Abstract
A growing body of literature has elucidated the involvement of the central renin–angiotensin system (RAS) in various neuropsychiatric diseases. While consensus on the exact mechanism of the central RAS in schizophrenia pathophysiology does not currently exist, increasing evidence reveals promise in harnessing the therapeutic potential of RAS modulation in the treatment of schizophrenia. In this review, we examine how the central RAS affects inflammation, glutamate, dopamine, gamma-aminobutyric acid (GABA), and peroxisome proliferator-activated receptor (PPAR)-γ, all of which are associated with schizophrenia etiology. In addition, a recent study has demonstrated the therapeutic potential of RAS modulators, especially angiotensin II type 1 receptor blockers (ARBs), as adjunctive therapy to the currently available antipsychotic medications for schizophrenia treatment. With a greater understanding of how RAS inhibition directly modulates neurotransmitter balance in the brain, it is possible that compounds with RAS-inhibiting properties could be used to optimize physiological levels of glutamate, dopamine, and GABA, and the balance among the three neurotransmitters, analogously to how antipsychotic medications mediate the dopaminergic pathways. It can be hoped that a novel approach based on this concept, such as adjunctive telmisartan therapy, may offer practical interventional strategies to address currently unmet therapeutic needs in patients with schizophrenia, especially those with treatment-resistant schizophrenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
While consensus on the exact mechanism remains inconclusive, the role of the intrinsic central renin–angiotensin system (RAS) in schizophrenia pathophysiology appears to be multifaceted, spanning neuroinflammation; neurotransmitter homeostasis of glutamate, dopamine, and gamma-aminobutyric acid (GABA); and peroxisome proliferator-activated receptor-γ activity. |
Given such broad-spectrum effects of the central RAS system, compounds with RAS-inhibiting properties (specifically, angiotensin II type 1 receptor blockers with high lipophilicity such as telmisartan), may confer therapeutic effects in patients with treatment-resistant schizophrenia. |
Specific target engagements and restoration of neurotransmitter balance with RAS inhibitors require further investigation. |
1 Introduction
Schizophrenia is ranked the 12th most disabling disorder, and its best-practice treatment outcomes remain dismal [1]. Only 13.5% of patients meet the clinical and functional recovery criteria [2], and one-third are considered to have treatment-resistant schizophrenia (TRS) with persistent symptoms such as delusions and hallucinations [3]. The suboptimal clinical management comes at a cost of $US60 billion in the USA alone every year [4]. Novel treatment approaches targeting alternative neural circuits and pathways are therefore urgently needed.
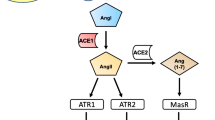
An intrinsic brain renin–angiotensin system (RAS), distinct from the peripheral RAS, was first reported in the seminal article by Ganten et al. [5] in 1971. Subsequent studies have shown a central RAS comprising all precursors and enzymes needed for the formation and metabolism of all biologically active forms of angiotensin, including angiotensinogen (precursor for angiotensins I, II, and III), renin, angiotensin-converting enzyme (ACE), aminopeptidases A and N, and specific receptor proteins [6, 7]. Angiotensin production was stimulated and replicated even after a nephrectomy, and messenger RNA (mRNA) expression for renin and angiotensinogen has been demonstrated in the brain [8, 9]. In addition, all major angiotensin receptors are expressed in the brain [10]. Different RAS components have been identified in various brain regions, including the hippocampus and amygdala, which are involved in cognition, emotion, and behaviors [11]. Furthermore, the established crosstalk between the central and peripheral RAS through circumventricular organs (CVO), the sites that lack the blood–brain barrier (BBB) [12], supports the broad-spectrum role for central RAS: regulation of cerebral spinal fluid (CSF), control of arterial pressure, neuroprotection, memory consolidation, thermoregulation, thirst, and even sexual behavior [13,14,15].
A growing body of literature has elucidated the involvement of the central RAS in various neuropsychiatric diseases, including Alzheimer’s and Parkinson’s diseases [16]. For example, losartan, an angiotensin II type 1 receptor (AT1R) blocker, prevented and rescued cerebrovascular, neuropathological, and cognitive deficits in an Alzheimer’s disease model [17]. Such effects have also been demonstrated in Parkinson’s disease animal models with both ACE inhibitors (captopril and perindopril) and AT1R antagonists (losartan, candesartan, and telmisartan) [18].
Consensus on the exact mechanism of brain RAS in schizophrenia pathophysiology is yet to be established, but increasing evidence reveals the promise in harnessing the therapeutic potential of RAS modulation for schizophrenia treatment. In this review, we examine the multipronged role of the brain angiotensin system in schizophrenia etiology and present systematic implications for RAS inhibition as a novel pharmacotherapeutic approach to refractory schizophrenia.
