Abstract
Osteoarthritis (OA) represents the most prevalent and disabling arthritis worldwide due to its heterogeneous and progressive articular degradation. However, effective and timely diagnosis and fundamental treatment for this disorder are lacking. Metabolomics, a growing field in life science research in recent years, has the potential to detect many metabolites and thus explains the underlying pathophysiological processes. Hence, new specific metabolic markers and related metabolic pathways can be identified for OA. In this review, we aimed to provide an overview of studies related to the metabolomics of OA in animal models and humans to describe the metabolic changes and related pathways for OA. The present metabolomics studies reveal that the pathogenesis of OA may be significantly related to perturbations of amino acid metabolism. These altered amino acids (e.g., branched-chain amino acids, arginine, and alanine), as well as phospholipids, were identified as potential biomarkers to distinguish patients with OA from healthy individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) represents the most prevalent and disabling arthritis worldwide, affecting several diarthrodial joints, but primarily the knees and hips. Its worldwide incidence rate is approximately 1/10 in male and 1/5 in female over 60 years of age [1]. Our understanding concerning the etiology of OA continues to grow. Many factors, including age, obesity, gender, genetics, and joint injury, have shown to contribute to the development of OA, among which increasing age and obesity are the principal factors [2]. OA is now considered a heterogeneous chronic disease involving multiple joint tissues. It is mainly characterized by the degradation of articular cartilage, subchondral bone sclerosis, osteophyte formation, variable degrees of synovitis, and ligament degeneration, eventually leading to disability in the end state of the disease [3]. The high rates of morbidity and disability associated with OA have led to a reduced quality of life and a great economic burden on society. The economic burden of OA has been estimated to be between 1.0 and 2.5% of the gross domestic product for Western countries [4]. Despite this challenge, there are no effective early diagnostics or main therapeutics for this disease [5]. These statistics could be improved if the understanding of the diagnostic biomarkers and metabolic alterations in OA were clearer [6].
As an emerging field in life science research and a member of the “-omics” family of sciences, metabolomics provides a powerful approach to identify small molecules for several disorders [7]. By measuring and mathematically modeling changes in the levels of products of metabolism, both diagnostic and prognostic biomarkers can be detected and identified for a variety of diseases [8]. Furthermore, metabolomics might provide an opportunity to explain the underlying pathophysiological processes associated with diseases [9, 10]. Metabolite research in OA has had an increase in interest and a relatively rapid development, as the majority of articles related to OA were published in recent years. In this review, we aimed to provide an overview of the studies related to the metabolomics of OA in animal models and humans to describe the metabolic changes and related pathways in the pathogenesis of OA.
Metabolomics
Recently, the metabolomics approach has been used to identify and quantify small molecules in systemic biology for measuring chemical intermediates or products and providing a biochemical “snapshot” of an organism’s metabolic state [11]. The types of samples are diverse, including biological fluids (e.g., plasma, urine, and saliva), cells, and tissue extracts. Metabolomics is an ideal tool to discover biomarkers and understand the systems-level effects of metabolites owing to its inherent sensitivity [12]. As a part of the biological system-based approach, metabolomics belongs to the family of -omics sciences, which also encompasses genomics, transcriptomics, and proteomics. In general, omics studies can define networks in which genetic variation (genomics) leads to changes in gene expression (transcriptomics) to affect protein expression (proteomics). Quite differently, metabolomics is a means for measuring chemical intermediates, or metabolites, in a variety of biological samples. In other words, metabolomics tells us what happened instead of what may have happened. With the aid of metabolomics to overcome the limitations of other -omics sciences, the family could provide valuable information on endogenous and exogenous factors [13]. Consequently, metabolomics brings significant benefits in the diagnosis, therapy assessment, and an understanding of the pathogenesis for many disorders, such as cancer [14] and cardiovascular disease [15]. Furthermore, it may also aid in monitoring the effects of medical interventions [16] and studying autophagy [17]. Metabolomics generally involves three steps: metabolism, spectroscopy, and multivariate statistical analysis [11]. Among these, spectroscopy has proven the most useful in providing data that gives us a more comprehensive understanding of metabolism [18].
Most analytical platforms for metabolic profiling are based on spectroscopic techniques. Currently, there are three leading methods in metabolomics studies: nuclear magnetic resonance (NMR) spectroscopy, liquid chromatography–mass spectrometry (LC–MS), and gas chromatography–mass spectrometry (GC–MS). These have similar abilities in providing comprehensive coverage of an organism’s metabolic state [19]. NMR spectroscopy has important advantages that include being non-invasive, non-destructive, not requiring elaborate sample preparations, and rich in quantitative information over a wide dynamic range. Therefore, it has played a fundamental role in the development of metabolomics research [20]. Furthermore, it is a powerful structural tool to detect information on isomers and molecular structure, of which similar molecular fragmentation may be difficult to acquire in both GC–MS and LC–MS. Contrarily, however, NMR spectroscopy also has some limitations. For example, it is not well suited for low concentrations. Besides, the low sensitivity and limited resolution of individual metabolites from complex samples are challenging [21].
Mass spectrometry-based metabolomics is superior in sensitivity to NMR. GC–MS is generally considered a versatile and reproducible analytical platform for low-molecular-weight and volatile analytes because of its robust, selective, and sensitive nature [22]. Currently, LC–MS is widely used in metabolomics for a broad range of metabolites, including not only metabolites from high- to low-molecular-weight but also from hydrophilic to hydrophobic molecules, due to its high sensitivity and reliable quantitation [19]. However, in contrast to the NMR approach which handles samples quickly and tends to be more reproducible, mass spectrometry-based techniques require complicated sample preparation steps and chemical derivation that may lead to both metabolite loss and differential adduct formation [23]. Overall, these analytical methods are complementary techniques and work for different analytes depending on the experimental objective and the sample type being investigated (Table 1).
The metabolomics of OA
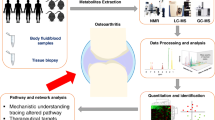
As an ideal tool to discover biomarkers and understand the systems-level effects of several disorders, metabolomics can also help elucidate OA. Figure 1 illustrates the overall flowchart of common metabolomics for OA, including the major steps from collecting samples to obtaining the most relevant pathways involved with OA.
