Abstract
Sarcopenia, the decline of muscle mass with age, causes impaired gait, disability and falls. It may therefore increase the risk of fracture for osteoporotic women. The aims of this study were to determine the prevalence of sarcopenia in osteopenic and osteoporotic women, and to determine if hormone replacement therapy (HRT), diet, or physical activity (PA) has a role in the prevention of sarcopenia. One hundred and thirty-one premenopausal and 82 postmenopausal (54 taking HRT) healthy women (17–77 years) volunteered for the study. Body composition was measured by dual X-ray absorptiometry (DXA). Sarcopenia was defined as a relative skeletal muscle index (RSMI) (appendicular skeletal muscle mass divided by height) below 5.45 kg/m2. Osteopenia was defined by a densitometric t -score for bone mineral density (BMD) (g/cm2) below −1.0 and osteoporosis by a t -score below −2.5. Nutrient intake was assessed using 3-day food records and physical activity (PA) was measured using the Baecke Physical Activity Questionnaire. Pearson chi-squared, independent t -tests, simple correlation and multiple regression were used to analyze the data. In premenopausal osteopenic women the prevalence of sarcopenia was 12.5%. In postmenopausal women it was 25% for those with osteopenia, and 50% for those with osteoporosis. PA was independently related to RSMI (β=0.222, p =0.0001), but diet and HRT were not. After adjusting for PA, RSMI was not significantly related to BMD. These data suggest that the relationship between RSMI, BMD and risk of osteoporosis may largely be mediated through participation in PA. Sarcopenia screening simultaneous to BMD examinations by DXA, may be of value in identifying osteoporotic women with sarcopenia, a group that may be most in need of exercise interventions to increase muscle and BMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is associated with several anatomical changes that are conducive to increased disability, frailty and falls. Much of this physical impairment is due to a gradual deterioration of bone (osteopenia) and a progressive decline in lean tissue. This loss of muscle mass is referred to as sarcopenia [1]. Observational studies have shown that muscle mass starts to decrease at approximately 1% per year following the fourth decade of life [2–3]. Sarcopenia is often marked by weight stability [4], which is due in part to other age-related changes in body composition, such as increased fat mass [5–6].
Several groups have reported the prevalence of sarcopenia [2,7–10], but these findings lack consistency because of different techniques used for the measurement of lean mass, and the use of different reference populations. Based on data from NHANES III, the prevalence of osteopenia and osteoporosis were estimated to be 42% and 17%, respectively, in women >50 years, and low bone density was found to be most common in white women [11]. British age-adjusted prevalence levels of osteopenia and osteoporosis are substantially lower than those reported in NHANES III [12]. Given that the proportion of elderly people (≥65 years) in the population is set to increase [13], this is likely to lead to increased incidence of sarcopenia and osteopenia. Undoubtedly, this will be a major health care concern, increasing the financial burden and decreasing the quality of life for the elderly.
Just as peak muscle mass is a determinant in the development of sarcopenia, the development of osteoporosis depends on the maximum bone mass achieved in young adulthood, the age at which bone loss begins and the rate of loss, all of which are influenced by endocrine, genetic and nutritional factors. Menopause has been linked to a reduction in lean mass (LM) and bone mineral density (BMD) [14–16], both of which are directly related to a reduced output of ovarian hormones that is differentiated from the aging process [17]. There is controversy, however, with regards to the role of menopause in the loss of LM, and to whether hormone replacement therapy (HRT) can prevent these changes.
Several studies have shown a positive relationship between LM and BMD [18–23], and osteoporotic women have been shown to have significantly lower appendicular skeletal muscle mass compared with matched non-osteoporotic controls [24]. Based on the theory that muscle mass is an indicator of BMD, it could be speculated that sarcopenia is a risk factor in the development of osteopenia, and that it is more prevalent among osteopenic individuals. However, to date this hypothesis has not been extensively tested. One cross-sectional study in France showed no association between osteoporosis and sarcopenia [24].
Consequently, the purpose of this study was (1) to determine if sarcopenia is more prevalent among osteopenic and osteoporotic women compared with normal women; (2) to assess the role of HRT in protection against sarcopenia; and (3) to identify predictors of muscle mass in women of varying ages, as potential areas to target for the prevention of sarcopenia.