2 Current Understanding of the Role of the Renin-Angiotensin System (RAS) in Schizophrenia
2.1 RAS, Inflammation, and Schizophrenia
The proinflammatory state in patients with schizophrenia has been well-established [19]. Both Naudin et al. [20] and Lin et al. [21] reported that serum levels of tumor necrosis factor (TNF)-α and interleukin (IL)-6 were significantly higher in patients with chronic schizophrenia than in controls. Various autoimmune diseases, including bullous pemphigoid, acquired hemolytic anemia, thyrotoxicosis, and celiac disease, have also been associated with a higher prevalence of schizophrenia [22]. A recent report on anti-N-methyl-D-aspartate (NMDA) receptor encephalitis psychosis, similar to that of schizophrenia, also shone light on potential direct pathologic immune involvement in the disease.
A large body of literature has suggested that the dysregulation of inflammatory and immunological processes is related to schizophrenia symptomatology. For example, our group first reported that higher serum levels of C-reactive protein (CRP) and white blood cell count (WBC) were correlated with worse psychopathology in patients with schizophrenia [23, 24]. Other studies have also suggested that inflammation may play a role in treatment response in patients with schizophrenia. Among 79 patients with schizophrenia who withdrew from haloperidol treatment up to 6 weeks, those with higher baseline levels of CSF IL-2 were associated with worsening psychotic symptoms during the withdrawal period [25]. In another 12-week study with 78 patients who received either risperidone or haloperidol, patients with lower serum IL-2 level showed greater clinical improvement [26]. More recently, the potential benefit of anti-inflammatory agents in the treatment of schizophrenia has also been examined. Muller et al. [27] used celecoxib, a selective cyclooxygenase (COX)-2 inhibitor, in adjunct to risperidone for patients with acute exacerbation of schizophrenia and found significant improvement in psychopathology. However, these data should be interpreted with caution, as celecoxib did not confer improvement in clinical symptoms or measures of disability in a different study sample of patients with schizophrenia [28].
While overall immune dysregulation in the setting of schizophrenia is no longer in question, the crux of the matter lies in whether such immune dysfunction is directly tied to a pathologic state of the central immune system or is secondary to various clinical and systemic factors associated with schizophrenia, including metabolic syndrome [29]. Albeit scarce compared with that of systemic inflammation, evidence of neuroinflammation in schizophrenia pathophysiology has increasingly been demonstrated by imaging and post-mortem studies [30, 31]. Specifically, in regard to imaging studies, researchers have harnessed the role of microglial activation in response to inflammatory stimuli, which can be captured by the increase in expression of the 18-kDa translocator protein (TSPO) [32] in vivo with positron emission tomography (PET) radiotracers. While various PET studies have replicated the elevated microglial density, ultimately suggesting increased cerebral activation of the immune system [30, 33], these studies include limitations, especially considering their small sample size, different tracer types (i.e., generation of the TSPO ligand), binding affinity, and disease time course. For instance, a recent meta-analysis showed that the statistical significance of elevation of TSPO binding in patients with schizophrenia was dependent on whether volume of distribution (null finding) or binding potential (positive finding) was used as the main outcome measure [30]. Post-mortem studies are also not without their drawbacks, including variable study designs, different methodologic approaches, and the large number of null studies. Despite the heterogeneity, some post-mortem brain studies have shown increased microglial activity and expression of proinflammatory genes [31, 34]. Microarray studies have also consistently demonstrated elevation in the expression of both serpin peptidase inhibitor clade A member 3 (SERPINA3, acute phase protein shown to increase with inflammation) and interferon-induced transmembrane protein (IFITM, immune-related protein suggested to be involved in neuroinflammation) [34], further indicating the role of neuroinflammatory processes in schizophrenia etiology.
The proinflammatory properties of angiotensin II are well-studied. Overactivity of AT1R has been shown to be an integral determinant of uncontrolled and excessive inflammation, alterations in cerebrovascular function, and abnormal response to stress [35, 36]. Some RAS blockers have been shown to have anti-inflammatory and immunomodulatory effects [37]. Aldosterone, an end hormone of the RAS pathway, has also been identified as a major contributor for the central inflammatory process. Following lipopolysaccharide administration, aldosterone has been shown to activate the mineralocorticoid receptors, which then further triggers production and release of proinflammatory cytokines [38].
Numerous studies support the anti-inflammatory effects of AT1R blockers (ARBs). For example, Miura et al. [39] studied the metabolic effects of telmisartan in place of valsartan or candesartan in patients with hypertension and type 2 diabetes mellitus (T2DM). These authors found significant increases in the serum levels of adiponectin and a decrease in the serum levels of CRP, both of which are known to be associated with protection from diabetes mellitus and atherosclerosis [39]. Koulouris et al. [40] also showed positive effects of ramipril, telmisartan, and combination therapy on inflammation and lipid peroxidation in patients with T2DM free of coronary artery disease [40]. A more recent clinical study reported anti-inflammatory and anti-oxidative-stress effects of irbesartan in patients with hypertension [41]. Previous studies collectively have demonstrated that ARBs may exert vascular and metabolic benefits by inhibiting the aforementioned angiotensin-mediated inflammation and oxidative stress. Therefore, using ARBs to inhibit RAS activity may mediate clinical symptoms of schizophrenia through their impact on the upregulated systemic and neuroinflammatory status in patients with schizophrenia.
