Abstract
Epilepsy is a chronic brain disease with a global prevalence of 70 million people. According to the World Health Organization, roughly 5 million new cases are diagnosed every year. Anti-seizure drugs are the treatment of choice. However, in roughly one third of the patients, these drugs fail to produce the desired effect. As a result, finding novel treatments for epilepsy becomes inevitable. Recently, angiotensin receptor blockers have been proposed as a treatment to reduce the over-excitation of neurons in epilepsy. For this purpose, we conducted a review using Medline/PubMed and Google Scholar using the relevant search terms and extracted the relevant data in a table. Our review suggests that this novel approach has a very high potential to treat epilepsy, especially in those patients who fail to respond to conventional treatment options. However, more extensive and human-based trials should be conducted to reach a decisive conclusion. Nevertheless, the use of ARBs in patients with epilepsy should be carefully monitored keeping the adverse effects in mind.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is one of the most common chronic brain diseases, with a prevalence of about 1–2% across the globe. Globally, 70 million people are estimated to have epilepsy, and according to the World Health Organization, 5 million new cases are diagnosed yearly [1,2,3]. It occurs due to disruption of the regular electrical activity in the brain and is characterized by recurrent seizures—which are sudden, short-lived, excessive, and result due to rapid firing of the neurons leading to transient alterations of brain function such as rhythmic muscular contractions, loss of tone, and loss of consciousness [4]. While a seizure is a single event of transitory signs and symptoms, it is usually termed epilepsy only when multiple seizures occur [3]. The epileptic seizures can be classified based on their appearance and the part of the cerebral cortex involved and can be focal or generalized in onset [5].
The diagnostic criteria for epilepsy include two or more unprovoked seizures 24 h apart, complete recovery between the two episodes, a single unprovoked seizure with a greater than 60% risk of another attack in the next 10 years, or those with epilepsy syndrome [3]. It is investigated using a diverse spectrum of investigations, including electroencephalogram (EEG), CT scan/MRI of the brain, intensive monitoring, genetic testing, and biochemical markers such as brain proteins like S100B or neuronal specific enolase, and neuroinflammatory proteins[6,7,8]. Epilepsy is considered a benign condition; in most cases, treatment is effective without any significant complications. Generally, the prognosis depends upon multiple factors such as the cause, type, and number of seizures; EEG abnormalities; early response to treatment; and other neurological symptoms [9].
The initial approach to managing epilepsy is using anti-seizure medications (ASMs). There are over 20 types of anti-seizure drugs available for use in various types of epilepsy syndromes with the aim to attain seizure freedom in about two-thirds of the patients [10, 11]. These drugs can be classified into narrow-spectrum and broad-spectrum agents and include the traditional phenytoin, phenobarbital, primidone, carbamazepine, valproic acid, and ethosuximide. The narrow-spectrum agents work only for certain types of seizures and worsen the prognosis for absence and myoclonic seizures, whereas broad-spectrum agents have better efficacy. The older agents have more adverse effects including fatigue, gastrointestinal symptoms, cognitive dysfunction, and mood changes. The choice of drug depends upon the type of seizure, the etiology, tolerance, and response in the patient, and the impact of side effects compared with the therapeutic effect [7]. Other treatment options include resection surgery, neuromodulation therapies such as deep brain stimulation (DBS) and vagus nerve stimulation (VNS), precision medicine, gene therapy, stem cell therapy, and anti-seizure devices for neurostimulation. However, these modalities have benefitted very few individuals and are associated with severe and life-threatening adverse events [12, 13]. There is a need to search for novel drugs and therapeutic approaches because the current modalities fail to work in a staggering one third of patients with epilepsy who are forced to try unconventional procedures to find a cure for their condition [11]. Apart from the patients with drug-resistant epilepsy (DRE) suffer from premature death, poor quality of life, and psychosocial disturbances, and for them, the development of new drugs is of utmost importance.
Recently, angiotensin receptor blockers (ARBs) have emerged as a potential drug in preclinical studies to decrease the overfiring of the neurons. These agents alter the renin–angiotensin–aldosterone system (RAAS), influencing the brain’s physiology and pathology. Furthermore, a genetic link between RAAS and epilepsy has also been established [14]. In a study conducted by Corina et al., involving 168,612 hypertensive patients treated with different drug therapies, and among 42,153 patients who received ARB therapy, there was a significant reduction in the incidence of epilepsy which highlights that fact that ARBs can be considered a novel approach in its therapeutic plan [15].