Subjects and methods
Subjects
Subjects were recruited from the Birmingham area of Alabama by word-of-mouth and advertisement in community and fitness centers. Two hundred thirteen healthy women (206 Caucasian and seven African-American), aged between 17 and 77 years were selected for the study. Criteria for exclusion were pregnancy, metabolic or endocrine disorders and intake of cholesterol or blood pressure lowering medication. Eighty-two of the volunteers were postmenopausal, 54 of which were taking HRT. Menopausal status was self-reported and defined as an absence of menses for at least 12 months prior to the investigation. The data were collected during a single laboratory visit, following an overnight fast. All participants provided informed consent, and the Institutional Review Board of the University of Alabama, at Birmingham, granted approval.
Anthropometry
Body weight was measured to the nearest 0.1 kg using a calibrated clinical scale, with each subject wearing a swimsuit. Height was determined bare foot to the nearest 0.5 cm, using a stadiometer.
Dual X-ray absorptiometry
Percentage body fat (%BF), total fat mass (FM), total fat-free mass (FFM), appendicular lean mass, BMD and bone mineral content were determined by total body scans (model DPX-L, software version 3.2; Lunar Radiation, Madison, WI, USA). Subjects were scanned in light, metal-free clothing while lying still, flat on their backs with their arms by their sides. All scans were performed and analyzed in the Division of Physiology and Metabolism at the Department of Nutrition Sciences. Osteopenia was distinguished by a densitometric t -score for BMD below −1.0 and osteoporosis by a t -score below −2.5. Sarcopenia was defined using the classification of Baumgartner et al. [2]. Relative skeletal muscle index (RSMI) was derived from appendicular muscle mass (kg) divided by height (m2). Clinically significant sarcopenia was defined as an RSMI value greater than 2 standard deviations (2 SD) below the sex-specific mean of a young healthy reference population (<5.45 kg/m2 for women).
Dietary assessment
Nutrient intakes were assessed using 3-day estimated food records, which were completed by subjects prior to their laboratory visit. During the visit they were checked for completeness by an investigator. All except two subjects recorded their daily dietary intake for 3 days, including 1 weekend day. Total energy, protein, fat, and carbohydrate intake were estimated using the US Department of Agriculture Dietary Analysis Program for microcomputers (National Technical Information Service Federal Computer Products Center, Springfield, VA). The average of the 3-day intakes was used for analyses.
Physical activity
Physical activity (PA) was assessed using the Baecke Questionnaire of Habitual Physical Activity [25]. All except one subject completed the questionnaires. This questionnaire has been validated previously [26–28] and the authors report a 3-month test-retest reliability coefficient of 0.74–0.9. Normal PA was assessed for three components: work, sport and leisure. All responses, with the exception of occupational activity and type of sport played, were pre-coded on a 5-point scale, with descriptors ranging from never [1] to very often [5]. Occupational activity was scored as low- [1], middle- [3] or high-level [5] activity. The sport score was calculated as the sum of (intensity code × duration code × code for proportion of the year) × 1.25. Each activity component could receive a maximum of 5 points, giving a maximum of 15 for the PA index. Each index was rounded to the nearest tenth of a point.
Statistics
All analyses were done using the Statistical Package for the Social Sciences, version 11 (SPSS, Chicago, IL). Descriptive statistics were used to determine the prevalence of sarcopenia, osteopenia and osteoporosis in the sample. Pearson chi-squared was used to analyze associations between sarcopenia, osteopenia and HRT. Two-sided independent t -tests were used to compare mean observations between premenopausal and postmenopausal women, and between sarcopenic and normal women. Simple zero-order correlations were used to analyze the relationship between RSMI, BMD, age, height, weight, BMI, %BF, PA and nutrient intake. To determine if PA was independently related to RSMI after adjusting for age and weight, a multiple regression model was developed with RSMI as the dependent variable. To determine if the relationship between RSMI and BMD was independent of age, weight and PA, multiple regression models were developed with BMD as the dependent variable. Premenopausal and postmenopausal women were tested separately for some analyses, due to obvious differences between each group. Statistical significance was set at p <0.05.
