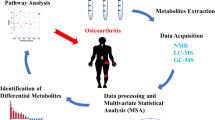
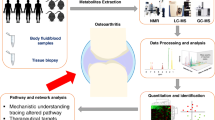
Abstract
Introduction
Osteoarthritis (OA) is a common degenerative disorder of the synovial joints and is usually an age-related disease that occurs due to continuous wear and tear of the cartilage in the joints. Presently, there is no proven medical management to halt the progression of the disease in the early stages. The purpose of our systematic review is to analyze the possible metabolites and metabolic pathways that are specifically involved in OA pathogenesis and early treatment of the disease.
Materials and Methods
The articles were collected from PubMed, Cochrane, Google Scholar, Embase, and Scopus databases. “Knee”, “Osteoarthritis”, “Proteomics”, “Lipidomics”, “Metabolomics”, “Metabolic Methods”, and metabolic* were employed for finding the articles. Only original articles with human or animal OA models with healthy controls were included.
Results
From the initial screening, a total of 458 articles were identified from the 5 research databases. From these, 297 articles were selected in the end for screening, of which 53 papers were selected for full-text screening. Finally, 50 articles were taken for the review based on body fluid: 6 urine studies, 15 plasma studies, 16 synovial fluid studies, 11 serum studies, 4 joint tissue studies, and 1 fecal study. Many metabolites were found to be elevated in OA. Some of these metabolites can be used to stage the OA Three pathways that were found to be commonly involved are the TCA cycle, the glycolytic pathway, and the lipid metabolism.
Conclusion
All these studies showed a vast array of metabolites and metabolic pathways associated with OA. Metabolites like lysophospholipids, phospholipids, arginine, BCCA, and histidine were identified as potential biomarkers of OA but a definite association was not identified, Three pathways (glycolytic pathway, TCA cycle, and lipid metabolic pathways) have been found as highly significant in OA pathogenesis. These metabolic pathways could provide novel therapeutic targets for the prevention and progression of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary osteoarthritis (OA), a degenerative joint condition, is the most prominent type of arthritis. Patients with OA are typically elderly patients, with almost 80% of them being at the age of 65 years or above [1]. The most affected joint is the knee where OA occurs mostly due to loss of balance between continuous wear and tear and remodeling occurring in the joint. This degenerative process is significantly influenced by inflammation as well. Besides the knee, hip, spine, and small joints are also involved with the slow progressive loss of cartilage. Although there is still a long way to go before total control over OA progression is reached, early detection of this condition can help us to better manage this condition and prevent morbidity. Currently, clinical and radiological methods are used to classify or grade OA.
Conservatively knee osteoarthritis is treated with NSAIDs, cartilage supplements, and calcium. Injections of hyaluronic acid [2] or intraarticular steroids are tried. Proximal fibular osteotomy (PFO) and high tibial osteotomy [3] are two joint preservation surgeries that would be instrumental in halting the initial phase of the disease. KOA is often diagnosed at an advanced stage when a patient exhibits medial joint line tenderness, joint crepitus, effusion, and deformity, which after initial conservative measures might eventually progress to require total knee arthroplasty (TKR).
A closer look at the molecular metabolites in KOA patients could help us better understand the disease which could potentially lead to the development of newer methods of identifying the disease and their treatment which would ultimately mean better management in KOA. Though many studies have been conducted recently to find and characterize biomarkers, however, none of these biomarkers have received clinical validation. It is possible to analyze thousands of different molecules all at once through a single targeted method using mass spectrometry (LC–MS), gas Chromatography–Mass Spectrometry (GC–MS), and H-NMR (proton nuclear magnetic resonance spectroscopy). Other techniques such as microarrays for deoxyribonucleic acid, ribonucleic acid, or protein can also simultaneously analyze thousands of molecules all at once [4]. Although metabolite signatures were identified, no published literature is available for the treatment of KOA. Proteomic, lipidomic, and metabolomic approaches aim to identify molecular profiles or signatures of different tissues such as cartilage, bone, synovium, meniscus, and tendon. Followed by synovial fluid, serum, and even urine and fecal samples, hoping to find certain predictive molecules or molecular classes responsible for OA development, disease progression, or possible therapeutic targets. The goal of this study is to uncover common molecular signatures that can be adopted in the future for the prognosis and treatment of KOA. Our objective is to provide a review of molecules in the synovial fluid or urine or other samples that can distinguish between KOA and non-KOA patients.
Materials and Methods
The systematic review was conducted following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) Guidelines [5]. The study protocol has been registered in PROSPERO with registration number CRD42024428345. The studies that met the PICO criteria that were included in the review are mentioned below. The review included studies that fulfilled the PICO criteria as mentioned below.
-
Population: Human or animal subjects with KOA
-
Intervention: Identification of KOA-specific metabolites from body fluid and tissue samples.
-
Comparator: Healthy controls or no comparator
-
Outcome: Identification of molecules specific to KOA in different fluid and tissue samples in the body. Their correlation with diagnosis, treatment, and prognosis of KOA.
Study Design
Any original studies qualifying the PICO criteria.
Search Strategy
On February 14th, 2023, the PubMed, Cochrane, Google Scholar, Embase, and Scopus databases were searched. Search keywords employed for the review were “Knee”, “Osteoarthritis”, “Proteomics”, “Lipidomics”, “Metabolomics”, “Metabolic Methods”, and metabolic* used in various combinations with the Boolean operators—“AND” “OR” and “NOT”. The review included the original studies on animal and human KOA models with established primary and surgically induced OA, respectively. Only English language studies were selected for review. Analytical, observational, and cross-section studies were included in the review. Review articles, conference papers, brief reports, opinions, and editorials were excluded from the review. The screening was done by two reviewers independently on the Rayyan QCRI tool. Any discrepancy between the reviewers was resolved by the involvement of the third reviewer.
Data Extraction
Two reviewers independently retrieved relevant data from articles included for analysis. The following data were extracted:
-
1.
Study characteristics: Year of publication, authors, the animal model used (if applicable), and methodology used for the study,
-
2.
Baseline characteristics: Method of induction of arthritis, number of subjects in both the groups, nature of the control group, names, and the number of metabolites isolated, and
-
3.