A scheme of the overall flowchart of metabolomics for OA. First, OA samples are collected and extracted for spectroscopy examinations by NMR, LC–MS, and GC–MS. Before Fourier transformations, raw spectral data need to be processed, which includes phase corrections, baseline corrections, and resonance alignment. Subsequently, sample data is normalized and subjected to multivariate statistical analysis, including principle component analysis, a partial least squares discriminant analysis, and orthogonal signal correction partial least squares discriminant analysis. Together with univariate data analysis, characteristic metabolites can be determined. Finally, the metabolic pathway analysis is applied to determine the most relevant pathways involved with OA
OA is best considered a disease of the whole “joint organ.” Hence, the metabolic changes of OA could arise in various periarticular tissues, including articular cartilage [22], subchondral bone [24], and the synovium [25]. Meanwhile, it is well-established that the types of metabolic samples are wide ranging and include biological fluids, cells, and tissue extracts. Evidently, those studies on pathogenic mechanisms of diseases are particularly focused on animal models [26]. However, several population-based OA studies can be found in the literature [27]. In 1989, Williamson et al. first used 1H NMR to investigate synovial fluid (SF) components in patients with joint diseases [28]. Nevertheless, the variations seen in humans are much greater than that of experimental animals, which could pose both problems and advantages for modeling. Thus, the analytical platforms used in animals may be more appropriate or affordable than in patients [29]. Significant metabolic changes in OA in various sample types are discussed below.
Metabolomics of tissues in OA
Tissue analysis is a powerful tool to study the localized responses of diseases and provide relevant metabolic information. A previous trial had investigated cartilage from patients with OA who underwent total knee arthroplasties and from non-OA healthy individuals using high-resolution magic angle spinning NMR spectroscopy. This trial found that the metabolite levels of alanine, N-acetylcholine, and glycine could accurately classify OA and healthy [30]. Another study investigated the synovium from human OA joints by GC–MS and LC–MS and found alterations in 11 metabolites. Among these, seven were present at higher levels in the end-stage of the OA group compared to the control, while four were relatively lower, which specifically indicated altered activities of collagen metabolism, branched-chain amino acids (BCAA) metabolism, energy metabolism, and tryptophan metabolism [31]. Now subchondral bone has received increasing attention in OA due to the pathological changes that of subchondral bone may play an important role in the initiation and progression of OA. The sclerotic subchondral bone from OA patients compared with the non-sclerotic subchondral bone was analyzed by Yang et al. and demonstrated that alteration in taurine and beta-alanine metabolism was clearly found to be associated with the sclerosis of subchondral bone [32].
Metabolomics of biological fluids in OA
Biological fluids are convenient for metabolic research as they are relatively easy to obtain from animal and human subjects [33]. These fluids include urine, saliva, cerebrospinal fluid, SF, semen, plasma, serum, and whole blood. These biofluids provide potential advantages to monitor the state of biological organisms. Therefore, in the future, the metabolomics of biological fluids could play a key role in public health [34].
Blood
Plasma and serum are excellent sources due to the large number of metabolites that can be detected from them, thus contributing to the novel markers associated with the OA risk [34, 35]. Zhang et al. [36] collected plasma from patients with OA and healthy donors. They found that six biochemicals are associated with OA, but arginine played the leading role in discriminating OA from controls. This study suggested that an arginine depletion in OA would result in an imbalance between cartilage repair and damage due to the overactivity of the arginine-to-ornithine pathway. Zhai et al. [37] studied serum-based metabolomics of OA in humans. They compared 123 female knee OA cases and 299 controls in the discovery sample and then designed an independent replication to verify their results. They found that 14 metabolite concentration ratios were significantly correlated to knee OA, especially the ratio of the BCAA to histidine, as well as valine to tryptophan, arginine, and glycine. These findings were later supported by Zhang et al. (2016a) and extended to the male population. Maher et al. [34] studied the serum of sheep that had undergone meniscal destabilization (MD), anterior cruciate ligament transaction (ACLT), or sham operations after 4 weeks and 12 weeks using 1H NMR spectroscopy. Their results suggested that metabolic changes in ACLT were more extensive compared to MD. They documented increased concentrations of dimethyl sulfone in MD after 4 weeks. An increase was also evident in the concentration of 3-methylhistidine and an obvious decreased level in BCAA in ACLT-induced OA over both time points. Furthermore, the increased concentrations of glutamine, creatine, and creatinine after 12 weeks are associated with altered muscle metabolism.
Urine
Urine samples serve as excellent tools compared to other biofluids since they are easy to sample and are non-invasive. Furthermore, urine needs minimum sample preparation due to its lower protein content. These advantages have ensured the widespread use of urine as an analytical tool in clinical practice [38].
A study had examined urine samples by NMR spectroscopy from 22 patients with OA and 22 controls who both underwent the Intensive Diet and Exercise for Arthritis and suggested that differences in metabolism could be useful in the progression of OA [39]. The Intensive Diet and Exercise for Arthritis study showed that a loss of body weight and a reduction of pain were associated with the levels of interleukin-6 and C-reactive protein in overweight and obese adults with knee OA [40]. This group documented increased concentrations of hydroxybutyrate, pyruvate, and glycerol. In addition, they reported an increased ratio of creatine-to-creatinine in patients with OA and elevated levels of methylhistamine and histidine [39]. Another metabolomic study based on GC–MS using urine was able to distinguish patients with OA from healthy controls. This study described significantly elevated levels of aconitate, isocitrate, citrate, and histamine in patients with OA compared with the healthy. In contrast, it also showed lower levels of histidine and glutamine in the urine from patients with OA [41].
Synovial fluid
SF could provide a real-time and joint-specific metabolic profile of OA because it is a bathing solution that is in direct contact with all the tissues of the entire joint. Therefore, SF could allow for comprehensive detection of the diseased joint [42].