Results
Subject characteristics
Subject characteristics, and diet and physical activity data are presented in Tables 1 and 2, respectively. Postmenopausal women ( n =82), were heavier and had higher %BF compared with the younger premenopausal women ( n =131). Sarcopenic women ( n =16), were older, lighter, had lower FFM and BMC compared with the normal postmenopausal women ( n =66). Nutrient intakes were not significantly different between groups, but PA was lower in postmenopausal compared with premenopausal women, and in sarcopenic compared with normal women.
Prevalence of sarcopenia, osteopenia and osteoporosis
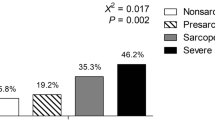
Fig. 1 shows the prevalence of sarcopenia, osteopenia and osteoporosis with age. In premenopausal women the prevalence of sarcopenia was 1.5%; prevalence of osteopenia was 6.1%, and the combination of having both sarcopenia and osteopenia was 0.8%. Osteoporosis was not present in the premenopausal women. For those with osteopenia, 12.5% had sarcopenia compared with 0.8% in those with normal BMD. However, Pearson chi-squared analysis with continuity correction of 1.264 showed no association between sarcopenia and osteopenia in premenopausal women ( p =0.261). In postmenopausal women, the prevalence of sarcopenia and osteopenia were each 19.5%, and prevalence of osteoporosis was 4.9%. The prevalence of combined sarcopenia and osteopenia was 7.3% in postmenopausal women. In those with normal BMD, the prevalence of sarcopenia was 16.1%, compared with 25% in the osteopenic and 50% in the osteoporotic postmenopausal women. As with the premenopausal women, Pearson chi-squared analysis showed no association between sarcopenia and osteopenia or osteoporosis in postmenopausal women ( p =0.209).
Influence of HRT on sarcopenia, osteopenia and osteoporosis
The prevalence of sarcopenia was 18.5% in women taking HRT, compared with 21.4% in those who were not. HRT did not appear to be protective against sarcopenia, and RSMI was not significantly different ( p =0.591) among those taking (6.2±0.8 kg/m2) and not taking (6.3±1.0 kg/m2) HRT. Linear regression also showed that HRT use was not a predictor of RSMI (β=0.155, p =0.164). The prevalence of osteopenia was 16.7% and osteoporosis was 1.9% in women taking HRT, compared with 25.0% and 10.7% in women not taking HRT. BMD was significantly higher ( p =0.027) in women taking HRT (1.13±0.08 g/cm2) compared with those who were not (1.08±0.11 g/cm2).
Predictors of RSMI and BMD
Simple zero-order correlations of RSMI and BMD with the various study measures are shown in Table 3. Total energy, protein, carbohydrate and fat intakes, and %BF were not associated with either BMD or RSMI. Multiple regression analysis (Table 4) showed that age, weight and PA were independently related to RSMI. In addition, multiple regression analysis found that the relationship between RSMI and BMD was not independent and was influenced by age, weight and PA (Table 5).
Discussion
The main aim of this study was to quantitatively test the hypothesis that sarcopenia is more prevalent in osteopenic and osteoporotic women compared to women with a normal BMD. Using the classification of Baumgartner et al. [2], the prevalence of sarcopenia in this sample was 11.7%, and was higher in premenopausal osteopenic women compared to those with normal BMD. In postmenopausal women the prevalence of sarcopenia was 8.9% higher in osteopenic women and 33.9% higher in osteoporotic women compared to the women with normal BMD. However, Pearson chi-squared analysis showed no association between sarcopenia and osteopenia or osteoporosis. This is consistent with the findings of Gillette-Guyonnet et al. [24], who did not show a positive association between sarcopenia and osteoporosis. RSMI was positively related to BMD ( r =0.402, p =0.000), however. This significant correlation disappeared after adjusting for PA, suggesting that the parallel progression of reduced bone density and muscle mass with age may at least in part be mitigated by low PA levels. In addition, aging itself was independently related to RSMI, BMD and PA index.