Outcomes: Clinical changes in patients, histologic changes in KOA, and metabolite level changes from baseline.
Risk of Bias and Quality Assessment
The risk of bias in the included studies was assessed by the JBI risk of bias tool [6] by two reviewers independently. A third reviewer was included to resolve any discrepancy between the reviewers.
Results
Search Results
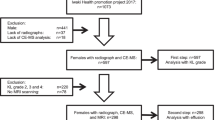
After title screening, 458 articles were retrieved from 5 databases. After eliminating the duplicates, the abstracts of 297 articles were subjected to screening. In the end, a total of 53 papers were selected for full-text screening. While screening all the included papers, one article was excluded because of the use of non-English language, one article did not have details of metabolites, and one article did not have any healthy controls. These three were excluded. To synthesize the data, 50 papers were included. The PRISMA flow diagram of the study selection is shown in Fig. 1.
Quality Assessment
The methodological quality of the included studies was assessed using the JBI tool and is included as supplementary file 1. The included studies did not show a high risk of bias to warrant exclusion.
General Characteristics
Thirteen studies focused exclusively on animal studies and the remaining thirty-seven papers focused on human studies. Based on samples used for metabolic analysis, 6 studies used urine samples which are shown in Table 1, 15 studies used plasma which is presented in Table 2, 16 studies used synovial fluid (Table 3), 1 study used fecal samples (Table 4). Four studies were based on joint tissue (Table 5), and eleven studies used serum samples (Table 6).
Metabolomics in Synovial Fluid
We analyzed 16 synovial fluid studies, of which 12 studies were human and 4 studies were animal. One study employed both synovium tissue and synovial fluid as their sample, whereas two other studies simultaneously evaluated metabolites in synovial fluid and plasma. The remaining 13 studies were evaluated based on synovial fluid as the sample.
Animal Studies
All the animal studies used different animal models. One study used male mice [7], another used 18 skeletally mature female Suffolk-cross sheep [8] and one study was conducted on white rabbits [9]. Two out of four studies demonstrated altered levels of branched-chain amino acids (BCCA). Levels of leucine, isoleucine, and valine were upregulated in OA models compared to healthy controls. All three studies did not show any common metabolites in their results. Mickiewicz et al. [8] indicated a fundamental reduction in the concentration of glucose in OA models. Yiwen et al. [9] revealed a consequential accumulation of arginine and proline levels in ACL transected rabbit knee and noted elevated levels of N1-acetylspermidine. In addition, tryptophan and its derivatives were also elevated in samples of OA models compared to sham controls. Hahn et al. [7] for their study used two groups of mice—control fat-fed (CF) and high fat-fed (HF) mice. These two groups were evaluated at the end of the 52 weeks. In CF mice, they revealed downregulation of metabolites like lysine degradation, pyruvate metabolism, fatty acid metabolism, steroid biosynthesis, and tryptophan metabolism; in HC mice on the other hand, there was a positive association with coenzyme A biosynthesis, short-chain fatty acid synthesis, and BCCA synthesis.
Human Studies
We analyzed 12 human studies, 6 of which showed an upregulation of the TCA cycle. Amino acid metabolism was altered in 8 out of 12 studies. One study indicated changed nucleotide metabolism, while seven studies revealed altered lipid metabolism. Arginine levels varied in ten studies, eight out of ten studies exhibited depletion of arginine levels in OA, but Carlson et al. [10] laid out an enhancement of glycine, serine, alanine threonine, lysine, proline, and arginine levels in OA. Weidong et al. [11] found that OA patient’s leucine levels were considerably higher when compared to healthy controls. Corina et al. [12] found 43 metabolites, among them 9 were important. But out of these nine, four were considered significant for the study, they were phospholipids, phosphatidylcholines, sphingomyelin, and ceramide. This study also revealed that phosphatidylcholine, diadenosine 5′,5′- diphosphate, and lysophosphatidylcholine levels were heightened in late OA. Zhang et al. [13] detected a total of 86 metabolites, among which they found that glycerophospholipids, sphingolipids, biogenic amine, and acylcarnitine were higher in OA patients. Anderson et al. [14] compared OA with rheumatoid arthritis (RA) controls. Citrate, creatinine, glucose, glutamine, glycerol, pyruvate, and taurine levels were raised in OA patients while 3-hydroxybutyrate, acetate, isoleucine, leucine, sarcosine, and threonine were higher in RA. Kim et al. [15] graded OA using different metabolites. From the synovial fluid of both early and late-stage OA, 114 metabolites were identified, of which 28 were the ones differentiating early OA from late OA. The key metabolites differentiating were higher levels of malate, ethanolamine, squalene, glycerol, myristic acid, oleic acid, lanosterol, heptadecanoic acid, and capric acid in late OA.
Metabolomics in Urine Sample
We analyzed six studies that evaluated urine metabolites, which consisted of two animal studies and four human studies.
Animal Studies
Both studies used Sprague–Dawley rats as their medium of analysis. Both studies similarly noted significant upregulation in the TCA cycle and fatty acid metabolism. Yin et al. [16] identified 14 metabolites of which glycine, hippuric acid, acetoacetic acid, 5 -hydroxy indole acetic, alanine, and threonine were upregulated but citric acid, adipic acid, glutamine, phenylacetic acid, azelaic acid, tryptophan, histidine, succinic acid were downregulated. Due to abnormal activity of the TCA cycle, citric acid and succinic acid levels were decreased in the OA rat’s urine samples. Jiang et al. [17] identified multiple components as potential biomarkers of OA including, alanine, alpha-ketoglutarate, asparagine, maltose, and glutamine all of which were significantly altered in OA compared to healthy controls.
Human Studies
All four studies in common exhibited elevated levels of amino acid metabolism, but only three studies showcased an upregulation of the TCA cycle. Abdelrazig et al. [18] recognized 26 altered metabolites in patients with inflammatory OA. Alterations in amino acid metabolism, pyruvate metabolism, and TCA cycle were also noted by them. According to Li et al. [19], OA patients with joint effusion had lower levels of glutamine and histidine and higher levels of aconite, isocitrate, citrate, and histamine. Loeser et al. [20] recognized that arginine and histidine levels were reduced in OA patients in contrast to healthy controls.