Methods
The relevant articles for this review were searched on Medline/PubMed and Google Scholar. Our search string comprised of “Angiotensin Receptor Blockers,” “ARBS,” “Epilepsy,” “Seizures,” and “Recurrent Seizures.” Articles in any language other than English were excluded. The final list of articles was generated based on relevance to our topic.
Mechanism of action of angiotensin receptor blockers
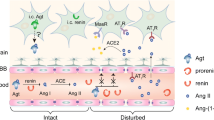
Angiotensin receptor blockers are widely used in treating hypertension. As the name indicates, they block the angiotensin II type 1 (AT1) receptors and prevent angiotensin II from binding to it. In doing this, ARBs disrupt the RAAS, which contributes to the body’s fluid and electrolyte balance [16]. The juxtaglomerular apparatus in the distal convoluted tubule detects a decrease in renal blood flow which triggers the secretion of the enzyme renin from the kidney that acts on angiotensinogen released by the liver to produce angiotensin I (Ang I), which is then converted to angiotensin II (Ang II) by the angiotensin-converting enzyme (ACE). Angiotensin II has multiple effects ranging from vasoconstriction to an increase in sympathetic activity and the release of aldosterone [17].
Even though the exact way these agents affect the brain is not known, they are now becoming famous for their neuroprotective actions [18]. Various studies have now established that Ang II is also a pro-inflammatory, proliferative, and pro-fibrotic agent that mediates the release of cytokines; promotes reactive oxygen species (ROS) production, apoptosis, cell growth migration, and differentiation; regulates gene expression and various intracellular signaling pathways; activates NFκB; and increases oxidative stress, etc.—mechanisms that can precipitate tissue injury [19].
While RAAS is majorly a peripherally acting system, all components have been found to have an impact on the brain, where they actively regulate several mechanisms, including exploratory behavior, stress, anxiety, learning, and memory acquisition [20]. Hence, Ang II is also considered a significant neurotransmitter because of its action on AT1 and AT2 receptors found in various brain regions [21]. A study from 2016 also discusses the presence of AT4 apart from AT1 and AT2 receptors in different areas of the brain, e.g., the area postrema, amygdala, caudate putamen, cerebellum, cortex, globus pallidus, hippocampus, lateral and medial septal areas, mesencephalon, and thalamus [22].
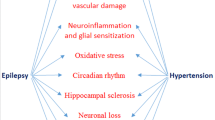
As depicted in Fig. 1, ARBs have been shown to improve cerebral blood flow, reduce cerebral hemorrhage, maintain the function of the blood–brain barrier, ameliorate the inflammatory effects, and protect neurons from apoptosis. By acting as a barrier between the AT1 receptor and angiotensin II, ARBS prevents damage to the brain and exerts a therapeutic effect that warrants their use in many brain disorders, including epilepsy [23, 24]. These drugs also influence the release of certain neurotransmitters; for example, they suppress the secretion of glutamate in response to oxygen-glucose depletion and reduce the production of nitric oxide and reactive oxygen species, which can cause harm to brain tissue [25]. Alpha-synuclein, a protein that accumulates and manifests in degenerative disorders like Parkinson’s, also works through the AT1 receptor, increasing its expression multifold. The use of ARBs in such conditions shields the dopaminergic neurons from the harmful effects of the pro-inflammatory cytokines TNF, IL-6, IL-1B, etc. [26].
Angiotensin system and epilepsy
Inevitably, RAAS would have a pathophysiological role in numerous neurodegenerative disorders as has been discovered in Alzheimer’s, Parkinson’s [27], and Huntington’s [28] diseases. One of the recently established disorders displaying a role of RAAS is epilepsy, on which extensive clinical trials have been conducted over the last decade. Currently, data on the parts played by brain RAAS components on comorbid conditions in epilepsy is inadequate. Usually, the brain maintains a balance between excitation and inhibition—remaining receptive to stimuli as well as resistant to uncontrolled unsolicited activity—via γ-aminobutyric acid (GABA) and glycine as the primary inhibitory neurotransmitters and glutamate as the main stimulatory transmitter. The upset of this narrowly regulated balance predisposes to dysfunctional neural action potential transmission and seizures resulting from high neuronal excitability. Over the last few years, scientists have collected growing evidence of upregulated levels of Ang II peptides, ACE molecules, Ang II receptors, and mRNA expression in limbic regions of the brain, creating a lower threshold for seizures, e.g., in the hippocampus of both genetic rat models [29] and those with an acquired temporal lobe epilepsy induced by pilocarpine [30], as well as human patients with temporal lobe epilepsies [31].