Regardless of the role of sarcopenia in bone loss through the muscle-bone relationship, it indirectly influences the risk of fracture because it causes impaired balance and falls [1]. In addition, the amount of muscle that surrounds a bone may act as a form of protection or padding during a fall, by attenuating the force of impact on the bone. FM could also act to protect bone in this way, but this study found no significant difference in the FM of sarcopenic and normal women. Therefore, these findings suggest that osteopenic and osteoporotic women with sarcopenia may have a higher risk of fracture compared with their non-sarcopenic counterparts. In the present study, 25% of osteopenic women and 50% of osteoporotic women have sarcopenia and consequently may have an increased fracture risk.
The prevalence of sarcopenia for women over 60 years in this sample (23.1%), is similar to that reported by Baumgartner et al. [2] and Iannuzzi-Sucich et al. [8]. The findings are not consistent, however, with those reported by Janssen et al. [9], where sarcopenia was defined using a different method and skeletal muscle mass was measured using bio impedance, or by Tankó et al. [10], where a different reference population was used. Newman et al. [29] proposed that FM should be considered when evaluating sarcopenia in women, and in overweight or obese individuals. An apparently normal muscle mass could mask sarcopenia in overweight individuals who have insufficient muscle mass relative to their FM or body size. These disparities highlight the need for a consensus definition of sarcopenia that can be used as a precise and accurate measurement of sarcopenia in epidemiological studies.
Osteoporosis costs $13.8 billion annually in the US, and those who experience hip fractures have an increased risk of death for up to 12 months following the fracture [13]. Guidelines based on systematic reviews and cost-effective analyses have suggested that it is worthwhile to measure BMD in white women aged 65 years and over [30]. Considering that sarcopenia increases the risk of falls and hence fractures, it may be worthwhile to screen for it concurrently to BMD examinations. DXA enables determination of BMD and LM, either in whole-body or selected regions, with higher accuracy and precision than any other noninvasive method [30]. Therefore, DXA could be currently regarded as the best available method for the diagnosis of sarcopenia, osteopenia and osteoporosis.
An additional aim of this study was to determine if HRT has a role in the prevention of sarcopenia. Following the results of the Women’s Health Initiative study in 2002, it is clear that HRT therapy is effective in the prevention of osteoporosis, but that it also causes a modest increase in breast cancer risk after 5 years of use [32]. Since then, concern over the safety of HRT has resulted in a greater than 30% decline in the use of Premarin and Prempro (conjugated estrogens), the most commonly used forms of HRT [33]. The possibility that HRT may also have a role in the prevention of muscle-mass decline could make it a more worthwhile option for women with both sarcopenia and osteopenia. Sørensen et al. [34] found that lean mass and BMD increased, and fat mass decreased during HRT use, in a placebo-controlled, crossover study. In contrast, a cross-sectional study of elderly women receiving HRT found no association between HRT and lean mass, or strength. In addition, it has been shown that there is no difference in the prevalence of sarcopenia in women taking and not taking HRT [35]. The findings of this study, that there was no significant difference in RSMI between women taking and not taking HRT, also suggest that HRT does not protect against muscle loss or sarcopenia.
The final aim of this study was to determine predictors of RSMI in premenopausal and postmenopausal women. PA was found to be the only manipulative predictor of RSMI, and is therefore an area that could be targeted in the prevention of sarcopenia and osteopenia. Several studies have shown that muscle strength and size are increased by resistance training [1, 36–38]. Improvements have also been observed in the BMD of older adults, following this type of exercise [39–40], and a proportional relationship was found between exercise intensity and change in BMD [41]. Aside from the direct effect of exercise on BMD, the results of this study suggest that the relationship between PA and BMD are also mediated through the role of PA in the maintenance of muscle mass, which indicates a potential use for strength training in reducing falls and consequent fractures in the elderly population. It has recently been suggested that muscle contractions could be the most significant component influencing BMC in the whole body [42]. In this study Capozza et al. found a stronger relationship between BMC and LM in the lower limbs compared with the whole body. Despite the presence of baseline muscle atrophy and ultrastructural damage, frail elders have been shown to respond to strength training with musculoskeletal remodeling and significant increases in muscle mass [36]. The American College of Sports Medicine [43], recommended strength training 2–3 days a week for optimal musculoskeletal fitness. It has also been shown that injury rate from strength training is low and that it is incompatible with few medical conditions [36]. The capacity of strength training to reduce or delay functional limitations and falls indicates its role for the prevention of sarcopenia and consequent osteoporotic fractures [44].
