Abstract
Purpose
There are no universally agreed guidelines regarding which types of physical activity are safe and/or recommended in the perioperative period for patients undergoing ventral hernia repair or abdominal wall reconstruction (AWR). This study is intended to identify and summarise the literature on this topic.
Methods
Database searches of PubMed, CINAHL, Allied & Complementary medicine database, PEDro and Web of Science were performed followed by a snowballing search using two papers identified by the database search and four hand-selected papers of the authors’ choosing. Inclusion—cohort studies, randomized controlled trials, prospective or retrospective. Studies concerning complex incisional hernia repairs and AWRs including a “prehabilitation” and/or “rehabilitation” program targeting the abdominal wall muscles in which the interventions were of a physical exercise nature. RoB2 and Robins-I were used to assess risk of bias. Prospero CRD42021236745. No external funding. Data from the included studies were extracted using a table based on the Cochrane Consumers and Communication Review Group’s data extraction template.
Results
The database search yielded 5423 records. After screening two titles were selected for inclusion in our study. The snowballing search identified 49 records. After screening one title was selected for inclusion in our study. Three total papers were included—two randomised studies and one cohort study (combined 423 patients). All three studies subjected their patients to varying types of physical activity preoperatively, one study also prescribed these activities postoperatively. The outcomes differed between the studies therefore meta-analysis was impossible—two studies measured hernia recurrence, one measured peak torque. All three studies showed improved outcomes in their study groups compared to controls however significant methodological flaws and confounding factors existed in all three studies. No adverse events were reported.
Conclusions
The literature supporting the advice given to patients regarding recommended physical activity levels in the perioperative period for AWR patients is sparse. Further research is urgently required on this subject.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ventral hernias and ventral hernia repairs (VHR) are common. A recent national database study found that five percent of all patients who had undergone a laparotomy in France during 2010 had subsequently undergone a repair of an incisional hernia resulting from that laparotomy by 2015 [1]. In the United States the number of ventral hernia repairs performed annually has increased by roughly 50% to around 500,000 in little more than a decade [2, 3].
Recurrence after VHR is also common and the risk increases with numerous factors including the complexity of the patient and their operation as well as the number of previous attempts at repair [4,5,6]. Complicated and multiply recurrent cases may need an abdominal wall reconstruction (AWR) approach. In order to reduce recurrence and optimise both the short and long term outcomes of AWR increasing attention has been paid to developing enhanced recovery after surgery (ERAS) protocols [7, 8]. These have tended to focus on well recognised risk factors such as obesity, diabetes control and smoking cessation. While prehabilitation has gained traction in recent years, published studies have largely avoided addressing one of the most common patient concerns in the perioperative period, namely physical activity. Post-surgical physical exercise in particular is often left to individual interpretation. AWR, with variable degrees of musculoaponeurotic realignment, reinforcement, reapproximation, division and/ or chemo-denervation is akin to musculoskeletal surgery (MSK) yet rehabilitation after AWR represents a physicians’ blind spot in contradistinction to the very well thought through and carefully planned physical therapy regimens after MSK. The purpose of this review was to identify and summarise the literature concerning physical activity levels both prior to and following AWR with a view to enabling clinicians to provide patients with evidence-based advice in the weeks and months either side of their surgery.
Method
Database literature search method
A systematic review protocol was devised, agreed upon by all authors and registered with the PROSPERO database (registration number CRD42021236745) [9]. PubMed, CINAHL, Allied & Complementary medicine database (AMED), PEDro and Web of Science were each searched by STA, NHB and LM with the most recent searches being conducted on 13th February 2021. The full search syntax is available in the supplemental material.
The inclusion criteria comprised of both randomized controlled trials (RCT) and cohort studies in order to minimize the risk of under-representing the literature thus providing an incomplete summary of the evidence. No restrictions were placed on the searches with regard to publication date or language of publication. The inclusion and exclusion criteria were shown as follows:
Inclusion criteria
-
Cohort studies, randomized controlled trials.