2.2 RAS, Glutamate, and Schizophrenia
The abnormal presynaptic dopamine transmission usually seen in schizophrenia is absent in TRS [42]. It therefore is no surprise that typical and atypical antipsychotic medications that modulate the dopamine pathway in varying degrees are unsuccessful in conferring clinical improvement in individuals with TRS. Increased glutamate levels have been reported in antipsychotic-naive or antipsychotic-free patients with schizophrenia [43]. Recent studies demonstrated higher glutamate levels in the anterior cingulate cortex of antipsychotic-treated first-episode patients with unremitted psychotic symptoms and in treatment-resistant patients versus medication responders [42, 44]. It has been postulated that abnormal glutamatergic signaling secondary to excessive stimulation of non-NMDA glutamate receptors (e.g., α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid [AMPA] and kainate) may result in calcium influx and neuronal injury, which likely lead to clinical symptoms, including cognitive impairment in patients with schizophrenia [45].
Various studies have shown that the RAS can affect glutamate-mediated cell injury. Angiotensin II type 2 receptor (AT2R) binding was found to be increased a few hours after glutamate exposure, and increased AT2R mRNA was suppressed by MK-801, an NMDA-receptor antagonist [46]. Positive findings also exist for AT1R: Fujita et al. [47] reported suppression of ischemia-induced extracellular glutamate activity and concomitant reduction of reactive oxygen species (ROS) production with administration of candesartan, an AT1R inhibitor. Whether ultimately via AT1R or AT2R, the RAS appears to contribute to glutamate-induced oxidative stress, a proinflammatory mechanism frequently associated with schizophrenia [48].
The ability of ARBs to confer neuroprotection partly results from restoration of neurotransmitter homeostasis. Wu et al. [49] recently described how ARBs attenuate glutamate neurotoxicity by inhibiting oxygen glucose depletion (OGD)-induced extracellular glutamate release, reactive oxygen species production, and nitric oxide generation via glutamate transporter 1 (GLT-1) upregulation. Telmisartan was shown to significantly reduce glutamate-induced neuronal injury and apoptosis in cultured rat primary cerebellar granule cells (CGCs) [50]. Presumably, ARBs such as telmisartan may have therapeutic effects on schizophrenia through their modulatory influence on glutamate activity in the brain.
2.3 RAS, Dopamine, and Schizophrenia
The dopamine hypothesis of schizophrenia postulates that hyperactivity of dopamine neurotransmission in subcortical and limbic brain regions contributes to positive symptoms such as delusions and hallucinations. On the other hand, negative and cognitive symptoms of schizophrenia can be attributed to the hypofunctionality of dopamine neurotransmission in the prefrontal cortex [51]. Recent studies have shown dopamine deficiency extending to most cortical regions and even some extra-striatal subcortical regions not previously considered hypodopaminergic in schizophrenia [52].
Both angiotensin and dopamine receptors are located in neurons, microglia, and astrocytes [53]. Previous reports suggest that a decrease in dopaminergic activity may induce compensatory upregulation of local RAS function in both dopaminergic neurons and glia. The subsequent overactivation of RAS can lead to microglia activation and inflammatory response, resulting in production of oxidative stress and neurotoxicity [54]. Therefore, inhibition of the brain RAS might be an effective neuroprotective strategy against dopaminergic deficit in various cortical and subcortical regions in schizophrenia and ultimately confer clinical improvement, especially in the domains of negative symptoms and cognition [55].
2.4 RAS, Gamma-Aminobutyric Acid (GABA), and Schizophrenia
Among the vast conflicting data regarding schizophrenia pathophysiology, one of the most consistent and well-replicated post-mortem findings is the reduction of mRNA that encodes the 67-kd isoform of glutamic acid decarboxylase (GAD67), an enzyme principally responsible for the synthesis of GABA [56]. GAD67 protein levels were also reported to be lower in patients with schizophrenia [57], which further corroborates the pathologic GABA transmission compromise associated with the disease.
GABA is considered a key player in brain function given its integral role in sustaining synchronous oscillations in cortical networks [58]. A hallmark of neuronal network function, oscillatory activity takes part in input selection, neuroplasticity, and consolidation and combination of learned information in the thalamus, hippocampus, and neocortex [59]. GABA-mediated cortical oscillations in fact have been shown to be critical in wide-ranging cognitive and perceptual processes pertaining to working memory [60], associative learning [61], and visual short-term memory [62]. Therefore, it has been hypothesized that altered cortical GABA transmission is involved in the molecular mechanism of cognitive deficit observed in schizophrenia. For instance, functional hypofrontality, working memory, and physiologic dysfunction of the dorsolateral prefrontal cortex have been linked to schizophrenia, and impaired performance of working memory on cognitive control tasks has been attributed to decreased GABA-mediated oscillatory activity [63, 64]. In fact, Frankle et al. [65] recently reported in vivo neuroimaging evidence of impaired GABA transmission in the context of cognitive disturbance in patients with schizophrenia by measuring the binding of [11C]flumazenil, a radiotracer that binds to the benzodiazepine site of the GABAA receptor.