Carlson et al. [43] found that 35 metabolites were statistically important in identifying the separation between human OA and healthy SF via LC–MS. These metabolites included phosphatidylcholine (PC), lysophosphatidylcholine (lysoPC), ceramides, myristate derivatives, and carnitine derivatives. Enrichment analyses of these significant metabolites suggest chondroitin sulfate degradation with arginine, proline, and nitric oxide (NO) metabolism upregulated in OA. Another study conducted by Mickiewicz et al. [44] utilized NMR compared SF collected from patients with OA and post mortem samples. Remarkable decreases of methionine, N-phenylacetyl glycine, ethanol, creatine, O-acetyl-carnitine, and 3-hydroxybutyrate concentrations were observed in the OA. Alternatively, fructose and citrate concentrations were higher in OA than in non-OA controls. In an ovine model, the NMR assessed significantly dysregulated in an anterior cruciate ligament reconstruction injury through SF. They measured 65 metabolites, and 6 of them, that were isobutyrate, glucose, hydroxyproline, asparagine, serine, and uridine, were relevant to early post-injury degenerative. Moreover, a large percent of them were associated with the hypoxic and acidotic conditions result from injured and inflamed joint [45].
In summary, several types of biological fluids and tissue extracts of OA have been examined and have shown significant metabolic changes between OA and non-OA (Table 2).
Metabolic pathways likely contributing to OA
During the initiation and development of OA, the majority of metabolomic profiling approaches reveal changes associated with energy metabolism (e.g., glucose metabolism, tricarboxylic acid cycle (TCA), and ß-oxidation pathway), lipid metabolism, the eicosanoid pathway, amino acid metabolism, and other metabolic factors [5]. The present studies reveal that the pathogenesis of OA may be significantly related to perturbations of amino acid metabolism. These altered amino acids (e.g., BCAA, arginine, and alanine), as well as phospholipids, were identified as potential biomarkers to distinguish patients with OA from healthy counterparts.
Branched-chain amino acids
Leucine, isoleucine, and valine are essential amino acids, meaning they are not produced by our bodies and must be taken in as part of our diet. They are also known as BCAA due to their non-linear aliphatic side chains. They make up approximately one-third of skeletal muscle essential amino acids and are essential fuels for body energy metabolism. A number of studies have indicated that significantly dysregulated BCAA concentrations are linked to OA [47, 48]. Recent studies have rekindled BCAA as biomarkers associated with obesity since weight reduction improves the BCAA profile. Moreover, BCAA have been suggested as a major predictor of insulin resistance and cardiovascular diseases [49]. Meanwhile, obesity has long been recognized as a strong risk factor for knee OA, type 2 diabetes, and cardiovascular diseases, and cardiovascular diseases could lead to metabolic OA [50, 51]. BCAA are activators of the mammalian target of rapamycin (mTOR) signaling pathway that regulates a variety of biological functions, such as autophagy [52]. The increased BCAA levels could lead to reduced autophagy and change cell survival and overall tissue homeostasis. BCAA promote mononuclear cell migrations via an activation of mTOR1, which suggests a potential for increased inflammation in OA. BCAA supplements are associated with the increased production of the main proinflammatory cytokines involved in the pathophysiology of OA, including IL-1 and 2, tumor necrosis factor–alpha, and interferon-gamma [53], which are implicated in the degeneration of the articular cartilage matrix [54]. Figure 2 shows a schematic overview of the BCAA metabolic pathway in relation to OA.
Schematic overview of the BCAA metabolic pathway in relation to OA. BCAA are activators of the mTOR signaling pathway that regulates autophagy. The increased BCAA levels would result in reduced autophagy to change tissue homeostasis through this pathway. BCAA promote mononuclear cell migration via an activation of mTOR1 that suggests the potential for increased inflammation in OA
Arginine
Arginine is classified as a semi-essential amino acid in humans and serves as the precursor for the synthesis of many molecules, including urea, NO, proline, glutamate, creatine, and agmatine [55]. Arginine may lead to inflammation-associated diseases, including OA, due to the functions of anti-inflammation and anti-oxidation [56]. Furthermore, recent evidence suggests that arginine concentrations are decreased in patients with OA, which has likely contributed to the progression of OA [57, 58]. Furthermore, there are competing metabolic pathways for arginine as a substrate by arginase (ARG) and NO synthase (NOS). ARG catalyzes L-arginine into L-ornithine in the liver to facilitate the generation of urea. Ornithine can form a metabolic precursor for proline, a key amino acid especially enriched in collagen that contributes to collagen and polyamine synthesis and cell proliferation resulting in fibrosis [59, 60]. Meanwhile, arginine is catabolized to NO and citrulline by NOS [61]. However, the role of NO in the development of OA remains inconclusive. Some studies have suggested that NO and its redox derivatives may play protective roles in a joint [62]. However, other studies have shown a destructive role in mediating the inflammatory response and apoptosis, inhibiting the synthesis of both collagen and proteoglycan, and activating matrix metalloproteinases [43, 63]. A schematic overview of the arginine metabolic pathway in relation to OA is shown in Fig. 3.
Schematic overview of the arginine metabolic pathway in relation to OA. NOS and ARG catalyze the conversion of arginine to NO and ornithine, respectively. Subsequently, ornithine is converted by OAT into proline, a contributor to collagen and polyamine synthesis, resulting in articular degradation. However, the role of NO in the development of OA is still inconclusive
Phospholipids
Phospholipids are important components of the SF that contribute to articular joint lubrication [43]. Kosinski et al. found that SF contains several phospholipid classes including PC, lysoPC, and phosphatidylethanolamine. Moreover, PC is the key phospholipid class in SF. Compared with the controls, SF from patients with OA had higher levels of phospholipids. Therefore, phospholipids, such as PC and lysoPC, may be associated, at least in part, with the pathogenesis of OA [64, 65]. PCs are converted into lysoPCs by phospholipase A2 (PLA2) [66]. The increased lysoPC-to-PC ratio [46] suggests an increased activity of PLA2 to convert PCs to lysoPCs, which reveals either an increased lysoPC concentration or a decreased PC concentration. Furthermore, the deficiency in PCs, especially unsaturated PCs, could lead to articular cartilage damage [67]. PLA2 acts as a chemoattractant at the sites of inflammation and promotes the inflammatory reaction [36], which suggests that PLA2 may be an important effector contributing to OA development. Subsequently, lysophosphatidic acid (LPA) is generated by autotaxin from lysoPCs [68]. An increased level of autotaxin has been detected in patients with OA compared to normal controls [69], and LPA is involved in the development of neuropathic pain and contributes to inflammation [70, 71]. In addition, the increased activity of PC to lysoPC could release free fatty acids, such as arachidonic acid, which may lead to OA joint symptoms, such as cartilage degradation [72]. A schematic overview of the phospholipid metabolic pathway in relation to OA is shown in Fig. 4.