Further research has discovered that Ang II has a dual effect on predisposition to seizures, depending upon the levels present in brain RAAS. At physiological levels, in acute seizure tests and naive subjects of the experiment, Ang II, Ang III, and Ang IV displayed anticonvulsant activity [32]. Particularly, Ang II and AT1 receptors alleviated seizure susceptibility [29, 33, 34]. However, chronic administration of intracerebroventricular Ang II in status epilepticus rats decreased the latent state. It increased the frequency of seizures [32], reiterating that increased levels of RAAS components cause a lower threshold of seizure vulnerability.
With Ang II as the major active peptide of RAAS mediating numerous mechanisms, it is crucial to understand its role in nervous action potential transmission. Interestingly, Ang II has been discovered to have a dual effect in terms of neuronal excitability, depending on the type of receptor it will activate. While AT1 is excitatory in nature, AT2 is inhibitory, as reviewed in [35,36,37]. A study from 2019 [38] explains the pathological role of RAAS in epilepsy as hyperactivating AT1 and ACE signaling in neuronal cells, e.g., astrocytes, oligodendrocytes, and microglia via its pro-inflammatory properties and blood–brain barrier dysfunction.
While anticonvulsants are the mainstay of therapy, they are primarily for symptom control rather than cure. Apart from this, there are reports of adverse effects involving behavioral changes, metabolic upset, and neurotoxicity [38].
With the established upregulation of Ang II in brain RAAS associated with seizures, it opens new gateways to intervene in the pathophysiological mechanisms that mediate a seizure, i.e., by administering agents to block the effect of brain RAAS active peptides. Recent research has explored the effectiveness of using Ang II receptor blockers (ARBs) to control the vulnerability to epileptic syndromes by blocking the inflammatory and tissue toxic effects of Ang II. The AT1 receptor antagonists have anticonvulsant, anti-inflammatory, antioxidant, behavioral, and neuroprotective properties in the epileptic state [32].
Evidence of ARBs in epilepsy
Traumatic or non-traumatic brain injury stands out as a highly concerning global health concern, often accompanied by significantly worrisome delayed neurological complications. Among these complications, post-traumatic epilepsy emerges as a particularly challenging condition (37). As mentioned earlier, brain injury has been associated with the gradual breakdown of the blood–brain barrier as well as an upregulation of ATR1 resulting in increased activation of the serum-derived albumin-induced TGF-β signaling pathways. Due to the absence of a specific FDA-approved TGF-β antagonist, losartan was initially used because of its inhibitory potential on this signaling pathway based on previous studies [14, 39]. ARBs antagonize the ATR1 and the TGF-β, resulting in a delayed breakdown and suppressing neuroinflammation. Evidence is obtained via several studies indicating a reduction in seizure frequency and epilepsy-related comorbidities such as cognitive dysfunction. A study conducted on rats suggested that the use of losartan curtails the neurotoxicity and oxidative stress in the ventrolateral medulla brought about by status epilepticus. Another study demonstrated longer seizure-free periods, reduced seizure frequency, and a better adaptation of epilepsy-associated behavioral changes with the use of losartan [40]. Not only this but losartan has also been hypothesized to reduce epilepsy-related mortality, in Sprague–Dawley rats, via the downregulation of superoxide anion generation [41]. Promising findings from the experiments demonstrate that including an ARB, specifically losartan, in standard anti-seizure treatment significantly reduces the occurrence of epileptic episodes by approximately 60%. A study involving rats and the amygdala kindling model discovered that administering losartan 1 h before inducing seizures effectively elevated the threshold stimuli needed to trigger seizures. Additionally, a separate investigation observed increased kindling stimulations required to generate an epileptic state. Notably, some rats exhibited an inability to reach the epileptic condition altogether when treated with ARBs [42].