The interplay between RAS and GABA transmission therefore becomes quite salient, as enhanced GABA activity through RAS modulation could produce cognitive benefits. Studies have revealed the interdependence of the two systems: sympathomimetic responses following acute blockade of GABAA receptors in the paraventricular nucleus (PVN) depends on AT1R activation [66]; bilateral microdialysis of the AT1R blocker ZD7155 into the rostral ventrolateral medulla (RVLM) increases GABA levels [67]. Sanchez-Lemus et al. [68] further revealed that cortical benzodiazepine 1 (BZ1) receptor expression, a modulator for GABA activity, is under AT1R control. They found that candesartan, an ARB, ameliorates stress-induced alterations in BZ1 receptor expression, thereby preventing alterations in the cortical GABAA complex [68]. This finding carries important clinical implications, as restoration of physiological GABA activity could potentially lead to alleviation of cognitive impairment.
Further, the anti-inflammatory effect of ARBs can be attributed partially to restored GABA expression. Being an inhibitory neurotransmitter, GABA carries a parallel inhibitory role in immune function [69]. Pathogenic T-lymphocyte and inflammatory cytokine production in peripheral macrophages were both shown to be mediated by extracellular GABA activity [70, 71]. Therefore, with enhanced GABA level via AT1R blockade, ARBs may confer their beneficial effects in patients with schizophrenia through the anti-inflammatory properties of GABA.
2.5 RAS, Peroxisome Proliferator-Activated Receptor-γ, and Schizophrenia
Peroxisome proliferator activated receptor-γ (PPAR-γ) is a nuclear transcription factor that exists in the form of a heterodimer complex with the retinoid X receptor-α. Activation of PPAR-γ causes the receptor complex to affect the expression of key target genes that mediate beneficial effects on glucose and lipid metabolism. The clinical fame of PPAR-γ is mostly derived from its use as the therapeutic target of insulin resistance, diabetes mellitus, and metabolic syndrome [72]. Two thiazolidinedione PPAR-γ agonists, pioglitazone and rosiglitazone, are approved by the US FDA for diabetes mellitus treatment. In fact, studies have also shown that these PPAR-γ agonists can decrease serum levels of CRP and WBC [73] and reduce nuclear factor (NF)-κB and monocyte chemoattractant protein (MCP)-1, which support the additional anti-inflammatory and antioxidative effects of PPAR-γ agonists [74].
The previously studied metabolic and cardioprotective roles of PPAR-γ have also been extrapolated to the brain. Pioglitazone treatment following traumatic brain injury was found to protect mitochondrial function, reduce inflammation, minimize cortical lesion, and improve cognitive function [75]. Experimental evidence from acute brain injuries, chronic neurodegenerative diseases, and animal models of focal cerebral ischemia, spinal cord injury, multiple sclerosis, and Alzheimer’s and Parkinson’s diseases have revealed that the beneficial effects of PPAR-γ agonists are mediated by the prevention of uncontrolled microglial activation, neutrophil/macrophage infiltration, inflammatory cytokine and chemokine expression, and proinflammatory transcription factor activation, along with promotion of antioxidant enzymatic activity [76].
Interestingly, the current literature supports an interplay between the seemingly unrelated RAS and PPAR-γ systems: PPAR-γ agonists have been shown to downregulate AT1R expression [77] and suppress AT1R-mediated inflammation [78] in vivo, whereas angiotensin II in turn reduces PPAR-γ mRNA expression [79]. In addition, the neuroprotective effects of telmisartan were decreased but not abolished in CGCs of AT1R-knock-out mice, and a PPAR-γ antagonist partially reversed telmisartan-induced neuroprotection [50], further supporting the two-pronged mechanism of telmisartan-induced neuroprotection involving both AT1R blockade and PPAR-γ agonism.
No proof yet exists of a causal relationship between PPAR-γ alterations and schizophrenia. However, a recent study reported an association between the PPAR-γ gene and clinical symptom profiles in patients with schizophrenia [80]. Given the decreased levels of PPAR-γ activity in schizophrenia [81], the use of ARBs with PPAR-γ agonist effects, such as telmisartan, as a therapy for schizophrenia appears promising.
3 Telmisartan: A Potentially Novel Treatment for Schizophrenia
We recently reported the positive clinical impact of adjunctive telmisartan treatment in patients with schizophrenia or schizoaffective disorder who were receiving either olanzapine or clozapine [82] in a 12-week randomized, double-blind, placebo-controlled study. The subject pool received adjunctive telmisartan 80 mg daily, and its effect on psychopathology and cognition was measured using the Positive and Negative Syndrome Scale (PANSS) and the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB). Albeit findings were negative for change in cognitive measures, the study revealed a significant decrease in the PANSS total score in the telmisartan group (N = 22) compared with the placebo group (N = 21), along with a significant reduction in plasma levels of IL-6, a well-established inflammatory marker. While larger sample sizes and longer treatment durations are warranted to further elucidate the specific clinical benefit of telmisartan therapy, the study attests to the promise of RAS modulation in schizophrenia treatment.