Metabolomics in a Fecal Sample
Only one fecal study which was based on a human model was analyzed in our study. This study revealed alterations in amino acid, tryptophan, and leukotriene metabolism. Rushing et al. [21] analyzed metabolite changes associated with gut microbiota in obese OA patients. More than 100 metabolites have been isolated but the most prominent differentiators between OA cases and controls were dipeptides and tripeptides. In comparison to controls, it was identified that OA patients’ fecal samples had lower hippuric acid (phenylalanine derivative).
Metabolomics in Joint Tissue Samples
Four studies analyzed joint tissue sampling for metabolic analysis in OA, of which three were based on human models and one study on an animal model. One human study utilized both synovial fluid and synovial tissue for analysis while the other three studies including the animal study focused only on joint tissue study. All four studies showed altered amino acid metabolism, but no similar metabolites were detected. Jessica et al. [22] showcased that 42 metabolites were isolated from synovial tissue and 29 metabolites from synovial fluid but only 3 metabolites (lactate, dimethylamine, and creatine) positively correlated in both samples. The most crucial metabolite to distinguish between low-grade and high-grade synovitis has been revealed to be glucose. Haudenschild et al. [23] used mice models with ACL resection, which were injected with intraperitoneal flavopiridol (cdk9 inhibitor). Flavopiridol downregulates transcription of early response genes, which were associated with joint damage. The study concluded that flavopiridol prevented the upregulation of vitamin D3, phylloquinone, and acetylcarnitine.
Metabolomics in Plasma Study
A total of 15 studies were analyzed, of which 3 were animal studies and 12 were human studies. In the 12 studies based on human models, 2 used plasma and synovial fluid for analysis.
Animal Studies
All the animal studies used mice models for analysis. Only lipid metabolism was commonly altered in all the studies, with elevated levels of Lysophosphatidylcholine and phosphatidylcholine derivatives. One study observed significant alteration in the TCA cycle and its metabolites. Another study analyzed gut metabolites targeting HIF 1alpha to inhibit OA. Zhiyuan et al. [24] noted enhanced levels of iron in OA models causing a deleterious impact on joint homeostasis. The study presented higher serum iron levels in OA patients compared to the control group but transferrin levels and TIBC were lower in OA models. Pousinis et al. [25] analyzed lipidomics study in mice models and found 24 altered metabolites, of which 6 metabolites were considered significant in OA models, these metabolites were related to steroid biosynthesis, sphingolipid metabolism, linoleic acid, alpha-linolenic acid, glycerophospholipid, and arachidonic acid metabolism.
Human Studies
A total of 12 human studies were analyzed, among which lipid metabolism is the only pathway commonly affected in most of the studies, followed by amino acid metabolism. Lysophosphatidylcholine: The phosphatidylcholine ratio was elevated in most of these studies, which proves to be a prominent biomarker for OA. Other significant metabolites identified were arginine, glycine, leucine, and histidine. Costello et al. [26] studied the correlation between metabolites and pain and functional non-responders in post-TKR patients—5 phosphatidylcholines (PC), 4 amino acids (proline was the most significant) 2 acylcarnitine, and 1 biogenic amine were found to be associated with pain and 14 PC (PC aaC36:8 was most significant), 7 amino acids, 1 LysoPCs and carnitine were associated with function non-responders in OA. Zhang et al. [27] studied the relevance of arginine in OA patients, out of six significant metabolites, arginine was the most significant metabolite in all stages of the study. Knee OA patients had on average 69 microM lower plasma arginine levels compared to controls. ROC analysis also showed that arginine had the greatest diagnostic value with an AUC of 0.984. The study also found 2.2 times higher levels of ornithine in OA patients compared to control. Zhai et al. [28] studied the significance of phenylalanine in the radiographic advancement of OA. After age, sex, BMI, and clinical site adjustment, the study revealed phenylalanine concentration was substantially linked to knee OA progression. Moreover, phenylalanine levels were highly linked with knee progression in females and not in men.
Metabolomics in Serum Study
Eleven studies were analyzed with serum samples, three were focused on animal models, and eight on human models. While five studies pointed out altered lipid metabolism and seven studies indicated altered amino acid metabolism. LysoPCs and PC were enhanced in three studies and altered levels of glycine, histidine, arginine, and leucine were exhibited in three studies.
Animal Studies
The animal study was based on two distinct models; one was on sheep and the other on mice. Both studies in common indicated enhanced amino acid metabolism. Tristan et al. [29] identified 17 significant metabolites after 72 h post-surgery (ACL resection in mice models), but at 4 weeks follow-up, only 8 metabolites were found significant and at 10 weeks follow-up, only 3 metabolites (decreased D-mannose levels and increased hexanoyl carnitine and butyryl carnitine) were elevated between OA models and sham models. Anthony et al. [30] following meniscal destabilization, at 4 weeks, identified that TMAO, glutamine, and acetate levels were increased, and lactate and glycine levels were decreased whereas at 12 weeks postop only TMAO and tyrosine were elevated.
Human Studies
In all the human studies, lipid metabolism and amino acid metabolisms were altered. Chen et al. [31] utilized targeted metabolomics analysis and detected 25 amino acids and 4 biogenic amines. The study found the metabolites with the most significant impact were found to be involved in the metabolism of alanine, aspartate, glutamate, arginine, and proline. Schadler et al. [32] studied the association of FABP4 and leptins with knee OA severity and BMI. Higher levels of FABP4 and leptin levels were found in the obese women population which is a predictor for OA progression. Xie et al. [33] studied the relationship between knee cartilage loss and associated metabolites. The study revealed that four metabolites were associated with patellar cartilage loss, and four metabolites each were associated with lateral and medial cartilage loss in OA patients compared to controls.
Discussion
Metabolomics is the comprehensive analysis of small molecules in a biological system. Metabolites are the ultimate end products of different metabolic pathways that project the genotypic, phenotypic, and environmental characteristics of various biological systems. We have analyzed 50 works of literature to detect metabolic perturbations in the urine, blood (plasma and serum), synovium, feces, and synovial fluid of animal models and human models [34].
Potential Biomarkers of OA
The study has identified various biomarkers that were significant in the detection and treatment of OA. These include various amino acids, lipids, nucleotides, and glucose metabolites.