These neuroprotective effects of ARBs, especially in epileptic scenarios, have been explored in several animal models to establish the association of ARBs in mitigating epilepsy. Some of the recent animal and human studies are highlighted in Table 1. An animal study was conducted by Pereira et al. on Wistar rats and WARs (Wistar audiogenic rats) to explore the relationship between RAAS inhibitors and temporal lobe epilepsy in a controlled environment. The rats were divided into three groups, and each group was pre-treated orally with enalapril (ACE inhibitor), losartan (ARB), and vehicle (water), respectively, for 21 days. After 7 days of treatment, acoustic stimulation was conducted until seizures appeared. These seizures were then gauged using the mesencephalic severity index and Racine’s scale. The results showed that losartan had a curbing effect on temporal lobe epilepsy and tonic–clonic seizures in rats [29]. Hanael et al. conducted a similar animal study on dogs. Ten dogs with idiopathic epilepsy were treated with telmisartan in addition to the antiepileptic protocol over 4 months. The pre- and post-treatment seizure frequencies were compared, and their serum creatinine and mean arterial pressure were recorded regularly; the results showed that seven out of ten dogs had less frequent seizures after being treated with telmisartan with no significant side effects causing drug discontinuation [43]. The outcomes of both these studies were at par with one another; however, the study conducted by Łukawski et al. on rats had a different outcome. In this investigation, an assortment of ACE inhibitors (namely captopril and enalapril), as well as AT1 antagonists (including losartan, telmisartan, and candesartan), was scrutinized in their efficacy against pentylenetetrazole (PTZ)-induced seizures in male Swiss mice. Several animals, with eight mice per group, were administered various doses of PTZ alone or in conjunction with anti-hypertensive medications. The rodents were individually situated and closely monitored for a duration of 30 min to observe the manifestation of clonic seizures. Among the examined drugs, solely captopril exhibited a discernible protective effect against convulsions brought about by PTZ, while the ARBs administered showed insignificant anticonvulsant effects [44]. To delve into the effect of ARBs in inhibiting TGF-β, an experiment was conducted. This experiment aimed to investigate the molecular mechanisms underlying albumin-induced TGF-β signaling in the brain and evaluate the efficacy of losartan as a preventive treatment for epilepsy. The study utilized primary neuronal cortical cultures and astrocytic cultures in rat models. Various reagents, including losartan and albumin, were employed. Protein and gene expression analysis techniques were utilized to assess the effects of albumin and TGF-β signaling. The outcomes revealed that albumin-induced TGF-β signaling led to rapid transcriptional changes, astrocytic transformation, inflammatory signaling, and downregulation of GABA-related genes, increasing network excitability. Losartan, an FDA-approved angiotensin II type 1 receptor antagonist, showed potential as a blocker of TGF-β signaling and preventive treatment for epilepsy [45].
To scrutinize this association with humans, a cohort study was conducted on patients with established underlying hypertension on one or more anti-hypertensive drugs. The IQVIA database was used to obtain data from around 168,612 patients. Propensity score matching was performed, and a Cox regression model was used to analyze the effectiveness of ARBs on hypertensive epileptic patients. The study’s results demonstrated a noteworthy reduction in the occurrence of epileptic episodes with the use of ARB in contrast to other anti-hypertensives [15]. Contrary to all these in vivo studies, an in vitro experiment conducted in 2019 on brain slices produced contrasting results. In this experiment, 35 human brain slices were obtained from 8 hippocampi surgically resected from patients with drug-resistant temporal lobe epilepsy. The slices were closely evaluated for the effect of losartan, and the amplitudes, rates, and duration of the epileptic events were recorded using glass electrodes. According to this experiment, losartan had no significant effect on the epileptiform activity in the human brain slices, and the mean amplitudes, rates, and durations remained unchanged. The unpredictable results of this experiment may be owed to its in vitro nature since multiple pathophysiological pathways influence in vivo studies [46].
Synergistic drug interactions of ARBs in epilepsy
Drug metabolism plays a crucial role in drug interactions, and some drugs alter the activity of certain enzymes involved in drug metabolism such as cytochrome P450 (CYP) [48]. It is mostly expressed in liver cells, but other organs including kidney, skin, and adrenal glands are also known to express it [49]. According to the available literature, it has been found that pharmacokinetic interactions of losartan with other drugs are mainly mediated via CYP2C9 and CYP3A4. Similarly, the role played by these enzymes in the metabolism of valsartan, candesartan, irbesartan, and azilsartan is moderate, whereas CYP has practically no role in the metabolism of telmisartan, eprosartan, and olmesartan [48].