Telmisartan is a drug of particular interest regarding its effect on the central RAS. Among other drugs in the ARB class that cross the BBB, telmisartan is a drug of highest lipophilicity that readily enters the CNS in a dose- and time-dependent manner to block centrally mediated effects of angiotensin II [83, 84]. Its slow clearance from the brain, as seen by consistently high brain/plasma ratio within the PET scanning period, also reflects its central long-acting pharmacokinetics [85]. It is an ARB with not only the strongest anti-inflammatory property but also the highest binding affinity for PPAR- γ [86].
4 Conclusion
Central RAS inhibition can reduce glutamate-mediated excitotoxicity, minimize dopamine deficiency-associated microglial activation, and restore GABA and PPAR-γ activity in the brain as well as reduce inflammatory response and oxidative stress. All these effects may lead to potential benefits in schizophrenia treatment (Fig. 1). The use of RAS modulators, especially ARBs, as an adjunct to the currently available antipsychotic medications for schizophrenia treatment appears potentially promising.
However, more research is still needed to fill the knowledge gap and address the following important questions: (1) the available evidence relating to RAS and glutamate or GABA mostly comes from animal studies, so more human data in both healthy or disease conditions are needed; (2) as the etiology of each of the three major types of symptoms of schizophrenia (positive symptoms such as delusions and hallucinations, negative symptoms such as social withdrawal and flat affect, and cognitive impairment) remains uncertain, which symptoms are more likely associated with RAS is unclear; (3) while the processes that ARBs may affect (inflammation, glutamate, dopamine, GABA, PPAR-γ) could be relevant to the etiology of schizophrenia, no direct evidence yet links these processes and an antipsychotic effect.
Our recent preliminary findings on the effects of adjunctive telmisartan in patients with schizophrenia receiving clozapine or olanzapine are encouraging, but they need to be replicated in larger-scale trials with a longer treatment duration. An ongoing follow-up study in our group will examine the target engagement in the brain of patients with schizophrenia who receive telmisartan treatment. Specifically, we will measure how telmisartan treatment affects glutamate and GABA levels in the brains of patients with TRS using proton magnetic resonance spectroscopy (1H-MRS). With a greater understanding of how RAS inhibition directly modulates neurotransmitter balance in the brain, perhaps compounds with RAS inhibition property could be used to optimize physiological levels of glutamate, dopamine, and GABA, and the balance among the three neurotransmitters, analogously to how antipsychotic medications affect the dopaminergic pathways. It can be hoped that the novel approach, such as adjunctive telmisartan, may offer practical interventional strategies to address currently unmet therapeutic needs in patients with schizophrenia, especially in those with TRS.
References
Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–59.
Jaaskelainen E, Juola P, Hirvonen N, McGrath JJ, Saha S, Isohanni M, et al. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull. 2013;39(6):1296–306.
Meltzer HY. Treatment-resistant schizophrenia–the role of clozapine. Curr Med Res Opin. 1997;14(1):1–20.
Marcus SC, Olfson M. Outpatient antipsychotic treatment and inpatient costs of schizophrenia. Schizophr Bull. 2008;34(1):173–80.
Ganten D, Boucher R, Genest J. Renin activity in brain tissue of puppies and adult dogs. Brain Res. 1971;33(2):557–9.
McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, et al. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol. 2003;35(6):901–18.
Farag E, Sessler DI, Ebrahim Z, Kurz A, Morgan J, Ahuja S, et al. The renin angiotensin system and the brain: new developments. J Clin Neurosci. 2017;46:1–8.
Unger T, Badoer E, Ganten D, Lang RE, Rettig R. Brain angiotensin: pathways and pharmacology. Circulation. 1988;77(6 Pt 2):I40–54.
Dzau VJ, Ingelfinger J, Pratt RE, Ellison KE. Identification of renin and angiotensinogen messenger RNA sequences in mouse and rat brains. Hypertension. 1986;8(6):544–8.
Saavedra JM. Brain angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cell Mol Neurobiol. 2005;25(3–4):485–512.
Tashev R, Stefanova M. Hippocampal asymmetry in angiotensin II modulatory effects on learning and memory in rats. Acta Neurobiol Exp. 2015;75(1):48–59.
Ferguson AV, Bains JS. Electrophysiology of the circumventricular organs. Front Neuroendocrinol. 1996;17(4):440–75.
Ciobica A, Bild W, Hritcu L, Haulica I. Brain renin-angiotensin system in cognitive function: pre-clinical findings and implications for prevention and treatment of dementia. Acta Neurol Belg. 2009;109(3):171–80.
von Bohlen und Halbach O, Albrecht D. The CNS renin-angiotensin system. Cell Tissue Res. 2006;326(2):599–616.