Schematic overview of phospholipid metabolic pathway in relation to OA. PCs are converted into lysoPCs by PLA2, and arachidonic acid, belonging to free fatty acid, is produced by the conversion of PCs to lysoPCs. Deficiencies in PCs and the eicosanoid pathway may lead to articular degradation. Subsequent metabolism of lysoPCs via autotaxin generates LPA, which is involved in the development of neuropathic pain and contributes to inflammation
Alanine
Alanine, one of the 20 amino acids that constitute the proteins of the human body, is synthesized in the liver and obtained from diet. The synthesis of carnosine in skeletal muscle is limited by the availability of beta-alanine. In addition, as a functional amino acid, alanine plays an important role in cell growth and physiological metabolism [73]. It is generally believed that alanine is dysregulated in OA [74, 75]. Through the alanine-glucose cycle, alanine is converted into glutamate and pyruvic acid. Subsequently pyruvic acid participates in the Cori cycle, and then both of them enter the TCA cycle to regulate energy metabolism [76]. Evidently, a decreased level of alanine concentration in cartilage was found that may be associated with degradation of the collagen framework with OA progression [30]. Adenosine triphosphate (ATP), besides being the elementary source of energy in humans, has also been shown to play a vital role in regulating chondrocyte function and repairing damaged cartilage [77], for the reason that the decrease of alanine may be attributed to the depletion of ATP in chondrocytes derived from cartilage. On the contrary, an increased level of alanine concentration related to the sclerosis of subchondral bone was also found [32]. We know that alanine can provide energy to increase work performance and power output in the muscle cell [78]. Energy consumption was increased in bone cells due to the increased activity of the osteoblast and osteoclast in the sclerotic subchondral bone. Therefore, it is supposed that alanine might have a similar role to increase energy consumption in both muscle cell and bone cells. This points out the significant effect of alanine in the pathogenesis, suggesting an abnormal alanine metabolism occurring in OA. Further, carefully designed studies are required to identify the different performances of alanine in different lesions of OA joint. In this way, we can use alanine as an indicator to fix the lesion to implement symptomatic treatment in the earlier stages of disease. Figure 5 shows a schematic overview of the alanine metabolic pathway in relation to OA.
Conclusions and future direction
Metabolomics has been applied to several disorders. Here, we have described the evidence in both animals and humans showing the potential of metabolomics as a promising tool for investigating OA. Many metabolic samples have been evaluated, such as blood, urine, SF, and tissue extracts. Table 2 summarizes the metabolic changes of OA in different sample types. Considering that OA is recognized as a tremendous heterogenous and multifactorial disease, multiple markers are essential. Hopefully, useful biomarkers can be provided for OA, therefore, allowing both diagnosis and prognosis. The current literature data suggest that the metabolic pathogenesis of OA may be significantly related to perturbations of amino acid metabolism. These altered amino acids (e.g., BCAA, arginine, and alanine), as well as phospholipids, were identified as potential biomarkers to distinguish OA from healthy individuals.
Recently, the ingestion of amino acids has actively been investigated in nutritional science, and BCAA, as well as arginine, could be used as novel nutraceuticals for OA as mentioned above. A present clinical study has shown that exercise therapy combined with BCAA supplements could lead to a significant improvement in contralateral hip abductor muscle strength in women with hip OA [79]. The elderly are more susceptible to OA, and the BCAA uptake response is reduced with aging [80]. Therefore, BCAA supplements may be effective for OA. Unlike BCAA, the body can synthesize L-arginine, but exogenous supplements of L-arginine may also be necessary. Oral supplementation with L-arginine is a benefit to cardiovascular functions and may help treat certain medical conditions [81, 82]. Meanwhile, L-arginine supplementation is used to enhance both tissue growth and general performance, potentiate the ergogenic potential and muscle tolerance to high intensive work and the gas exchange threshold, decrease the recovery performance period, and improve wound healing [83]. Arginine is also a potentially novel anti-obesity amino acid and may be beneficial for the treatment of obesity, which is a risk factor of OA [84]. Moreover, PCs are converted into lysoPCs by PLA2, which also produce LPA and free fatty acids. Thus, PLA2 may be a novel target to develop new drugs to improve OA. In parallel, LPA could be an attractive therapeutic target for both pain and OA pathogenesis. Alanine is identified as a potential biomarker of OA. As mentioned, the performance of alanine varies at different lesions of the OA joint, such as cartilage and subchondral bone. Therefore, it is likely used as an indicator to fix the lesion region to implement symptomatic treatment in the earlier stages of the disease. While carefully designed studies are required to confirm these findings, metabolomics is an exciting field in systems biology that may broadly apply to clinical applications in the foreseeable future and minimize the negative effects of OA in society.
