cells, we assumed that there are no harmful effects to them. Furthermore, the photomicrograph at 7 days shows there are no undesirable effects due to protocol 2 (Fig. 2c).
The results indicate that LLLT, using this protocol, stimulated the early bone regeneration stage. The histomorphometry results of newly formed bone (Fig. 3) in the period of 15 days indicated higher bone formation in group 2 in comparison with control group (p < 0.05) (ANOVA, Tukey). Results of 45 days were the same for all groups. These results indicate that the LLLT is more effective in the early stages of the regeneration process and is in agreement with Ozawa and Fukuhara [16, 18] who reported the LLLT efficacy being greater in the cell proliferation phase, in relation to the bone matrix maturation and mineralization phases and also with Pretel et al. [3] who also irradiated directly on the defect site and found enhancement in bone formation in the LLLT-treated group.
Vascular endothelial growth factor (VEGF), osteocalcin (OC), and osteopontin (OP) immunohistochemical staining were performed in all specimens. VEGF is a signal protein produced by cells that stimulates vasculogenesis and angiogenes. OP and OC are extracellular matrix proteins synthesized and secreted during the process of osteoblast differentiation and mineralization [21]. While OP is an early and effective marker of bone formation, OC indicates the later phase of bone formation. Our results at 7 days in the central part of defect showed more intense labeling for OC in samples of protocol 2 and for OP for protocol 1. Thus, LLLT in both protocols improved osteoblast differentiation. The more intense expression of OC in samples of protocol 2 indicates a more advanced stage in the bone remodeling process, consistent with data from histomorphometry at 15 days, in which the amount of newly formed bone was higher compared to other groups.
Protocol 2 showed to be efficient, as already mentioned, since the fraction of newly formed bone is greater than the control group at 15 days. Thus, this protocol provides an alternative with clinical feasibility, taking into account the reduction in the number of applications, resulting in the reduction of clinic visits and consequently reducing the costs.
Conclusion
LLLT is a valuable technique presenting ability to accelerate bone regeneration. The protocol presented in this work demonstrates that LLLT works in the early stages of the bone regeneration process.
References
Buser D (2009) 20 Years of guided bone regeneration in implant dentistry, 2nd edn. Quintessence book, London
Paiva-Oliveira EL, Lima NC, Silva PH, Sousa NTA, Barbosa FS, Orsini M, Silva JG (2012) Low-level laser therapy (LLLT) reduces inflammatory infiltrate and enhances skeletal muscle repair: histomorphometric parameters. Laser Phys 22(9):1425–1430
Pretel H, Lizarelli RFZ, Ramalho LTO (2007) Effect of low-level laser therapy on bone repair: histological study in rats. Lasers Surg Med 39(10):788–796
Poppi R, Silva A, Nacer R, Vieira R, Oliveira L, Faria-Junior NS, Tarso Camilo Carvalho P (2011) Evaluation of the osteogenic effect of low-level laser therapy (808 nm and 660 nm) on bone defects induced in the femurs of female rats submitted to ovariectomy. Lasers Med Sci 26(4):515–522
Jayasree RS, Gupta AK, Rathinam K, Mohanan PV, Mohanty M (2001) The influence of photodynamic therapy on the wound healing process in rats. J Appl Biomater 15(3):176–186
Tiphlova O, Karu T (1989) Role of primary photoacceptors in low-power laser effects: action of He-Ne laser radiation on bacteriophage T4-Escherichia coli interaction. Lasers Surg Med 9(1):67–69
Karu T, Pyatibrat L, Kalendo G (1995) Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol B: Biol 27(3):219–223
Pinheiro AB, Soares LP, Barbosa AS, Ramalho LP, Santos J (2012) Does LED phototherapy influence the repair of bone defects grafted with MTA, bone morphogenetic proteins, and guided bone regeneration? A description of the repair process on rodents. Lasers Med Sci 27(5):1013–1024
Ribeiro TP, Nascimento SB, Cardoso C, Alessandra D, Hage R, Almeida JD, Arisawa EAL (2012) Low-level laser therapy and calcitonin in bone repair: densitometric analysis. Int J Photoenergy. doi:10.1155/2012/829587
Omasa S, Motoyoshi M, Arai Y, Ejima K, Shimizu N (2012) Low-level laser therapy enhances the stability of orthodontic mini-implants via bone formation related to BMP-2 expression in a rat model. Photomed Laser Surg 30(5):255–261
Kawasaki K, Shimizu N (2000) Effects of low-energy laser irradiation on bone remodeling during experimental tooth movement in rats. Lasers Surg Med 26(3):282–291
Ninomiya T, Hosoya A, Nakamura H, Sano K, Nishisaka T, Ozawa H (2007) Increase of bone volume by a nanosecond pulsed laser irradiation is caused by a decreased osteoclast number and an activated osteoblasts. Bone 40(1):140–148
Kim YD, Kim SS, Hwang DS, Kim SG, Kwon YH, Shin SH, Kim UK, Kim JR, Chung IK (2007) Effect of low-level laser treatment after installation of dental titanium implant-immunohistochemical study of RANKL, RANK, OPG: an experimental study in rats. Lasers Surg Med 39(5):441–450
Fujita S, Yamaguchi M, Utsunomiya T, Yamamoto H, Kasai K (2008) Low-energy laser stimulates tooth movement velocity via expression of RANK and RANKL. Orthod Craniofac Res 11(3):143–155
Matsumoto MA, Ferino RV, Monteleone GF, Ribeiro DA (2009) Low-level laser therapy modulates cyclo-oxygenase-2 expression during bone repair in rats. Lasers Med Sci 24(2):195–201
Ribeiro DA, Matsumoto MA (2008) Low-level laser therapy improves bone repair in rats treated with anti-inflammatory drugs. J Oral Rehabil 35(12):925–933
Fukuhara E, Goto T, Matayoshi T, Kobayashi S, Takahashi T (2006) Optimal low-energy laser irradiation causes temporal G2/M arrest on rat calvarial osteoblasts. Calcif Tissue Int 79(6):443–450
Ozawa Y, Shimizu N, Kariya G, Abiko Y (1998) Low-energy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone 22(4):347–354
Barbosa D, Souza R, Xavier M, Silva F, Arisawa E, Villaverde A (2013) Effects of low-level laser therapy (LLLT) on bone repair in rats: optical densitometry analysis. Lasers Med Sci 28(2):651–656
Janet CG, Barbee RW, Bielitzki JT, Clayton LA, Donovan JC, Hendriksen CFM, Kohn DF, Lipman NS, Locke PA, Melcher J, Quimby FW, Turner PV, Wood GA, Würbel H (2011) Guide for the care and use of laboratory animals, 8th edn. National Academies Press, Washington DC
Nagata M, Messora M, Okamoto R, Campos N, Pola N, Esper L, Sbrana M, Fucini S, Garcia V, Bosco A (2009) Influence of the proportion of particulate autogenous bone graft/platelet-rich plasma on bone healing in critical-size defects: an immunohistochemical analysis in rat calvaria. Bone 5(2):339–345
Acknowledgement
The authors would like to thank CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the fellowship to LM and Wilson Orcini and Maira Couto for the technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marques, L., Holgado, L.A., Francischone, L.A. et al. New LLLT protocol to speed up the bone healing process—histometric and immunohistochemical analysis in rat calvarial bone defect. Lasers Med Sci 30, 1225–1230 (2015). https://doi.org/10.1007/s10103-014-1580-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-014-1580-x