Uijl E, Ren L, Danser AHJ. Angiotensin generation in the brain: a re-evaluation. Clin Sci. 2018;132(8):839–50.
Saavedra JM. Beneficial effects of Angiotensin II receptor blockers in brain disorders. Pharmacol Res. 2017;125(Pt A):91–103.
Ongali B, Nicolakakis N, Tong XK, Aboulkassim T, Papadopoulos P, Rosa-Neto P, et al. Angiotensin II type 1 receptor blocker losartan prevents and rescues cerebrovascular, neuropathological and cognitive deficits in an Alzheimer’s disease model. Neurobiol Dis. 2014;68:126–36.
Perez-Lloret S, Otero-Losada M, Toblli JE, Capani F. Renin-angiotensin system as a potential target for new therapeutic approaches in Parkinson’s disease. Expert Opin Investig Drugs. 2017;26(10):1163–73.
Sirota P, Meiman M, Herschko R, Bessler H. Effect of neuroleptic administration on serum levels of soluble IL-2 receptor-alpha and IL-1 receptor antagonist in schizophrenic patients. Psychiatry Res. 2005;134(2):151–9.
Naudin J, Capo C, Giusano B, Mege JL, Azorin JM. A differential role for interleukin-6 and tumor necrosis factor-alpha in schizophrenia? Schizophr Res. 1997;26(2–3):227–33.
Lin A, Kenis G, Bignotti S, Tura GJ, De Jong R, Bosmans E, et al. The inflammatory response system in treatment-resistant schizophrenia: increased serum interleukin-6. Schizophr Res. 1998;32(1):9–15.
Eaton WW, Byrne M, Ewald H, Mors O, Chen CY, Agerbo E, et al. Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry. 2006;163(3):521–8.
Fan X, Pristach C, Liu EY, Freudenreich O, Henderson DC, Goff DC. Elevated serum levels of C-reactive protein are associated with more severe psychopathology in a subgroup of patients with schizophrenia. Psychiatry Res. 2007;149(1–3):267–71.
Fan X, Liu EY, Freudenreich O, Park JH, Liu D, Wang J, et al. Higher white blood cell counts are associated with an increased risk for metabolic syndrome and more severe psychopathology in non-diabetic patients with schizophrenia. Schizophr Res. 2010;118(1–3):211–7.
McAllister CG, van Kammen DP, Rehn TJ, Miller AL, Gurklis J, Kelley ME, et al. Increases in CSF levels of interleukin-2 in schizophrenia: effects of recurrence of psychosis and medication status. Am J Psychiatry. 1995;152(9):1291–7.
Zhang XY, Zhou DF, Cao LY, Zhang PY, Wu GY, Shen YC. Changes in serum interleukin-2, -6, and -8 levels before and during treatment with risperidone and haloperidol: relationship to outcome in schizophrenia. J Clin Psychiatry. 2004;65(7):940–7.
Muller N, Riedel M, Scheppach C, Brandstatter B, Sokullu S, Krampe K, et al. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry. 2002;159(6):1029–34.
Rapaport MH, Delrahim KK, Bresee CJ, Maddux RE, Ahmadpour O, Dolnak D. Celecoxib augmentation of continuously ill patients with schizophrenia. Biol Psychiatry. 2005;57(12):1594–6.
Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277–86.
Marques TR, Ashok AH, Pillinger T, Veronese M, Turkheimer FE, Dazzan P, et al. Neuroinflammation in schizophrenia: meta-analysis of in vivo microglial imaging studies. Psychol Med. 2018. https://doi.org/10.1017/S0033291718003057.
van Kesteren CF, Gremmels H, de Witte LD, Hol EM, Van Gool AR, Falkai PG, et al. Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry. 2017;7(3):e1075.
Cosenza-Nashat M, Zhao ML, Suh HS, Morgan J, Natividad R, Morgello S, et al. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol. 2009;35(3):306–28.
Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [(11)C]PBR28 PET brain imaging study. Am J Psychiatry. 2016;173(1):44–52.
Trepanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry. 2016;21(8):1009–26.
Saavedra JM, Sanchez-Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: therapeutic implications. Psychoneuroendocrinology. 2011;36(1):1–18.
Phillips MI, de Oliveira EM. Brain renin angiotensin in disease. J Mol Med (Berl). 2008;86(6):715–22.
Capettini LS, Montecucco F, Mach F, Stergiopulos N, Santos RA, da Silva RF. Role of renin-angiotensin system in inflammation, immunity and aging. Curr Pharm Des. 2012;18(7):963–70.
Grossmann C, Gekle M. New aspects of rapid aldosterone signaling. Mol Cell Endocrinol. 2009;308(1–2):53–62.
Miura Y, Yamamoto N, Tsunekawa S, Taguchi S, Eguchi Y, Ozaki N, et al. Replacement of valsartan and candesartan by telmisartan in hypertensive patients with type 2 diabetes: metabolic and antiatherogenic consequences. Diabetes Care. 2005;28(3):757–8.