Research has shown that particular nutrients may play a role in the prevention of sarcopenia [45] and osteopenia [46–49]. However, the current study found no association between macronutrient intake and sarcopenia, osteopenia or osteoporosis.
The limitations of the current study are acknowledged. The subjects were not randomly selected; they were healthy volunteers, and, consequently, they may not be truly representative of the general female population. In addition, there were no subjects over 77 years, so the results may not be representative or typical for very old women. Osteopenia and osteoporosis were diagnosed by total body scans alone, because regional DXA scans of the femoral neck and lumbar spine were not taken. In addition to these limitations, PA and nutrient intake were measured by self-reporting, a method shown to bear discrepancies, such as over-reporting of physical activity [50–51] and under-reporting of energy intake [52–53]. Although not as common as female osteoporosis, the incidence of osteoporosis and fractures in men is increasing [11]. Therefore, future efforts to assess sarcopenia in terms of osteoporosis should include both males and females and represent more categories of old age.
Conclusion
This study has found that 12.5% of osteopenic premenopausal women, 25% of osteopenic postmenopausal women and 50% of osteoporotic postmenopausal women have sarcopenia, and therefore may have an increased risk of fracture compared to osteopenic women and osteoporotic women with a normal RSMI. Screening for sarcopenia by DXA simultaneous to osteoporosis screening would identify these women, who are clearly those most in need of strength-training intervention. This study also highlights the need for a consensus definition of sarcopenia.
Abbreviations
- BF :
-
Body fat
- BMC :
-
Bone mineral content
- BMD :
-
Bone mineral density
- DXA :
-
Dual X-ray absorptiometry
- FFM :
-
Fat free mass
- FM :
-
Fat mass
- LM :
-
Lean mass
- HRT :
-
Hormone replacement therapy
- PA :
-
Physical activity
- RSMI :
-
Relative skeletal muscle index
References
Rosenberg IR, Roubenoff R (1995) Stalking sarcopenia. Ann Intern Med 123:727–728
Baumgartner RN, Koehler KM, Romero LJ, Lindeman RD, Garry PJ (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:744-63
Janssen I, Heymsfield S, Wang Z, Ross R (2000) Skeletal muscle mass and distribution in 468 men and women aged 18–88 years. J Appl Physiol 89:81–88
Gallagher D, Ruts E, Visser M, Heshka, Baumgartner RN, Wang J, Pierson RN, Pi-Sunyer FX, Heymsfield SB (2000) Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab 279:E366–375
Martini G, Valenti R, Giovani S, Nuti R (1997) Age-related changes in body composition of healthy and osteoporotic women. Maturitas 27:25–33
Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA (2002) Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr 76:473–481
Kyle UG, Genton L, Hans D, Karsegard VL, Michel JP, Slosman DO, Pichard C (2001) Total body mass, fat mass, fat-free mass, and skeletal muscle in older people: cross-sectional differences in 60-year-old persons. J Am Geriatr Soc 49:1633–1640
Iannuzzi-Sucich M, Prestwood KM, Kenny AM (2002) Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy older men and women. J Gerontol A Biol Sci Med Sci 57:M772–777
Janssen I, Heymsfield SB, Ross R (2002) Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50:889–896
Tankó LB, Movsesyan L, Mouritzen U, Christiansen C, Svendsen OL (2002) Appendicular lean tissue mass and the prevalence of sarcopenia among healthy women. Metabolism 51:69–74
Looker AC, Orwoll ES, Johnston Jr CC, Lindsay RL, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP (1997) Prevalence of low femoral bone density in older US adults from NHANES III. J Bone Miner Res 12:1769–1771