Proteomics in OA
Arginine is a semi-essential amino acid in humans, which is one of the most important metabolites we identified in OA patients. Arginase stimulates collagen formation and cell proliferation through the urea and L-ornithine pathway which causes fibrosis in OA [35]. According to Zhang et al. [27], patients with knee OA had a plasma concentration of ornithine that was 2.2 times greater than that of the control. The arginine:proline ratio was lower in OA patients than in controls, thereby suggesting that OA is caused by overactivity related to arginine depletion. Five major metabolites were identified by De Sousa et al. [36] as being related to OA; however, arginine was determined to be the notable metabolic marker. BCCA includes valine, leucine, and isoleucine as essential amino acids, which act as a medium for various protein and energy metabolism and precursors to other amino acids. Our study concluded that BCCA were significant OA markers. Anthony et al. [30] identified decreased levels of serum BCCA in OA subjects and the ratio of BCCA to histidine was also determined as a biomarker for knee OA. Zhai et al. [37] showed an association between valine:histidine and leucine:histidine ratios and knee OA was statistically significant. Also, the study showed only the BCCA:histidine ratio was significant but not the serum concentration of histidine alone as a marker for OA. Phenylalanine and tryptophan are essential aromatic amino acids involved in protein synthesis. According to Guangju Zhai et al. [28], phenylalanine levels in the plasma of OA progressors were reportedly higher than those of non-progressors. The study proved no association of WOMAC pain scores with phenylalanine levels in OA. In contrast, Chen et al. [31] quantified significantly lower levels of phenylalanine in a serum study of OA patients. Mickiewicz et al. [8] identified elevated levels of phenylalanine synovial fluid in mice models. Rushing et al. [21] showed an association of tryptophan metabolism with the maintenance of intestinal health, through kynurenine and serotonin pathways.
Lipidomics in OA
Phosphatidylcholine and LysoPCs and their ratios are identified as potential biomarkers of OA in our study. Corina et al. [12] in their study had identified phosphatidylcholine, diadenosine 5′,5′-diphosphate, and lysophosphatidylcholine had higher values in late OA. According to Hahn et al. [38], OA patients undergoing joint replacements typically had lower PC levels than controls. Bingyong et al. [39] found abnormal levels of glycerophospholipids in synovial fluid samples from advanced OA patients. Alyssa et al. [40] compared synovial fluid between OA, RA, and healthy controls and found 19 significant metabolites were altered in diseased synovium compared to healthy control of which more than half were phospholipids. Poulami et al. [41] compared the progression of OA between high-fat diet-fed mice and lean-diet mice and found four potential biomarkers of OA in high-fat-fed mice which were 3 lysophosphatidylcholine (LysoPCs) analogs and one phosphatidylcholine (PC) analog. In the analysis of the plasma of 109 candidates, Zhang et al. [42] identified that the LysoPCs:PC ratio has shown great specificity and sensitivity in differentiating between healthy controls and advanced OA. Sphingomyelins, ceramides, and long-chain fatty acids were also identified in many of our studies as significant markers of OA. Meessen et al. [43] studied a significant association between fatty acid chain length and stages of OA, especially end-stage OA, but the study showed no association between fatty acid chain length and OA progression.
Metabolic Pathways Affected in OA
TCA Cycle
Most of our studies including blood, synovium, synovial fluid, and urine studies showed that the most affected pathway in OA pathology was the TCA cycle. The oxidation of acetyl coenzyme A (CoA) which is produced from proteins, fatty acids, and carbohydrates is how the TCA cycle harvests energy [44]. Abdelrazig et al. [18] examined urine samples from 74 patients with OA and found elevated levels of fumarate, acetyl phosphate, and S-lactoylglutathione which suggest that the disrupted metabolism in cartilage cells is causing increased activity of the pyruvate pathway and the TCA cycle. After analyzing 27 urine samples from OA patients, Li et al. [19] stated that the TCA cycle was more active in OA patients due to increased expression of aconitic acid, isocitric acid, and citric acid.
Glycolysis/Pyruvate Pathway
Glycolysis is commonly affected in the progression of OA. Chondrocytes’ main energy source is heavily reliant on glycolysis due to a hypoxic environment [45]. Other studies revealed a strong relationship between the advancement of OA and lactate levels which were found to be markedly raised in synovial fluid of OA patients [46]. Jessica et al. [22] analyzed synovial fluid and synovium in 21 and 37 OA patients, respectively, and found upregulation of the glycolytic cycle with increased levels of glucose, lactate, and pyruvate in synovial samples.
Lipid Metabolism
A close association between OA and lipid metabolism has been observed. Lipolysis breaks down triacylglycerol into glycerol and fatty acids, which exert an important influence on the articular microenvironment and cellular function of joints. Lipolysis induces the production of excessive adipokines causing inflammation of local tissue [47]. According to Onurol et al. [48], certain glycerophospholipids were elevated in knee OA and can serve as biomarkers of OA. Altered lipid metabolism also causes elevation of phosphatidylethanolamine, LysoPCs, and PA levels in OA patients compared to the control group. After analyzing data from 70 individuals with OA of the knee and hip, Tootsi et al. [49] concluded that the disruption of lipid metabolism is a key pathophysiological characteristic of OA. Alteration in lipid metabolism causes elevation of levels of potential metabolites like glycerophospholipids (PC and LysoPC) and sphingolipids causing activation of proinflammatory mediators.
Markers of OA Staging
-
a.
Early and late markers of OA: Metabolomics can be applied in staging OA by identifying markers for assessing the progression and severity of the disease. Our study has analyzed some markers for early and late OA (Table 7). Corina et al. [12] showed heme was found at higher levels in early OA. Studies have also found elevated levels of phosphatidylcholine, diadenosine 5′,5′-diphosphate, and phosphatidylcholine in late OA.
-
b.
Obesity-related markers of OA: Some biomarkers were associated with OA in obese patients. Onurol et al. [48] in their study, additionally identified that obesity in OA patients affected the acidosis and metabolites concerning oxidative stress. Markers related to obesity in OA are mentioned in Table 7.
-
c.