Abbreviations
- ACLT:
-
anterior cruciate ligament transaction
- ARG:
-
arginase
- ATP:
-
adenosine triphosphate
- BCAA:
-
branched-chain amino acids
- GC–MS:
-
gas chromatography–mass spectrometry
- LC–MS:
-
liquid chromatography–mass spectrometry
- LysoPC:
-
lysophosphatidylcholine
- LPA:
-
lysophosphatidic acid
- MD:
-
meniscal destabilization
- mTOR:
-
mammalian target of rapamycin
- NMR:
-
nuclear magnetic resonance
- NO:
-
nitric oxide
- NOS:
-
NO synthase
- OA:
-
osteoarthritis
- OAT:
-
ornithine aminotransferase
- PC:
-
phosphatidylcholine
- PLA2:
-
phospholipase A2
- SF:
-
synovial fluid
- TCA:
-
tricarboxylic acid
- UPLC-MS:
-
ultra-performance liquid chromatography-tandem mass spectrometry
References
Woolf AD, Pfleger B (2003) Burden of major musculoskeletal conditions. Bull World Health Organ 81(9):646–656
Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, Goldring SR, Jones G, Teichtahl AJ, Pelletier JP (2016) Osteoarthritis. Nature reviews Disease primers 2:16072. https://doi.org/10.1038/nrdp.2016.72
Loeser RF, Goldring SR, Scanzello CR, Goldring MB (2012) Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 64(6):1697–1707. https://doi.org/10.1002/art.34453
Hiligsmann M, Cooper C, Arden N, Boers M, Branco JC, Luisa Brandi M, Bruyère O, Guillemin F, Hochberg MC, Hunter DJ, Kanis JA, Kvien TK, Laslop A, Pelletier JP, Pinto D, Reiter-Niesert S, Rizzoli R, Rovati LC, Severens JL, Silverman S, Tsouderos Y, Tugwell P, Reginster JY (2013) Health economics in the field of osteoarthritis: an expert’s consensus paper from the European Society for Clinical and Economic Aspects of osteoporosis and osteoarthritis (ESCEO). Semin Arthritis Rheum 43(3):303–313. https://doi.org/10.1016/j.semarthrit.2013.07.003
Zhai G (2019) Alteration of metabolic pathways in osteoarthritis. Metabolites 9(1). https://doi.org/10.3390/metabo9010011
Nepple JJ, Thomason KM, An TW, Harris-Hayes M, Clohisy JC (2015) What is the utility of biomarkers for assessing the pathophysiology of hip osteoarthritis? A systematic review. Clin Orthop Relat Res 473(5):1683–1701. https://doi.org/10.1007/s11999-015-4148-6
Showiheen SAA, Sun AR, Wu X, Crawford R, Xiao Y, Wellard RM, Prasadam I (2019) Application of metabolomics to osteoarthritis: from basic science to the clinical approach. Curr Rheumatol Rep 21(6):26. https://doi.org/10.1007/s11926-019-0827-8
Nicholson JK, Lindon JC (2008) Systems biology: metabonomics. Nature 455(7216):1054–1056. https://doi.org/10.1038/4551054a
Priori R, Scrivo R, Brandt J, Valerio M, Casadei L, Valesini G, Manetti C (2013) Metabolomics in rheumatic diseases: the potential of an emerging methodology for improved patient diagnosis, prognosis, and treatment efficacy. Autoimmun Rev 12(10):1022–1030. https://doi.org/10.1016/j.autrev.2013.04.002
Cui L, Zheng D, Lee YH, Chan TK, Kumar Y, Ho WE, Chen JZ, Tannenbaum SR, Ong CN (2016) Metabolomics investigation reveals metabolite mediators associated with acute lung injury and repair in a murine model of influenza pneumonia. Sci Rep 6:26076. https://doi.org/10.1038/srep26076
Weljie AM, Newton J, Mercier P, Carlson E, Slupsky CM (2006) Targeted profiling: quantitative analysis of 1H NMR metabolomics data. Anal Chem 78(13):4430–4442. https://doi.org/10.1021/ac060209g
Johnson CH, Ivanisevic J, Siuzdak G (2016) Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol 17(7):451–459. https://doi.org/10.1038/nrm.2016.25
Gupta L, Ahmed S, Jain A, Misra R (2018) Emerging role of metabolomics in rheumatology. Int J Rheum Dis 21(8):1468–1477. https://doi.org/10.1111/1756-185x.13353
Halama A (2014) Metabolomics in cell culture--a strategy to study crucial metabolic pathways in cancer development and the response to treatment. Arch Biochem Biophys 564:100–109. https://doi.org/10.1016/j.abb.2014.09.002
McGarrah RW, Crown SB, Zhang GF, Shah SH, Newgard CB (2018) Cardiovascular metabolomics. Circ Res 122(9):1238–1258. https://doi.org/10.1161/circresaha.117.311002
Tavares G, Venturini G, Padilha K, Zatz R, Pereira AC, Thadhani RI, Rhee EP, Titan SMO (2018) 1,5-Anhydroglucitol predicts CKD progression in macroalbuminuric diabetic kidney disease: results from non-targeted metabolomics. Metabolomics: official Journal of the Metabolomic Society 14(4):39. https://doi.org/10.1007/s11306-018-1337-9
Weckmann K, Diefenthäler P, Baeken MW, Yusifli K, Turck CW, Asara JM, Behl C, Hajieva P (2018) Metabolomics profiling reveals differential adaptation of major energy metabolism pathways associated with autophagy upon oxygen and glucose reduction. Sci Rep 8(1):2337. https://doi.org/10.1038/s41598-018-19421-y
Zhang A, Sun H, Yan G, Wang P, Han Y, Wang X (2014) Metabolomics in diagnosis and biomarker discovery of colorectal cancer. Cancer Lett 345(1):17–20. https://doi.org/10.1016/j.canlet.2013.11.011
Jacob M, Lopata AL, Dasouki M, Abdel Rahman AM (2019) Metabolomics toward personalized medicine. Mass Spectrom Rev 38(3):221–238. https://doi.org/10.1002/mas.21548
Markley JL, Brüschweiler R, Edison AS, Eghbalnia HR, Powers R, Raftery D, Wishart DS (2017) The future of NMR-based metabolomics. Curr Opin Biotechnol 43:34–40. https://doi.org/10.1016/j.copbio.2016.08.001
Heather LC, Wang X, West JA, Griffin JL (2013) A practical guide to metabolomic profiling as a discovery tool for human heart disease. J Mol Cell Cardiol 55:2–11. https://doi.org/10.1016/j.yjmcc.2012.12.001