Holt G, Khaw KT, Reid DM, Compston JE, Bhalla A, Woolf AD, Crabtree NJ, Dalzell N, Wardley-Smith B, Lunt M, Reeve J (2002) Prevalence of osteoporotic bone mineral density at the hip in Britain differs substantially from the US over 50 years of age: implications for clinical densitometry. Br J Radiol 75:736–742
US Department of Health and Human Services (2000) Healthy People 2010: understanding and improving health. US Government Printing Office, Washington DC
Aloia JF, McGowan GM, Vaswani AN, Ross P, Cohn GD (1991) Relationship of menopause to skeletal and muscle mass. Am J Clin Nutr 53:1378–1383
Ley CJ, Lees B, Stevenson JC (1992) Sex- and menopause-associated changes in body fat distribution. Am J Clin Nutr 55:950–954
Douchi T, Yamamoto S, Nackamura S (1998) The effect of menopause on regional and body lean mass. Maturitas 29:247–252
Douchi T, Yamamoto S, Yoshimitsu N, Andoh T, Matsuo T, Nagata Y (2002) Relative contribution of aging and menopause to changes in lean and fat mass in segmental regions. Maturitas 42:301–306
Douchi T, Oki T, Nakamura S, Ijuin H, Yamamoto S, Nagata Y (1997) The effect of body composition on bone density in pre- and post-menopausal women. Maturitas 27:55–60
Visser M, Kiel DP, Langois J, Hannan MT, Felson DT, Wilson PW, Harris TB (1998) Muscle mass and fat mass in relation to bone mineral density in very old men and women: the Framingham Heart Study. Appl Radiat Isot 49:745–747
Douchi T, Yamamoto S, Oki T, Maruta K, Kuwahata R, Nagata Y (2000) Relationship between body fat distribution and bone mineral density in premenopausal Japanese women. Obstet Gynecol 95:722–755
Winters KM, Snow CM (2000) Body composition predicts bone mineral density and balance in premenopausal women. J Womens Health Gend Based Med 9:865–872
Blain H, Vuillemin A, Teissier A, Hanesse B, Guillemin F, Jeandel C (2001) Influence of muscle strength and body weight and composition on regional bone mineral density in healthy women aged 60 years and over. Gerontology 47:207–212
Bakker I, Twisk JW, Van Mechelen W, Kemper HC (2003) Fat-free body mass is the most important body composition determinant of 10-year longitudinal development of lumbar bone in adult men and women. J Clin Endocrinol Metab 88:2607–2613
Gillette-Guyonnet S, Nourhashemi F, Laque S, Grandjean H, Vellas B (2000) Body composition and osteoporosis in elderly women. Gerontology 46:189–193
Baecke JAH, Burema J, Frijters JER (1982) A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 36:936–942
Jacobs DR Jr, Ainsworth BE, Hartman TJ, Leon AS (1993) A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc 25:81–91
Philippaerts RM, Westerterp KR, Lefevre J (1999) Doubly labelled water validation of three physical activity questionnaires. Int J Sports Med 20:284–289
Bonnefoy M, Normand S, Pachiaudi C, Lacour JR, Laville M, Kostka T (2001) Simultaneous validation of ten physical activity questionnaires in older men: a doubly labelled water study. J Am Geriatr Soc 49:28–35
Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB (2003) Sarcopenia: Alternative definitions and associations with lower extremity function. J Am Geriatr Soc 51:1602–1609
Cummings SR, Bates D, Black DM (2002) Clinical use of bone densitometry: scientific review. JAMA 288:1889–1897
Visser M, Fuerst T, Lang T, Salamone L, Harris T (1999) Validity of fan-beam dual X-ray absorptiometry for measuring the fat-free mass and leg muscle mass. J Appl Physiol 87:1513–1520
Burger H (2003) Hormone replacement therapy in the post-women’s health initiative era. Report of a meeting held in Funchal, Maderia, February 24–25, 2003. Climacteric 6[Suppl 1]:11–36
Austin P, Mamdani M, Tu K, Jaakkimainen L (2003) Prescriptions for estrogen replacement therapy in Ontario before and after publication of the Women’s Health Initiative Study. JAMA 289:3241–3242