The interaction between ARBs (particularly losartan and telmisartan) and anti-seizure drugs (AED), both old and new, was investigated in a model of maximal electroshock in mice conducted by Łukawski et al. [50, 51]. ARBs failed to enhance the action of phenobarbital, phenytoin, and carbamazepine. Still, telmisartan and losartan profoundly increased the anticonvulsant activity of valproate by its pharmacodynamic action (total AED concentration in the brain remained unchanged) [50]. Similarly, newer anti-seizure drugs also showed variable results. Losartan potentiated the positive effects of lamotrigine (via pharmacodynamics) [51] and gabapentin (via pharmacokinetics) [52], while telmisartan yielded a similar result with topiramate (via pharmacokinetics) [51]. The pharmacokinetic effect led to an increase in the anti-seizure drug’s total brain and plasma concentration. Oxcarbazepine and tiagabine failed to show any change in their activity when it was given with losartan and telmisartan. Likewise, levetiracetam did not exhibit any increase in its anticonvulsant activity when it was given with losartan and candesartan [53].
Coadministration of gabapentin and losartan demonstrated an increased risk of neurotoxicity and warranted more caution in its use. Similarly, tiagabine, when given with either losartan or telmisartan, showed a greater risk of developing motor impairment [14].
Conclusion
This review has identified the potential role of ARBs in treating patients with epilepsy which if explored further can be groundbreaking. However, our study was limited by the absence of human-based trials, and still, there is a long way to consider ARBs for epilepsy. More in-depth research on pharmacokinetics and pharmacodynamics along with a focus on human trials must be carried out to accurately extrapolate and navigate the efficacy of these drugs on epileptic patients. Another important practical implication is that both short- and long-term adverse effects along with appropriate dosing must be reported to evaluate the safety and tolerability of ARBs for epilepsy.
References
Thijs RD, Surges R, O’Brien TJ, Sander JW (2019) Epilepsy in adults. Lancet 393(10172):689–701. https://doi.org/10.1016/S0140-6736(18)32596-0
“Epilepsy.” [Online]. Available: https://www.who.int/news-room/fact-sheets/detail/epilepsy. Accessed: 18 Oct 2023
Falco-Walter J (2020) Epilepsy-definition, classification, pathophysiology, and epidemiology. Semin Neurol 40(6):617–623. https://doi.org/10.1055/s-0040-1718719
“Epilepsy.” [Online]. Available: https://www.who.int/news-room/fact-sheets/detail/epilepsy. Accessed: 08 Jul 2023
“Epilepsy: a clinical overview - PubMed.” [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/33775643/. Accessed: 18 Oct 2023
“The role of genetic testing in epilepsy diagnosis and management: expert review of molecular diagnostics: Vol 17, No 8.” [Online] Available: https://www.tandfonline.com/doi/full/10.1080/14737159.2017.1335598. Accessed: 08 Jul 2023
“Diagnosis and treatment of epilepsy | psychiatric services.” [Online]. Available: https://ps.psychiatryonline.org/doi/abs/10.1176/ps.34.6.540. Accessed: 08 Jul 2023
RK Banote, S Akel, J Zelano (2022) “Blood biomarkers in epilepsy”. Acta Neurol Scand 146(4). https://doi.org/10.1111/ane.13616
“The natural history and prognosis of epilepsy - Beghi - 2015 - Epileptic Disorders - Wiley Online Library.” [Online]. Available: https://onlinelibrary.wiley.com/doi/10.1684/epd.2015.0751. Accessed: 18 Oct 2023
Perucca P, Scheffer IE, Kiley M (2018) The management of epilepsy in children and adults. Med J Aust 208(5):226–233. https://doi.org/10.5694/mja17.00951
“Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options | pharmacological reviews.” [Online]. Available: https://pharmrev.aspetjournals.org/content/72/3/606. Accessed: 08 Jul 2023