Kobayashi T, Yoshihara Y, Yamada H, Fujikawa K (2000) Procollagen IIC-peptide as a marker for assessing mechanical risk factors of knee osteoarthritis: effect of obesity and varus alignment. Ann Rheum Dis 59(12):982–984. https://doi.org/10.1136/ard.59.12.982
Dettmer K, Aronov PA, Hammock BD (2007) Mass spectrometry-based metabolomics. Mass Spectrom Rev 26(1):51–78. https://doi.org/10.1002/mas.20108
Blair-Levy JM, Watts CE, Fiorentino NM, Dimitriadis EK, Marini JC, Lipsky PE (2008) A type I collagen defect leads to rapidly progressive osteoarthritis in a mouse model. Arthritis Rheum 58(4):1096–1106. https://doi.org/10.1002/art.23277
Hui AY, McCarty WJ, Masuda K, Firestein GS, Sah RL (2012) A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley interdisciplinary reviews Systems biology and medicine 4(1):15–37. https://doi.org/10.1002/wsbm.157
Whitelaw CB, Sheets TP, Lillico SG, Telugu BP (2016) Engineering large animal models of human disease. J Pathol 238(2):247–256. https://doi.org/10.1002/path.4648
Carlson AK, Rawle RA, Wallace CW, Brooks EG, Adams E, Greenwood MC, Olmer M, Lotz MK, Bothner B, June RK (2019) Characterization of synovial fluid metabolomic phenotypes of cartilage morphological changes associated with osteoarthritis. Osteoarthr Cartil 27(8):1174–1184. https://doi.org/10.1016/j.joca.2019.04.007
Williamson MP, Humm G, Crisp AJ (1989) 1H nuclear magnetic resonance investigation of synovial fluid components in osteoarthritis, rheumatoid arthritis and traumatic effusions. Br J Rheumatol 28(1):23–27. https://doi.org/10.1093/rheumatology/28.1.23
Holmes E, Wilson ID, Nicholson JK (2008) Metabolic phenotyping in health and disease. Cell 134(5):714–717. https://doi.org/10.1016/j.cell.2008.08.026
Shet K, Siddiqui SM, Yoshihara H, Kurhanewicz J, Ries M, Li X (2012) High-resolution magic angle spinning NMR spectroscopy of human osteoarthritic cartilage. NMR Biomed 25(4):538–544. https://doi.org/10.1002/nbm.1769
Adams SB Jr, Setton LA, Kensicki E, Bolognesi MP, Toth AP, Nettles DL (2012) Global metabolic profiling of human osteoarthritic synovium. Osteoarthr Cartil 20(1):64–67. https://doi.org/10.1016/j.joca.2011.10.010
Yang G, Zhang H, Chen T, Zhu W, Ding S, Xu K, Xu Z, Guo Y, Zhang J (2016) Metabolic analysis of osteoarthritis subchondral bone based on UPLC/Q-TOF-MS. Anal Bioanal Chem 408(16):4275–4286. https://doi.org/10.1007/s00216-016-9524-x
Nguyen LT, Sharma AR, Chakraborty C, Saibaba B, Ahn ME, Lee SS (2017) Review of prospects of biological fluid biomarkers in osteoarthritis. Int J Mol Sci 18(3). https://doi.org/10.3390/ijms18030601
Maher AD, Coles C, White J, Bateman JF, Fuller ES, Burkhardt D, Little CB, Cake M, Read R, McDonagh MB, Rochfort SJ (2012) 1H NMR spectroscopy of serum reveals unique metabolic fingerprints associated with subtypes of surgically induced osteoarthritis in sheep. J Proteome Res 11(8):4261–4268. https://doi.org/10.1021/pr300368h
Costello CA, Hu T, Liu M, Zhang W, Furey A, Fan Z, Rahman P, Randell EW, Zhai G (2019) Metabolomics signature for non-responders to total joint replacement surgery in primary osteoarthritis patients: the Newfoundland Osteoarthritis Study. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. doi:https://doi.org/10.1002/jor.24529
Zhang W, Sun G, Likhodii S, Liu M, Aref-Eshghi E, Harper PE, Martin G, Furey A, Green R, Randell E, Rahman P, Zhai G (2016) Metabolomic analysis of human plasma reveals that arginine is depleted in knee osteoarthritis patients. Osteoarthr Cartil 24(5):827–834. https://doi.org/10.1016/j.joca.2015.12.004
Zhai G, Wang-Sattler R, Hart DJ, Arden NK, Hakim AJ, Illig T, Spector TD (2010) Serum branched-chain amino acid to histidine ratio: a novel metabolomic biomarker of knee osteoarthritis. Ann Rheum Dis 69(6):1227–1231. https://doi.org/10.1136/ard.2009.120857
Ryan D, Robards K, Prenzler PD, Kendall M (2011) Recent and potential developments in the analysis of urine: a review. Anal Chim Acta 684(1–2):8–20. https://doi.org/10.1016/j.aca.2010.10.035
Loeser RF, Pathmasiri W, Sumner SJ, McRitchie S, Beavers D, Saxena P, Nicklas BJ, Jordan J, Guermazi A, Hunter DJ, Messier SP (2016) Association of urinary metabolites with radiographic progression of knee osteoarthritis in overweight and obese adults: an exploratory study. Osteoarthr Cartil 24(8):1479–1486. https://doi.org/10.1016/j.joca.2016.03.011
Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, Beavers DP, Hunter DJ, Lyles MF, Eckstein F, Williamson JD, Carr JJ, Guermazi A, Loeser RF (2013) Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. Jama 310(12):1263–1273. https://doi.org/10.1001/jama.2013.277669
Li X, Yang SB, Qiu YP, Zhao T, Chen TL, Su MM, Chu LX, Lv AP, Liu P, Jia W (2010) Urinary metabolomics as a potentially novel diagnostic and stratification tool for knee osteoarthritis. Metabolomics: official journal of the Metabolomic Society 6(1):109–118. https://doi.org/10.1007/s11306-009-0184-0
Blanco FJ (2014) Osteoarthritis year in review 2014: we need more biochemical biomarkers in qualification phase. Osteoarthr Cartil 22(12):2025–2032. https://doi.org/10.1016/j.joca.2014.09.009