Markers associated with KL staging of OA: Epidemiological studies of OA have frequently employed the Kellgren–Lawrence classification of diseases as a research tool that graded OA from 0 to 4 grades with increasing severity of the disease [50]. Kim et al. [15] analyzed the synovial fluid of 15 patients and noted a clear separation of metabolites for each stage, KL 1 showed increased levels of arabitol, galactose, glucose, mannose, and tagatose. KL 2 showed increased levels of urate, beta-alanine, pyruvate, and terephthalate. KL 3 and 4 showed increased levels of fatty acids, proline, phenylalanine, squalene, and trehalose-6-phosphate. Peyton et al. [8] analyzed 36 articles which were based on 4 samples (urine, plasma, synovial fluid, and serum) and they concluded that there are more than 10 metabolic pathways and a vast array of metabolites that are related to OA. They could only identify the tryptophan pathway as the only association with OA pathogenesis. Akhbari et al. [51] reviewed only 17 articles which consisted of only synovial fluid samples, which makes their research inconclusive. They had shortlisted 24 biomarkers that are not specific to OA but also to RA and other inflammatory arthropathies. Haartmans et al. [4] focused solely on the method of analysis using 24 studies in different samples and concluded that mass spectrometry was the best tool for metabolomic analysis; however, no metabolites or metabolic pathways associated with OA were identified. Even though numerous metabolites were shortlisted as significant, no direct correlation could be mapped from their analysis. Through the analysis of 50 articles which focused on samples of 6 different samples (urine, synovial fluid, serum, plasma, feces, and joint tissue), we were able to arrive at a definite conclusion to our review. Our study precisely narrowed down three specific pathways associated with OA pathogenesis which are TCA cycle, glycolysis, and lipid pathways. We were also able to narrow down metabolites that are specific for each stage of OA pathogenesis.
Limitations
Our study had some limitations. Heterogeneity is the foremost limitation in our study because selected populations were different in each study irrespective of age, gender, and ethnicity, and samples were also different in each study with various platforms for analysis differing in terms of coverage, sensitivity, and selectivity among the different studies. Each of these studies selected was a retrospective case–control study. These studies make it difficult to pinpoint the exact temporal sequence in which exposure factors and output occurrence occur which restricts the capability to determine the causality. There were numerous metabolites from different studies that were associated with OA, but no common association was identified in all studies. However, three metabolic pathways identified were common in all studies and could be narrowed down to a few significant pathways.
Future Recommendations
As per our current study, the use of metabolites in different samples can be tried for accurate analysis and identification of the progression of OA. Different metabolites were identified with different fluid samples which gave us a core idea about assessing the severity and progression of OA. Especially amino acids like arginine and valine, molecules like CAT, and fatty acid metabolites like LysoPC and PC could become targets for newer treatment development for OA but are not specific. Metabolic pathways like glycolysis, TCA cycle, and lipid pathways can be potential key targets for preventing OA progression, but further research in the field of metabolite identification specific to OA is required which will bridge the gap between current evolving ideas and concepts of metabolomics with early diagnosis, treatment, and disease control.
Conclusion
All these studies showed a vast array of metabolites and metabolic pathways associated with OA. Metabolites like lysophospholipids, phospholipids, arginine, BCCA, and histidine were identified as potential biomarkers of OA but a definite association was not identified. Three pathways (glycolytic pathway, TCA cycle, and lipid metabolic pathways) have been found as highly significant in OA pathogenesis. These metabolic pathways could provide novel therapeutic targets for the prevention and progression of the disease.
Data availability
All data is contained within the manuscript.
References
Lawrence, R. C., Felson, D. T., Helmick, C. G., Arnold, L. M., Choi, H., Deyo, R. A., et al. (2008). Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis and Rheumatism, 58(1), 26–35. https://doi.org/10.1002/art.23176
Kohn, M. D., Sassoon, A. A., & Fernando, N. D. (2016). Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clinical Orthopaedics and Related Research, 474(8), 1886–1893. https://doi.org/10.1007/s11999-016-4732-4
Wu, Z.-X., Ren, W.-X., & Wang, Z.-Q. (2022). Proximal fibular osteotomy versus high tibial osteotomy for treating knee osteoarthritis: A systematic review and meta-analysis. Journal of Orthopaedic Surgery and Research, 17(1), 470. https://doi.org/10.1186/s13018-022-03299-8
Haartmans, M. J. J., Emanuel, K. S., Tuijthof, G. J. M., Heeren, R. M. A., Emans, P. J., & Cillero-Pastor, B. (2021). Mass spectrometry-based biomarkers for knee osteoarthritis: A systematic review. Expert Review of Proteomics, 18(8), 693–706. https://doi.org/10.1080/14789450.2021.1952868
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ, 339, b2535. https://doi.org/10.1136/bmj.b2535
Moola, S., Munn, Z., Sears, K., Sfetcu, R., Currie, M., Lisy, K., et al. (2015). Conducting systematic reviews of association (etiology): The Joanna Briggs Institute’s approach. International Journal of Evidence-Based Healthcare, 13(3), 163–169. https://doi.org/10.1097/XEB.0000000000000064
Hahn, A. K., Batushansky, A., Rawle, R. A., Prado Lopes, E. B., June, R. K., & Griffin, T. M. (2021). Effects of long-term exercise and a high-fat diet on synovial fluid metabolomics and joint structural phenotypes in mice: An integrated network analysis. Osteoarthritis and Cartilage, 29(11), 1549–1563. https://doi.org/10.1016/j.joca.2021.08.008