Mesraoua B et al (2019) Novel therapies for epilepsy in the pipeline. Epilepsy Behav 97:282–290. https://doi.org/10.1016/j.yebeh.2019.04.042
“Neuromodulation in epilepsy: state-of-the-art approved therapies - The Lancet Neurology.” [Online]. Available: https://www.thelancet.com/journals/laneur/article/PIIS1474-4422(21)00300-8/fulltext. Accessed: 08 Jul 2023
“IJMS | Free Full-Text | Role of the angiotensin pathway and its target therapy in epilepsy management.” [Online]. Available: https://www.mdpi.com/1422-0067/20/3/726. Accessed: 08 Jul 2023
Doege C, Luedde M, Kostev K (2022) Association between angiotensin receptor blocker therapy and incidence of epilepsy in patients with hypertension. JAMA Neurol 79(12):1296–1302. https://doi.org/10.1001/jamaneurol.2022.3413
“Angiotensin receptor blockers: new considerations in their mechanism of action - Sica - 2006 - The Journal of Clinical Hypertension - Wiley Online Library.” [Online]. Available: https://onlinelibrary.wiley.com/doi/10.1111/j.1524-6175.2005.05141.x. Accessed: 08 Jul 2023
Patel S, Rauf A, Khan H, Abu-Izneid T (2017) Renin-angiotensin-aldosterone (RAAS): the ubiquitous system for homeostasis and pathologies. Biomed Pharmacother 94:317–325. https://doi.org/10.1016/j.biopha.2017.07.091
“Angiotensin II causes neuronal damage in stretch-injured neurons: protective effects of losartan, an angiotensin T1 receptor blocker | SpringerLink.” [Online]. Available: https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s12035-017-0812-z. Accessed: 08 Jul 2023
“Angiotensin II revisited: new roles in inflammation, immunology and aging | EMBO Molecular Medicine.” [Online]. Available: https://www.embopress.org/doi/full/10.1002/emmm.201000080. Accessed: 08 Jul 2023
“Brain angiotensin II: new developments, unanswered questions and therapeutic opportunities | SpringerLink.” [Online]. Available: https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s10571-005-4011-5. Accessed: 08 Jul 2023
Wright JW, Harding JW (2011) Brain renin-angiotensin—a new look at an old system. Prog Neurobiol 95(1):49–67. https://doi.org/10.1016/j.pneurobio.2011.07.001
Singh KD, Karnik SS (2016) Angiotensin receptors: structure, function, signaling and clinical applications. J Cell Signal 1(2):111. https://doi.org/10.4172/jcs.1000111
“Neuroprotective effects of angiotensin receptor blockers | American Journal of Hypertension | Oxford Academic.” [Online]. Available: https://academic.oup.com/ajh/article/28/3/289/145322. Accessed: 08 Jul 2023
“The renin-angiotensin system and the cerebrovascular diseases: experimental and clinical evidence | Bentham Science.” [Online]. Available: https://www.eurekaselect.com/article/103034. Accessed: 08 Jul 2023
“Angiotensin receptor type 1 antagonists protect against neuronal injury induced by oxygen–glucose depletion - Wu - 2010 - British Journal of Pharmacology - Wiley Online Library.” [Online]. Available: https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/j.1476-5381.2010.00840.x. Accessed: 18 Oct 2023
Rodriguez-Perez AI et al (2018) Angiotensin type 1 receptor antagonists protect against alpha-synuclein-induced neuroinflammation and dopaminergic neuron death. Neurotherapeutics 15(4):1063–1081. https://doi.org/10.1007/s13311-018-0646-z
ArrazolaSastre A et al (2020) Small GTPases of the Ras and Rho families switch on/off signaling pathways in neurodegenerative diseases. Int J Mol Sci 21(17):6312. https://doi.org/10.3390/ijms21176312
Qu L et al (2019) The Ras superfamily of small GTPases in non-neoplastic cerebral diseases. Front Mol Neurosci 12:121. https://doi.org/10.3389/fnmol.2019.00121