Koulouris S, Symeonides P, Triantafyllou K, Ioannidis G, Karabinos I, Katostaras T, et al. Comparison of the effects of ramipril versus telmisartan in reducing serum levels of high-sensitivity C-reactive protein and oxidized low-density lipoprotein cholesterol in patients with type 2 diabetes mellitus. Am J Cardiol. 2005;95(11):1386–8.
Umebayashi R, Uchida HA, Okuyama Y, Kakio Y, Hanayama Y, Shikata K, et al. The clinical efficacy of angiotensin II type1 receptor blockers on inflammatory markers in patients with hypertension: a multicenter randomized-controlled trial; MUSCAT-3 study. Biomarkers. 2018. https://doi.org/10.1080/1354750X.2018.1548033.
Demjaha A, Murray RM, McGuire PK, Kapur S, Howes OD. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry. 2012;169(11):1203–10.
de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Stephano S, Favila R, Diaz-Galvis L, et al. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry. 2013;70(10):1057–66.
Egerton A, Brugger S, Raffin M, Barker GJ, Lythgoe DJ, McGuire PK, et al. Anterior cingulate glutamate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharmacology. 2012;37(11):2515–21.
Deutsch SI, Rosse RB, Schwartz BL, Mastropaolo J. A revised excitotoxic hypothesis of schizophrenia: therapeutic implications. Clin Neuropharmacol. 2001;24(1):43–9.
Makino I, Shibata K, Shibaguchi H, Niwa M, Katsuragi T, Furukawa T. The increase in angiotensin type-2 receptor mRNA level by glutamate stimulation in cultured rat cortical cells. Brain Res. 1998;804(2):296–305.
Fujita T, Hirooka K, Nakamura T, Itano T, Nishiyama A, Nagai Y, et al. Neuroprotective effects of angiotensin II type 1 receptor (AT1-R) blocker via modulating AT1-R signaling and decreased extracellular glutamate levels. Invest Ophthalmol Vis Sci. 2012;53(7):4099–110.
Boskovic M, Vovk T, Kores Plesnicar B, Grabnar I. Oxidative stress in schizophrenia. Curr Neuropharmacol. 2011;9(2):301–12.
Wu X, Kihara T, Hongo H, Akaike A, Niidome T, Sugimoto H. Angiotensin receptor type 1 antagonists protect against neuronal injury induced by oxygen-glucose depletion. Br J Pharmacol. 2010;161(1):33–50.
Wang J, Pang T, Hafko R, Benicky J, Sanchez-Lemus E, Saavedra JM. Telmisartan ameliorates glutamate-induced neurotoxicity: roles of AT(1) receptor blockade and PPARgamma activation. Neuropharmacology. 2014;79:249–61.
Toda M, Abi-Dargham A. Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiatry Rep. 2007;9(4):329–36.
Weinstein JJ, Chohan MO, Slifstein M, Kegeles LS, Moore H, Abi-Dargham A. Pathway-specific dopamine abnormalities in schizophrenia. Biol Psychiatry. 2017;81(1):31–42.
Labandeira-Garcia JL, Rodriguez-Pallares J, Dominguez-Meijide A, Valenzuela R, Villar-Cheda B, Rodriguez-Perez AI. Dopamine-angiotensin interactions in the basal ganglia and their relevance for Parkinson’s disease. Mov Disord. 2013;28(10):1337–42.
Rodriguez-Pallares J, Rey P, Parga JA, Munoz A, Guerra MJ, Labandeira-Garcia JL. Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH-derived ROS. Neurobiol Dis. 2008;31(1):58–73.
Labandeira-Garcia JL, Garrido-Gil P, Rodriguez-Pallares J, Valenzuela R, Borrajo A, Rodriguez-Perez AI. Brain renin-angiotensin system and dopaminergic cell vulnerability. Front Neuroanat. 2014;8:67.
Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6(4):312–24.
Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57(11):1061–9.
Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8(1):45–56.
Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–9.
Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13(12):1369–74.
Miltner WH, Braun C, Arnold M, Witte H, Taub E. Coherence of gamma-band EEG activity as a basis for associative learning. Nature. 1999;397(6718):434–6.
Tallon-Baudry C, Kreiter A, Bertrand O. Sustained and transient oscillatory responses in the gamma and beta bands in a visual short-term memory task in humans. Vis Neurosci. 1999;16(3):449–59.
Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155(9):1285–7.
Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43(2):114–24.
Frankle WG, Cho RY, Prasad KM, Mason NS, Paris J, Himes ML, et al. In vivo measurement of GABA transmission in healthy subjects and schizophrenia patients. Am J Psychiatry. 2015;172(11):1148–59.
Chen QH, Toney GM. Responses to GABA-A receptor blockade in the hypothalamic PVN are attenuated by local AT1 receptor antagonism. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R1231–9.
Patel D, Bohlke M, Phattanarudee S, Kabadi S, Maher TJ, Ally A. Cardiovascular responses and neurotransmitter changes during blockade of angiotensin II receptors within the ventrolateral medulla. Neurosci Res. 2008;60(3):340–8.