Van Pevenage, P. M., Birchmier, J. T., & June, R. K. (2023). Utilizing metabolomics to identify potential biomarkers and perturbed metabolic pathways in osteoarthritis: A systematic review. Seminars in Arthritis and Rheumatism, 59, 152163. https://doi.org/10.1016/j.semarthrit.2023.152163
Hu, Y., Wu, Q., Qiao, Y., Zhang, P., Dai, W., Tao, H., et al. (2021). Disturbances in metabolic pathways and the identification of a potential biomarker panel for early cartilage degeneration in a rabbit anterior cruciate ligament transection model. Cartilage, 13(2_suppl), 1376S-1387S. https://doi.org/10.1177/1947603520921434
Carlson, A. K., Rawle, R. A., Wallace, C. W., Brooks, E. G., Adams, E., Greenwood, M. C., et al. (2019). Characterization of synovial fluid metabolomic phenotypes of cartilage morphological changes associated with osteoarthritis. Osteoarthritis and Cartilage, 27(8), 1174–1184. https://doi.org/10.1016/j.joca.2019.04.007
Zhang, W., Sun, G., Likhodii, S., Aref-Eshghi, E., Harper, P., Randell, E., et al. (2016). Metabolomic analysis of human synovial fluid and plasma reveals that phosphatidylcholine metabolism is associated with both osteoarthritis and diabetes mellitus. Metabolomics, 12, 1–10. https://doi.org/10.1007/s11306-015-0937-x
Bocsa, D.-C., Socaciu, C., Iancu, S., Pelea, M.-A., Roșca, R.-I., Leopold, N., et al. (2022). Stage related metabolic profile of synovial fluid in patients with acute flares of knee osteoarthritis. Medicine and Pharmacy Reports, 95, 438–445. https://doi.org/10.15386/mpr-2454
Zhang, W., Likhodii, S., Zhang, Y., Aref-Eshghi, E., Harper, P. E., Randell, E., et al. (2014). Classification of osteoarthritis phenotypes by metabolomics analysis. British Medical Journal Open, 4(11), e006286. https://doi.org/10.1136/bmjopen-2014-006286
Anderson, J. R., Chokesuwattanaskul, S., Phelan, M. M., Welting, T. J. M., Lian, L.-Y., Peffers, M. J., et al. (2018). 1H NMR metabolomics identifies underlying inflammatory pathology in osteoarthritis and rheumatoid arthritis synovial joints. Journal of Proteome Research, 17(11), 3780–3790. https://doi.org/10.1021/acs.jproteome.8b00455
Kim, S., Hwang, J., Kim, J., Ahn, J. K., Cha, H.-S., & Kim, K. H. (2017). Metabolite profiles of synovial fluid change with the radiographic severity of knee osteoarthritis. Joint, Bone, Spine, 84(5), 605–610. https://doi.org/10.1016/j.jbspin.2016.05.018
Yin, H., Wang, L., Li, Q., Zhang, J., Zhang, L., & Wang, X. (2017). Metabolomic analysis of biochemical changes in urine of osteoarthritis rat and interventional effects of Bushen-Huoxue herb couple. Chinese Herbal Medicines, 9(4), 369–375. https://doi.org/10.1016/S1674-6384(17)60117-5
Jiang, H., Liu, J., Qin, X.-J., Chen, Y.-Y., Gao, J.-R., Meng, M., et al. (2018). Gas chromatography-time of flight/mass spectrometry-based metabonomics of changes in the urinary metabolic profile in osteoarthritic rats. Experimental and Therapeutic Medicine, 15(3), 2777–2785. https://doi.org/10.3892/etm.2018.5788
Abdelrazig, S., Ortori, C. A., Doherty, M., Valdes, A. M., Chapman, V., & Barrett, D. A. (2021). Metabolic signatures of osteoarthritis in urine using liquid chromatography-high resolution tandem mass spectrometry. Metabolomics, 17(3), 29. https://doi.org/10.1007/s11306-021-01778-3
Li, X., Yang, S., Qiu, Y., Zhao, T., Chen, T., Su, M., et al. (2010). Urinary metabolomics as a potentially novel diagnostic and stratification tool for knee osteoarthritis. Metabolomics, 6(1), 109–118. https://doi.org/10.1007/s11306-009-0184-0
Loeser, R. F., Pathmasiri, W., Sumner, S. J., McRitchie, S., Beavers, D., Saxena, P., et al. (2016). Association of urinary metabolites with radiographic progression of knee osteoarthritis in overweight and obese adults: An exploratory study. Osteoarthritis and Cartilage, 24(8), 1479–1486. https://doi.org/10.1016/j.joca.2016.03.011
Rushing, B. R., McRitchie, S., Arbeeva, L., Nelson, A. E., Azcarate-Peril, M. A., Li, Y.-Y., et al. (2022). Fecal metabolomics reveals products of dysregulated proteolysis and altered microbial metabolism in obesity-related osteoarthritis. Osteoarthritis and Cartilage, 30(1), 81–91. https://doi.org/10.1016/j.joca.2021.10.006
Murillo-Saich, J. D., Coras, R., Meyer, R., Llorente, C., Lane, N. E., & Guma, M. (2022). Synovial tissue metabolomic profiling reveal biomarkers of synovial inflammation in patients with osteoarthritis. Osteoarthritis and Cartilage Open, 4(3), 100295. https://doi.org/10.1016/j.ocarto.2022.100295
Haudenschild, D. R., Carlson, A. K., Zignego, D. L., Yik, J. H. N., Hilmer, J. K., & June, R. K. (2019). Inhibition of early response genes prevents changes in global joint metabolomic profiles in mouse post-traumatic osteoarthritis. Osteoarthritis and Cartilage, 27(3), 504–512. https://doi.org/10.1016/j.joca.2018.11.006
Wang, S., Song, Y., Xu, F., Liu, H., Shen, Y., Hu, L., et al. (2023). Identification and validation of ferroptosis-related genes in lipopolysaccharide-induced acute lung injury. Cellular Signalling, 108, 110698. https://doi.org/10.1016/j.cellsig.2023.110698
Pousinis, P., Gowler, P. R. W., Burston, J. J., Ortori, C. A., Chapman, V., & Barrett, D. A. (2020). Lipidomic identification of plasma lipids associated with pain behaviour and pathology in a mouse model of osteoarthritis. Metabolomics, 16(3), 32. https://doi.org/10.1007/s11306-020-01652-8
Costello, C. A., Hu, T., Liu, M., Zhang, W., Furey, A., Fan, Z., et al. (2020). Differential correlation network analysis identified novel metabolomics signatures for non-responders to total joint replacement in primary osteoarthritis patients. Metabolomics, 16(5), 61. https://doi.org/10.1007/s11306-020-01683-1
Zhang, W., Sun, G., Likhodii, S., Liu, M., Aref-Eshghi, E., Harper, P. E., et al. (2016). Metabolomic analysis of human plasma reveals that arginine is depleted in knee osteoarthritis patients. Osteoarthritis and Cartilage, 24(5), 827–834. https://doi.org/10.1016/j.joca.2015.12.004