Carlson AK, Rawle RA, Adams E, Greenwood MC, Bothner B, June RK (2018) Application of global metabolomic profiling of synovial fluid for osteoarthritis biomarkers. Biochem Biophys Res Commun 499(2):182–188. https://doi.org/10.1016/j.bbrc.2018.03.117
Mickiewicz B, Kelly JJ, Ludwig TE, Weljie AM, Wiley JP, Schmidt TA, Vogel HJ (2015) Metabolic analysis of knee synovial fluid as a potential diagnostic approach for osteoarthritis. Journal of orthopaedic research: official publication of the Orthopaedic Research Society 33(11):1631–1638. https://doi.org/10.1002/jor.22949
Mickiewicz B, Heard BJ, Chau JK, Chung M, Hart DA, Shrive NG, Frank CB, Vogel HJ (2015) Metabolic profiling of synovial fluid in a unilateral ovine model of anterior cruciate ligament reconstruction of the knee suggests biomarkers for early osteoarthritis. Journal of orthopaedic research: official publication of the Orthopaedic Research Society 33(1):71–77. https://doi.org/10.1002/jor.22743
Zhang W, Sun G, Aitken D, Likhodii S, Liu M, Martin G, Furey A, Randell E, Rahman P, Jones G, Zhai G (2016) Lysophosphatidylcholines to phosphatidylcholines ratio predicts advanced knee osteoarthritis. Rheumatology (Oxford, England) 55(9):1566–1574. https://doi.org/10.1093/rheumatology/kew207
Borel M, Pastoureau P, Papon J, Madelmont JC, Moins N, Maublant J, Miot-Noirault E (2009) Longitudinal profiling of articular cartilage degradation in osteoarthritis by high-resolution magic angle spinning 1H NMR spectroscopy: experimental study in the meniscectomized Guinea pig model. J Proteome Res 8(5):2594–2600. https://doi.org/10.1021/pr8009963
Senol O, Gundogdu G, Gundogdu K, Miloglu FD (2019) Investigation of the relationships between knee osteoarthritis and obesity via untargeted metabolomics analysis. Clin Rheumatol 38(5):1351–1360. https://doi.org/10.1007/s10067-019-04428-1
Zhenyukh O, Civantos E, Ruiz-Ortega M, Sánchez MS, Vázquez C, Peiró C, Egido J, Mas S (2017) High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic Biol Med 104:165–177. https://doi.org/10.1016/j.freeradbiomed.2017.01.009
Kluzek S, Newton JL, Arden NK (2015) Is osteoarthritis a metabolic disorder? Br Med Bull 115(1):111–121. https://doi.org/10.1093/bmb/ldv028
Lynch CJ, Adams SH (2014) Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 10(12):723–736. https://doi.org/10.1038/nrendo.2014.171
Bonvini A, Coqueiro AY, Tirapegui J, Calder PC, Rogero MM (2018) Immunomodulatory role of branched-chain amino acids. Nutr Rev 76(11):840–856. https://doi.org/10.1093/nutrit/nuy037
Bassit RA, Sawada LA, Bacurau RF, Navarro F, Costa Rosa LF (2000) The effect of BCAA supplementation upon the immune response of triathletes. Med Sci Sports Exerc 32(7):1214–1219. https://doi.org/10.1097/00005768-200007000-00005
Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H (2011) Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 7(1):33–42. https://doi.org/10.1038/nrrheum.2010.196
Morris SM Jr (2006) Arginine: beyond protein. The American Journal of Clinical Nutrition 83(2):508s–512s. https://doi.org/10.1093/ajcn/83.2.508S
Li Y, Xiao W, Luo W, Zeng C, Deng Z, Ren W, Wu G, Lei G (2016) Alterations of amino acid metabolism in osteoarthritis: its implications for nutrition and health. Amino Acids 48(4):907–914. https://doi.org/10.1007/s00726-015-2168-x
Ohnishi A, Osaki T, Matahira Y, Tsuka T, Imagawa T, Okamoto Y, Minami S (2013) Correlation of plasma amino acid concentrations and chondroprotective effects of glucosamine and fish collagen peptide on the development of osteoarthritis. J Vet Med Sci 75(4):497–502. https://doi.org/10.1292/jvms.12-0241
Tootsi K, Vilba K, Märtson A, Kals J, Paapstel K, Zilmer M (2020) Metabolomic signature of amino acids, biogenic amines and lipids in blood serum of patients with severe osteoarthritis. Metabolites 10(8). https://doi.org/10.3390/metabo10080323
Rockel JS, Kapoor M (2018) The metabolome and osteoarthritis: possible contributions to symptoms and pathology. Metabolites 8(4). https://doi.org/10.3390/metabo8040092
Wehling-Henricks M, Jordan MC, Gotoh T, Grody WW, Roos KP, Tidball JG (2010) Arginine metabolism by macrophages promotes cardiac and muscle fibrosis in mdx muscular dystrophy. PLoS One 5(5):e10763. https://doi.org/10.1371/journal.pone.0010763
Abramson SB, Amin AR, Clancy RM, Attur M (2001) The role of nitric oxide in tissue destruction. Best Pract Res Clin Rheumatol 15(5):831–845. https://doi.org/10.1053/berh.2001.0196
Xia W, Szomor Z, Wang Y, Murrell GA (2006) Nitric oxide enhances collagen synthesis in cultured human tendon cells. Journal of orthopaedic research: official publication of the Orthopaedic Research Society 24(2):159–172. https://doi.org/10.1002/jor.20060
Abramson SB (2008) Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Research & Therapy 10(Suppl 2):S2. https://doi.org/10.1186/ar2463
Kosinska MK, Liebisch G, Lochnit G, Wilhelm J, Klein H, Kaesser U, Lasczkowski G, Rickert M, Schmitz G, Steinmeyer J (2013) A lipidomic study of phospholipid classes and species in human synovial fluid. Arthritis Rheum 65(9):2323–2333. https://doi.org/10.1002/art.38053
Pousinis P, Gowler PRW, Burston JJ, Ortori CA, Chapman V, Barrett DA (2020) Lipidomic identification of plasma lipids associated with pain behaviour and pathology in a mouse model of osteoarthritis. Metabolomics: official journal of the Metabolomic Society 16(3):32. https://doi.org/10.1007/s11306-020-01652-8