“Inhibition of the renin–angiotensin system prevents seizures in a rat model of epilepsy | Clinical Science | Portland Press.” [Online]. Available: https://portlandpress.com/clinsci/article-abstract/119/11/477/68702/Inhibition-of-the-renin-angiotensin-system?redirectedFrom=fulltext. Accessed: 08 Jul 2023
Gouveia TLF et al (2012) The levels of renin–angiotensin related components are modified in the hippocampus of rats submitted to pilocarpine model of epilepsy. Neurochem Int 61(1):54–62. https://doi.org/10.1016/j.neuint.2012.04.012
Argañaraz GA et al (2008) The renin-angiotensin system is upregulated in the cortex and hippocampus of patients with temporal lobe epilepsy related to mesial temporal sclerosis. Epilepsia 49(8):1348–1357. https://doi.org/10.1111/j.1528-1167.2008.01581.x
Ivanova N, Tchekalarova J (2019) The potential therapeutic capacity of inhibiting the brain renin–angiotensin system in the treatment of co-morbid conditions in epilepsy. CNS Drugs 33(11):1101–1112. https://doi.org/10.1007/s40263-019-00678-4
Tchekalarova JD et al (2014) Antiepileptogenic and neuroprotective effects of losartan in kainate model of temporal lobe epilepsy. Pharmacol Biochem Behav 127:27–36. https://doi.org/10.1016/j.pbb.2014.10.005
Tchekalarova JD et al (2016) Long-term treatment with losartan attenuates seizure activity and neuronal damage without affecting behavioral changes in a model of co-morbid hypertension and epilepsy. Cell Mol Neurobiol 36(6):927–941. https://doi.org/10.1007/s10571-015-0278-3
“Dzau VJ, Bernstein K, Celermajer D, Cohen J, Dahlöf B, Deanfeld J et al (2001) The relevance of tissue angiotensin-converting enzyme: manifestations in mechanistic and endpoint data. Am J Cardiol 88:1–20. https://doi.org/10.1016/S0002-9149(01)01878-1.-Google Search.” Accessed: Jul. 08, 2023. [Online]. Available: https://www.google.com/search?q=Dzau+VJ/2C+Bernstein+K/2C+Celermajer+D/2C+Cohen+J/2C+Dahl/C3/B6f+B/2C+Deanfeld+J/2C+et+al.+The+relevance+of+tissue+angiotensin-converting+enzyme/3A+manifestations+in+mechanistic+and+endpoint+data.+Am+J+Cardiol.+2001/3B88/3A1/E2/80/9320.+https/3A/2F/2Fdoi.org/2F10.1016/2FS0002+-9149(01)01878-1&oq=Dzau+VJ/2C+Bernstein+K/2C+Celermajer+D/2C+Cohen+J/2C+Dahl/C3/B6f+B/2C+Deanfeld+J/2C+et+al.+The+relevance+of+tissue+angiotensin-converting+enzyme/3A+manifestations+in+mechanistic+and+endpoint+data.+Am+J+Cardiol.+2001/3B88/3A1/E2/80/9320.+https/3A/2F/2Fdoi.org/2F10.1016/2FS0002+-9149(01)01878-1&gs_lcrp=EgZjaHJvbWUyBggAEEUYOdIBCDg3MjdqMGo0qAIAsAIA&sourceid=chrome&ie=UTF-8
Kaschina E, Unger T (2003) Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press 12(2):70–88. https://doi.org/10.1080/08037050310001057
“Update on the role of the AT2 receptor : current opinion in nephrology and hypertension.” [Online]. Available: https://journals.lww.com/co-nephrolhypertens/Abstract/2005/01000/Update_on_the_role_of_the_AT2_receptor.11.aspx. Accessed: 08 Jul 2023
“Elucidating the potential side effects of current anti-seizure drugs for epilepsy | Bentham Science.” [Online]. Available: https://www.eurekaselect.com/article/117497. Accessed: 08 Jul 2023
“Should losartan be administered following brain injury?: Expert review of neurotherapeutics: Vol 14, No 12.” [Online]. Available: https://www.tandfonline.com/doi/abs/10.1586/14737175.2014.972945. Accessed: 08 Jul 2023
Tchekalarova J, Loyens E, Smolders I (2015) Effects of AT1 receptor antagonism on kainate-induced seizures and concomitant changes in hippocampal extracellular noradrenaline, serotonin, and dopamine levels in Wistar-Kyoto and spontaneously hypertensive rats. Epilepsy Behav 46:66–71. https://doi.org/10.1016/j.yebeh.2015.03.021