Zhai, G., Sun, X., Randell, E. W., Liu, M., Wang, N., Tolstykh, I., et al. (2021). Phenylalanine is a novel marker for radiographic knee osteoarthritis progression: The MOST study. The Journal of Rheumatology, 48(1), 123–128. https://doi.org/10.3899/jrheum.200054
Maerz, T., Sherman, E., Newton, M., Yilmaz, A., Kumar, P., Graham, S. F., et al. (2018). Metabolomic serum profiling after ACL injury in rats: A pilot study implicating inflammation and immune dysregulation in post-traumatic osteoarthritis. Journal of Orthopaedic Research, 36(7), 1969–1979. https://doi.org/10.1002/jor.23854
Maher, A. D., Coles, C., White, J., Bateman, J. F., Fuller, E. S., Burkhardt, D., et al. (2012). 1H NMR spectroscopy of serum reveals unique metabolic fingerprints associated with subtypes of surgically induced osteoarthritis in sheep. Journal of Proteome Research, 11(8), 4261–4268. https://doi.org/10.1021/pr300368h
Chen, R., Han, S., Liu, X., Wang, K., Zhou, Y., Yang, C., et al. (2018). Perturbations in amino acids and metabolic pathways in osteoarthritis patients determined by targeted metabolomics analysis. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences, 1085, 54–62. https://doi.org/10.1016/j.jchromb.2018.03.047
Schadler, P., Lohberger, B., Thauerer, B., Faschingbauer, M., Kullich, W., Stradner, M. H., et al. (2022). The association of blood biomarkers and body mass index in knee osteoarthritis: A cross-sectional study. Cartilage, 13(1), 19476035211069252. https://doi.org/10.1177/19476035211069251
Xie, Z., Aitken, D., Liu, M., Lei, G., Jones, G., Cicuttini, F., et al. (2022). Serum metabolomic signatures for knee cartilage volume loss over 10 years in community-dwelling older adults. Life, 12(6), 869. https://doi.org/10.3390/life12060869
Adams, S., Setton, L., & Nettles, D. (2013). The role of metabolomics in osteoarthritis research. The Journal of the American Academy of Orthopaedic Surgeons, 21, 63–64. https://doi.org/10.5435/JAAOS-21-01-63
Rockel, J. S., & Kapoor, M. (2018). The metabolome and osteoarthritis: Possible contributions to symptoms and pathology. Metabolites, 8(4), 92. https://doi.org/10.3390/metabo8040092
Berenguer, N. I., Canet, V. J. S., Soler-Canet, C., Segarra, S., García de Carellán, A., & Serra Aguado, C. I. (2024). Changes in the serum metabolome in an inflammatory model of osteoarthritis in rats. International Journal of Molecular Sciences, 25(6), 3158. https://doi.org/10.3390/ijms25063158
Zhai, G., Wang-Sattler, R., Hart, D. J., Arden, N. K., Hakim, A. J., Illig, T., et al. (2010). Serum branched-chain amino acid to histidine ratio: A novel metabolomic biomarker of knee osteoarthritis. Annals of the Rheumatic Diseases, 69(6), 1227–1231. https://doi.org/10.1136/ard.2009.120857
Piccionello, A., Sassaroli, S., Pennasilico, L., Rossi, G., Di Cerbo, A., Riccio, V., et al. (2023). Comparative study of 1H-NMR metabolomic profile of canine synovial fluid in patients affected by four progressive stages of spontaneous osteoarthritis. Scientific Reports, 14(1), 3627. https://doi.org/10.21203/rs.3.rs-3627758/v1
Xu, B., Su, H., Wang, R., Wang, Y., & Zhang, W. (2021). Metabolic networks of plasma and joint fluid base on differential correlation. PLoS ONE, 16(2), e0247191. https://doi.org/10.1371/journal.pone.0247191
Carlson, A. K., Rawle, R. A., Adams, E., Greenwood, M. C., Bothner, B., & June, R. K. (2018). Application of global metabolomic profiling of synovial fluid for osteoarthritis biomarkers. Biochemical and Biophysical Research Communications, 499(2), 182–188. https://doi.org/10.1016/j.bbrc.2018.03.117
Datta, P., Zhang, Y., Parousis, A., Sharma, A., Rossomacha, E., Endisha, H., et al. (2017). High-fat diet-induced acceleration of osteoarthritis is associated with a distinct and sustained plasma metabolite signature. Scientific Reports. https://doi.org/10.1038/s41598-017-07963-6
Zhai, G., Pelletier, J.-P., Liu, M., Randell, E. W., Rahman, P., & Martel-Pelletier, J. (2019). Serum lysophosphatidylcholines to phosphatidylcholines ratio is associated with symptomatic responders to symptomatic drugs in knee osteoarthritis patients. Arthritis Research & Therapy, 21, 224. https://doi.org/10.1186/s13075-019-2006-8
Meessen, J. M. T. A., Saberi-Hosnijeh, F., Bomer, N., den Hollander, W., van der Bom, J. G., van Hilten, J. A., et al. (2020). Serum fatty acid chain length associates with prevalent symptomatic end-stage osteoarthritis, independent of BMI. Scientific Reports, 10(1), 15459. https://doi.org/10.1038/s41598-020-71811-3
Choi, I., Son, H., & Baek, J.-H. (2021). Tricarboxylic acid (TCA) cycle intermediates: Regulators of immune responses. Life (Basel, Switzerland), 11(1), 69. https://doi.org/10.3390/life11010069
Tan, C., Li, L., Han, J., Xu, K., & Liu, X. (2022). A new strategy for osteoarthritis therapy: Inhibition of glycolysis. Frontiers in Pharmacology, 13, 1057229. https://doi.org/10.3389/fphar.2022.1057229
Song, Z., Li, X., Xie, J., Han, F., Wang, N., Hou, Y., et al. (2024). Associations of inflammatory cytokines with inflammatory bowel disease: A Mendelian randomization study. Frontiers in Immunology. https://doi.org/10.3389/fimmu.2023.1327879
Cao, X., Cui, Z., Ding, Z., Chen, Y., Wu, S., Wang, X., et al. (2022). An osteoarthritis subtype characterized by synovial lipid metabolism disorder and fibroblast-like synoviocyte dysfunction. Journal of Orthopaedic Translation, 33, 142–152. https://doi.org/10.1016/j.jot.2022.02.007
Senol, O., Gundogdu, G., Gundogdu, K., & Miloglu, F. D. (2019). Investigation of the relationships between knee osteoarthritis and obesity via untargeted metabolomics analysis. Clinical Rheumatology, 38(5), 1351–1360. https://doi.org/10.1007/s10067-019-04428-1
Tootsi, K., Kals, J., Zilmer, M., Paapstel, K., Ottas, A., & Märtson, A. (2018). Medium- and long-chain acylcarnitines are associated with osteoarthritis severity and arterial stiffness in end-stage osteoarthritis patients: A case-control study. International Journal of Rheumatic Diseases, 21(6), 1211–1218. https://doi.org/10.1111/1756-185X.13251
Wen, D. Y. (2000). Intra-articular hyaluronic acid injections for knee osteoarthritis. American Family Physician, 62(3), 565–570.