Murakami M, Nakatani Y, Atsumi GI, Inoue K, Kudo I (2017) Regulatory functions of phospholipase A2. Crit Rev Immunol 37(2–6):121–179. https://doi.org/10.1615/CritRevImmunol.v37.i2-6.20
Chen Y, Crawford RW, Oloyede A (2007) Unsaturated phosphatidylcholines lining on the surface of cartilage and its possible physiological roles. J Orthop Surg Res 2:14. https://doi.org/10.1186/1749-799x-2-14
Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K (2002) Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem 277(42):39436–39442. https://doi.org/10.1074/jbc.M205623200
Mabey T, Taleongpong P, Udomsinprasert W, Jirathanathornnukul N, Honsawek S (2015) Plasma and synovial fluid autotaxin correlate with severity in knee osteoarthritis. Clinica chimica acta; international journal of clinical chemistry 444:72–77. https://doi.org/10.1016/j.cca.2015.01.032
Uchida H, Nagai J, Ueda H (2014) Lysophosphatidic acid and its receptors LPA1 and LPA3 mediate paclitaxel-induced neuropathic pain in mice. Mol Pain 10:71. https://doi.org/10.1186/1744-8069-10-71
Gustin C, Van Steenbrugge M, Raes M (2008) LPA modulates monocyte migration directly and via LPA-stimulated endothelial cells. American journal of physiology Cell physiology 295(4):C905–C914. https://doi.org/10.1152/ajpcell.00544.2007
Zhai G, Randell EW, Rahman P (2018) Metabolomics of osteoarthritis: emerging novel markers and their potential clinical utility. Rheumatology (Oxford, England) 57(12):2087–2095. https://doi.org/10.1093/rheumatology/kex497
Harris RC, Tallon MJ, Dunnett M, Boobis L, Coakley J, Kim HJ, Fallowfield JL, Hill CA, Sale C, Wise JA (2006) The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 30(3):279–289. https://doi.org/10.1007/s00726-006-0299-9
Chen R, Han S, Liu X, Wang K, Zhou Y, Yang C, Zhang X (2018) Perturbations in amino acids and metabolic pathways in osteoarthritis patients determined by targeted metabolomics analysis. J Chromatogr B Anal Technol Biomed Life Sci 1085:54–62. https://doi.org/10.1016/j.jchromb.2018.03.047
Anderson JR, Phelan MM, Foddy L, Clegg PD, Peffers MJ (2020) Ex vivo equine cartilage explant osteoarthritis model: a metabolomics and proteomics study. J Proteome Res 19(9):3652–3667. https://doi.org/10.1021/acs.jproteome.0c00143
Jiang H, Liu J, Qin XJ, Chen YY, Gao JR, Meng M, Wang Y, Wang T (2018) Gas chromatography-time of flight/mass spectrometry-based metabonomics of changes in the urinary metabolic profile in osteoarthritic rats. Experimental and therapeutic medicine 15(3):2777–2785. https://doi.org/10.3892/etm.2018.5788
Millward-Sadler SJ, Wright MO, Flatman PW, Salter DM (2004) ATP in the mechanotransduction pathway of normal human chondrocytes. Biorheology 41(3–4):567–575
Quesnele JJ, Laframboise MA, Wong JJ, Kim P, Wells GD (2014) The effects of beta-alanine supplementation on performance: a systematic review of the literature. International journal of sport nutrition and exercise metabolism 24(1):14–27. https://doi.org/10.1123/ijsnem.2013-0007
Ikeda T, Jinno T, Masuda T, Aizawa J, Ninomiya K, Suzuki K, Hirakawa K (2018) Effect of exercise therapy combined with branched-chain amino acid supplementation on muscle strengthening in persons with osteoarthritis. Hong Kong physiotherapy journal: official publication of the Hong Kong Physiotherapy Association Limited = Wu li chih liao 38(1):23–31. https://doi.org/10.1142/s1013702518500038
Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR (2005) Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 82(5):1065–1073. https://doi.org/10.1093/ajcn/82.5.1065
Lin CC, Tsai WC, Chen JY, Li YH, Lin LJ, Chen JH (2008) Supplements of L-arginine attenuate the effects of high-fat meal on endothelial function and oxidative stress. Int J Cardiol 127(3):337–341. https://doi.org/10.1016/j.ijcard.2007.06.013
Siasos G, Tousoulis D, Vlachopoulos C, Antoniades C, Stefanadi E, Ioakeimidis N, Andreou I, Zisimos K, Papavassiliou AG, Stefanadis C (2008) Short-term treatment with L-arginine prevents the smoking-induced impairment of endothelial function and vascular elastic properties in young individuals. Int J Cardiol 126(3):394–399. https://doi.org/10.1016/j.ijcard.2007.04.057
Hristina K, Langerholc T, Trapecar M (2014) Novel metabolic roles of L-arginine in body energy metabolism and possible clinical applications. J Nutr Health Aging 18(2):213–218. https://doi.org/10.1007/s12603-014-0015-5
Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, Carroll RJ, Meininger CJ, Wu G (2005) Dietary L-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J Nutr 135(4):714–721. https://doi.org/10.1093/jn/135.4.714
Funding
This work was supported by the National Natural Science Foundation of China (81871848).
Author information
Authors and Affiliations
Contributions
All authors discuss the concept of the manuscript. JTL wrote the manuscript. GXN conceived the study; ZN, ZPY, and TL prepared some materials. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, JT., Zeng, N., Yan, ZP. et al. A review of applications of metabolomics in osteoarthritis. Clin Rheumatol 40, 2569–2579 (2021). https://doi.org/10.1007/s10067-020-05511-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-05511-8