“Brain-derived neurotrophic factor ameliorates brain stem cardiovascular dysregulation during experimental temporal lobe status epilepticus | PLOS ONE.” [Online]. Available: https://journals.plos.org/plosone/article?id=https://doi.org/10.1371/journal.pone.0033527 . Accessed: 08 Jul 2023
Klein P et al (2020) Repurposed molecules for antiepileptogenesis: missing an opportunity to prevent epilepsy? Epilepsia 61(3):359–386. https://doi.org/10.1111/epi.16450
“Telmisartan as an add-on treatment for dogs with refractory idiopathic epilepsy: a nonrandomized, uncontrolled, open-label clinical trial in: Journal of the American Veterinary Medical Association Volume 260 Issue 7 (2022).” [Online]. Available: https://avmajournals.avma.org/view/journals/javma/260/7/javma.20.12.0683.xml. Accessed: 08 Jul 2023
“Effect of ACE inhibitors and AT1 receptor antagonists on pentylenetetrazole-induced convulsions in mice | SpringerLink.” [Online]. Available: https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s10072-014-2040-x. Accessed: 08 Jul 2023
“Losartan prevents acquired epilepsy via TGF‐β signaling suppression - Bar‐Klein - 2014 - Annals of Neurology - Wiley Online Library.” Accessed: Jul. 08, 2023. [Online]. Available: https://onlinelibrary.wiley.com/doi/abs/https://doi.org/10.1002/ana.24147
Reyes-Garcia SZ, Scorza CA, Ortiz-Villatoro NN, Cavalheiro EA (2019) Losartan fails to suppress epileptiform activity in brain slices from resected tissues of patients with drug resistant epilepsy. J Neurol Sci 397:169–171. https://doi.org/10.1016/j.jns.2019.01.008
Russo E et al (2017) Cerebral small vessel disease predisposes to temporal lobe epilepsy in spontaneously hypertensive rats. Brain Res Bull 130:245–250. https://doi.org/10.1016/j.brainresbull.2017.02.003
R. Yang et al., “Drug interactions with angiotensin receptor blockers: role of human cytochromes P450,” Curr. Drug Metab., vol. 17, no. 7, pp. 681–691. [Online]. Available: https://www.eurekaselect.com/article/75897. Accessed: 09 Jul 2023
Zhao M et al (2021) Cytochrome P450 enzymes and drug metabolism in humans. Int J Mol Sci 22(23):12808. https://doi.org/10.3390/ijms222312808
Łukawski K, Janowska A, Jakubus T, Tochman-Gawda A, Czuczwar SJ (2010) Angiotensin AT1 receptor antagonists enhance the anticonvulsant action of valproate in the mouse model of maximal electroshock. Eur J Pharmacol 640(1):172–177. https://doi.org/10.1016/j.ejphar.2010.04.053
“Interactions between angiotensin AT1 receptor antagonists and second‐generation antiepileptic drugs in the test of maximal electroshock - Łukawski - 2014 - Fundamental & Clinical Pharmacology - Wiley Online Library.” [Online]. Available: https://onlinelibrary.wiley.com/doi/10.1111/fcp.12023. Accessed: 08 Jul 2023
Łukawski K, Janowska A, Jakubus T, Raszewski G, Czuczwar SJ (2013) Combined treatment with gabapentin and drugs affecting the renin–angiotensin system against electroconvulsions in mice. Eur J Pharmacol 706(1):92–97. https://doi.org/10.1016/j.ejphar.2013.02.054
Łukawski K, Raszewski G, Czuczwar SJ (2014) Interactions between levetiracetam and cardiovascular drugs against electroconvulsions in mice. Pharmacol Rep 66(6):1100–1105. https://doi.org/10.1016/j.pharep.2014.07.008
Author information
Authors and Affiliations
Contributions
TGS: topic idea, writing, and reviewing; SFSH: writing, editing, and figure; HA: writing, literature search, and table; AIK: writing and compilation; and RM: writing and compilation.
Corresponding author
Ethics declarations
Ethical approval
No ethical approval was required.
Conflict of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shaikh, T.G., Hasan, S.F.S., Ahmed, H. et al. The role of angiotensin receptor blockers in treating epilepsy: a review. Neurol Sci 45, 1437–1445 (2024). https://doi.org/10.1007/s10072-023-07249-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07249-y