Sanchez-Lemus E, Honda M, Saavedra JM. Angiotensin II AT1 receptor blocker candesartan prevents the fast up-regulation of cerebrocortical benzodiazepine-1 receptors induced by acute inflammatory and restraint stress. Behav Brain Res. 2012;232(1):84–92.
Bhat R, Axtell R, Mitra A, Miranda M, Lock C, Tsien RW, et al. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci USA. 2010;107(6):2580–5.
Bjurstom H, Wang J, Ericsson I, Bengtsson M, Liu Y, Kumar-Mendu S, et al. GABA, a natural immunomodulator of T lymphocytes. J Neuroimmunol. 2008;205(1–2):44–50.
Reyes-Garcia MG, Hernandez-Hernandez F, Hernandez-Tellez B, Garcia-Tamayo F. GABA (A) receptor subunits RNA expression in mice peritoneal macrophages modulate their IL-6/IL-12 production. J Neuroimmunol. 2007;188(1–2):64–8.
Chiquette E, Ramirez G, Defronzo R. A meta-analysis comparing the effect of thiazolidinediones on cardiovascular risk factors. Arch Intern Med. 2004;164(19):2097–104.
Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, Freed MI. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002;106(6):679–84.
Mohanty P, Aljada A, Ghanim H, Hofmeyer D, Tripathy D, Syed T, et al. Evidence for a potent antiinflammatory effect of rosiglitazone. J Clin Endocrinol Metab. 2004;89(6):2728–35.
Sauerbeck A, Gao J, Readnower R, Liu M, Pauly JR, Bing G, et al. Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Exp Neurol. 2011;227(1):128–35.
Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci. 2008;13:1813–26.
Zhao SM, Shen LH, Li HW, Wang L, Chen H, Wang YL, et al. Down-regulation of the expression of angiotensin II type 1 receptor in neonatal rat cardiac fibroblast by activation of PPARgamma signal pathway. Chin J Physiol. 2008;51(6):357–62.
Ji Y, Liu J, Wang Z, Liu N, Gou W. PPARgamma agonist, rosiglitazone, regulates angiotensin II-induced vascular inflammation through the TLR4-dependent signaling pathway. Lab Invest. 2009;89(8):887–902.
Tham DM, Martin-McNulty B, Wang YX, Wilson DW, Vergona R, Sullivan ME, et al. Angiotensin II is associated with activation of NF-kappaB-mediated genes and downregulation of PPARs. Physiol Genom. 2002;11(1):21–30.
Liu YR, Hu TM, Lan TH, Chiu HJ, Chang YH, Chen SF, et al. Association of the PPAR-gamma gene with altered glucose levels and psychosis profile in schizophrenia patients exposed to antipsychotics. Psychiatry investig. 2014;11(2):179–85.
Martinez-Gras I, Perez-Nievas BG, Garcia-Bueno B, Madrigal JL, Andres-Esteban E, Rodriguez-Jimenez R, et al. The anti-inflammatory prostaglandin 15d-PGJ2 and its nuclear receptor PPARgamma are decreased in schizophrenia. Schizophr Res. 2011;128(1–3):15–22.
Fan X, Song X, Zhao M, Jarskog LF, Natarajan R, Shukair N, et al. The effect of adjunctive telmisartan treatment on psychopathology and cognition in patients with schizophrenia. Acta Psychiatr Scand. 2017;136(5):465–72.
Wincewicz D, Braszko JJ. Telmisartan attenuates cognitive impairment caused by chronic stress in rats. Pharmacol Rep. 2014;66(3):436–41.
Gohlke P, Weiss S, Jansen A, Wienen W, Stangier J, Rascher W, et al. AT1 receptor antagonist telmisartan administered peripherally inhibits central responses to angiotensin II in conscious rats. J Pharmacol Exp Ther. 2001;298(1):62–70.
Noda A, Fushiki H, Murakami Y, Sasaki H, Miyoshi S, Kakuta H, et al. Brain penetration of telmisartan, a unique centrally acting angiotensin II type 1 receptor blocker, studied by PET in conscious rhesus macaques. Nucl Med Biol. 2012;39(8):1232–5.
Kakuta H, Kurosaki E, Niimi T, Gato K, Kawasaki Y, Suwa A, et al. Distinct properties of telmisartan on agonistic activities for peroxisome proliferator-activated receptor gamma among clinically used angiotensin II receptor blockers: drug-target interaction analyses. J Pharmacol Exp Ther. 2014;349(1):10–20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct this review or prepare this manuscript.
Conflict of interest
XF has received research support or honoraria from Alkermes, Neurocrine, Avanir, Allergen, Otsuka, Lundbeck, Boehringer Ingelheim, and Janssen. SJO reports no competing interests.
Rights and permissions
About this article
Cite this article
Oh, S.J., Fan, X. The Possible Role of the Angiotensin System in the Pathophysiology of Schizophrenia: Implications for Pharmacotherapy. CNS Drugs 33, 539–547 (2019). https://doi.org/10.1007/s40263-019-00632-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-019-00632-4