Sørensen MB, Rosenfalck AM, Højgaad L, Ottsen B (2001) Obesity and sarcopenia after menopause are reversed by sex hormone replacement therapy. Obes Res 9:622–626
Kenny AM, Dawson L, Kleppinger A, Iannuzzi-Sucich, Judge JO (2003) Prevalence of sarcopenia and predictors of skeletal muscle mass in non-obese women who are long-term users of estrogen replacement therapy. J Gerontol A Biol Sci Med Sci 58A:436–440
Fiatarone MA, Marks E, Ryan N, Meredith C, Lipsitz, Evans W (1990) High intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 263:1038–1044
Fiatarone Singh MA, Ding W, Manfredi TJ, Solares GS, O’Neill EF (1999) Insulin-like growth factor 1 in skeletal muscle after weight-lifting exercise in frail elders. Am J Physiol 277:E135–143
Hunter GR, Treuth MS (1995) Relative training intensity and increases in strength in older women. J Strength Cond Res 9:188–191
Nelson M, Fiatarone MA, Morganti C, Trice I, Greenberg R, Evans W (1994) Effect of high intensity strength training on multiple risk factors for osteoporotic fracture. JAMA 272:1909–1914
Kohrt W, Ehsani A, Birge S (1997) Effects of exercise involving predominantly either joint-reaction or ground-reaction forces on bone mineral density in older women. J Bone Miner Res 12:1253–1261
Cussler E, Lohman T, Going S (2003) Weight lifted in strength training predicts bone change in postmenopausal women. Med Sci Sports Exerc 35:10–17
Capozza RF, Cointry GR, Cure-Ramírez PC, Ferretti JL, Cure-Cure C (2004) A DXA study of muscle-bone relationships in the whole body and limbs of 2512 normal men and pre- and post-menopausal women. Bone 35:283–295
American College of Sports Medicine (1998) Position stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc 30:975–991
Hunter GR, McCarthy JP, Bamman MM (2004) Effects of resistance training on older adults. Sports Med 34:329–348
Friedman DR (2003) Are you doing all you can to fight sarcopenia? Tufts University Health and Nutrition Letter 21:1–5
Kerstetter JE, Looker AC, Insogna KL (2000) Low protein intake and low bone density. Calcif Tissue Int 66:313–321
Kerstetter JE, O’Brien KO, Insogna KL (2003) Dietary protein, calcium metabolism, and skeletal homeostasis revisited. Am J Clin Nutr 78:584S–592S
Chiechi LM, Secreto G, D’Amore M, Fanelli E, Venturelli F, Cantatore F, Loizzi P (2002) Efficacy of a soy rich diet in preventing postmenopausal osteoporosis: the Menfis randomised trial. Maturitas 42:295–300
Delmas PD (2002) Treatment of postmenopausal osteoporosis. Lancet 359:2018–2026
Klesges RC, Eck LH, Melton MW, Fulliton W, Sommes GW, Hanson CL (1990) The accuracy of self reports of physical activity. Med Sci Sports Exerc 22:690–697
Irwin MI, Ainsworth BE, Conway JM (2001) Estimation of energy expenditure from physical activity measures: Determinants of accuracy. Obes Res 9:517–525
Hill RJ, Davies PSW (2001) The validity of self-reported energy intake as determined using the doubly labelled water technique. Br J Nutr 85:415–430
Hebert JR, Patterson RE, Gorfine M, Ebbelling CB, Jeor ST, Chlebowski RT (2003) Differences between estimated calorie requirements and self-reported calorie intake in the Women’s Health Initiative. Ann Epidemiol 13:629–637
Acknowledgements
This study was supported by the Fogarty International Research Grant, Department of Health and Human Services, Public Health Service, AL, USA (1 FO5 TWO 4694-02). Sincere thanks to the volunteers for their participation, and to Robert Petri for his invaluable technical assistance
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walsh, M.C., Hunter, G.R. & Livingstone, M.B. Sarcopenia in premenopausal and postmenopausal women with osteopenia, osteoporosis and normal bone mineral density. Osteoporos Int 17, 61–67 (2006). https://doi.org/10.1007/s00198-005-1900-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-005-1900-x