Akhbari, P., Karamchandani, U., Jaggard, M. K. J., Graça, G., Bhattacharya, R., Lindon, J. C., et al. (2020). Can joint fluid metabolic profiling (or “metabonomics”) reveal biomarkers for osteoarthritis and inflammatory joint disease? Bone & Joint Research, 9(3), 108–119. https://doi.org/10.1302/2046-3758.93.BJR-2019-0167.R1
Lamers, R. J. A. N., van Nesselrooij, J. H. J., Kraus, V. B., Jordan, J. M., Renner, J. B., Dragomir, A. D., et al. (2005). Identification of an urinary metabolite profile associated with osteoarthritis. Osteoarthritis and Cartilage, 13(9), 762–768. https://doi.org/10.1016/j.joca.2005.04.005
Mickiewicz, B., Kelly, J. J., Ludwig, T. E., Weljie, A. M., Wiley, J. P., Schmidt, T. A., et al. (2015). Metabolic analysis of knee synovial fluid as a potential diagnostic approach for osteoarthritis. Journal of Orthopaedic Research, 33(11), 1631–1638. https://doi.org/10.1002/jor.22949
Kosinska, M. K., Liebisch, G., Lochnit, G., Wilhelm, J., Klein, H., Kaesser, U., et al. (2014). Sphingolipids in human synovial fluid—A lipidomic study. PLoS ONE, 9(3), e91769. https://doi.org/10.1371/journal.pone.0091769
Chandra, D., Ashraf, D., Yadav, P., & Raghuvanshi, V. (2023). Synovial fluid proteomics and serum metabolomics reveal molecular and metabolic changes in osteoarthritis. Molecular Biology and Biochemistry, 1, 1–9. https://doi.org/10.5281/zenodo.10255533
Rockel, J. S., Layeghifard, M., Rampersaud, Y. R., Perruccio, A. V., Mahomed, N. N., Davey, J. R., et al. (2022). Identification of a differential metabolite-based signature in patients with late-stage knee osteoarthritis. Osteoarthritis and Cartilage Open, 4(3), 100258. https://doi.org/10.1016/j.ocarto.2022.100258
Rockel, J. S., Zhang, W., Shestopaloff, K., Likhodii, S., Sun, G., Furey, A., et al. (2018). Metabolomics with severity of radiographic knee osteoarthritis and early phase synovitis in middle-aged women from the Iwaki Health Promotion Project: A cross-sectional study. PLoS ONE, 13(6), e0199618. https://doi.org/10.1371/journal.pone.0199618
Sasaki, E., Yamamoto, H., Asari, T., Matsuta, R., Ota, S., Kimura, Y., et al. (2022). Metabolomics with severity of radiographic knee osteoarthritis and early phase synovitis in middle-aged women from the Iwaki Health Promotion Project: A cross-sectional study. Arthritis Research & Therapy, 24(1), 145. https://doi.org/10.1186/s13075-022-02830-w
Werdyani, S., Liu, M., Sun, G., Furey, A., Randell, E., Rahman, P., et al. (2020). Plasma metabolomics identified three distinct endotypes of primary osteoarthritis patients. Osteoarthritis and Cartilage, 28, S23–S24. https://doi.org/10.1016/j.joca.2020.02.036
Huang, Z., He, Z., Kong, Y., Liu, Z., & Gong, L. (2020). Insight into osteoarthritis through integrative analysis of metabolomics and transcriptomics. Clinica Chimica Acta, 510, 323–329. https://doi.org/10.1016/j.cca.2020.07.010
Sun, S., Chen, M., Zhang, T., Wang, Y., Shen, W., Zhang, T., et al. (2024). Identification of key factors in cartilage tissue during the progression of osteoarthritis using a non-targeted metabolomics strategy. Phenomics. https://doi.org/10.1007/s43657-023-00123-z
Welhaven, H. D., Welfley, A. H., Brahmachary, P., Bergstrom, A. R., Houske, E., Glimm, M., et al. (2024). Metabolomic profiles and pathways in osteoarthritic human cartilage: A comparative analysis with healthy cartilage. Metabolites, 14(4), 183. https://doi.org/10.1101/2024.01.25.577269
Tootsi, K., Vilba, K., Märtson, A., Kals, J., Paapstel, K., & Zilmer, M. (2020). Metabolomic signature of amino acids, biogenic amines and lipids in blood serum of patients with severe osteoarthritis. Metabolites, 10(8), 323. https://doi.org/10.3390/metabo10080323
Zhang, Q., Li, H., Zhang, Z., Yang, F., & Chen, J. (2015). Serum metabolites as potential biomarkers for diagnosis of knee osteoarthritis. Disease Markers, 2015, 684794. https://doi.org/10.1155/2015/684794
Acknowledgements
Nil.
Funding
Nil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Standard Statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed Consent
For this type of study informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arjun, A., Chellamuthu, G., Jeyaraman, N. et al. Metabolomics in Osteoarthritis Knee: A Systematic Review of Literature. JOIO 58, 813–828 (2024). https://doi.org/10.1007/s43465-024-01169-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43465-024-01169-5