-
Prospective or retrospective.
-
Studies concerning self-defined complex incisional hernia repairs and AWRs.
-
Studies including the description of a “prehabilitation” and/or “rehabilitation” program targeting the abdominal wall muscles.
-
Studies concerning “prehabilitation” or “rehabilitation” interventions.
-
of a physical exercise nature and
-
focused primarily on the kinesiological function of the abdominal wall structures.
Exclusion criteria
-
Case series, case reports, review articles with no original data.
-
Studies involving patients aged under 18 years.
-
Studies primarily describing an ERAS program.
The search results were then checked by STA and duplicates were excluded before STA, NHB and LM screened the remaining papers initially by title, then abstract and finally by full article. The three independent reviewers were blinded to each other’s decisions. At the end of each stage the lists were compared and any discrepancies were settled by discussion and mutual agreement. Where necessary, corresponding authors were contacted if clarification was required in order to determine suitability for inclusion.
The data from the final list of included studies was extracted using a table based on the Cochrane Consumers and Communication Review Group’s data extraction template [10]. These data are shown in Table 1. The risk of bias for the included studies was assessed using the Robins-I tool for included cohort studies and RoB2 for included randomized studies [11, 12]. Draft characteristics of included studies tables were compiled by STA, NHB and LM independently with the other two members of the team then checking each other’s tables and, as before, settling discrepancies by discussion and mutual agreement to produce the final consensus table (Table 1).
Snowballing technique search method and rationale
Following the screening process only two papers were identified from the database searches as meeting our inclusion criteria [13]. In response to this low yield it was agreed by the authors that the scope of the study should be widened to additionally include any papers identified via a second search performed by LM and NHB using the snowballing technique as described by Wohlin [14]. The starter set was comprised of six articles including both papers retrieved from the database search, Liang et al. and Pezeshk et al. [15, 16]. The other four papers comprising our starter set were hand-selected by the authors as being likely to yield relevant articles owing to their topics and content despite not meeting our inclusion criteria in themselves [17,18,19,20]. The resulting titles were screened by STA, NHB and LM using the same method as was applied following the database search.
Results
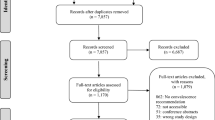
As shown in Fig. 1 the database literature search yielded a total of 5423 records. Of these, 5117 were excluded based on their titles alone and 287 were identified as being duplicates. The remaining 19 records were screened as abstracts with a further 12 not meeting our inclusion criteria. The seven records that were screened as full papers identified an additional five that were excluded for being expert opinion only or because they did not assess either physical activity or AWR. The database search thus yielded two titles which were included in our study. The snowballing search identified 49 records after three iterations by NHB and four iterations by LM of backward and forward snowball searching. Of these there were six duplicates. Ten records were excluded following the screening of their abstracts. Of the 33 records that were screened as full papers 32 were excluded for being systematic reviews or evaluations of a local ERAS protocol or because they did not assess either physical activity or AWR. The snowballing search, therefore, yielded one title which was included in our study bringing the total number of included studies to three.
Summaries of the three included studies are shown in Tables 1 and 2. The three included studies had markedly different methodological designs making direct comparison impossible. The reasons for exclusion of the 37 studies which were excluded after assessment as full papers are shown in Table 3.
Liang et al. is a RCT containing 118 subjects which investigated the impact of an intensive, individualized, MDT-derived prehabilitation program versus a generic standardized counselling approach prior to abdominal wall hernia repair [15]. Patients were assessed clinically for evidence of hernia recurrence and/or complications after a one month postoperative follow-up period [15]. 69.5% of the study group (SG) versus 47.5% of the control group (CG) were hernia and complication free at one month post-operation; however, this was largely due to more of the SG undergoing surgery [15] (Table 2).
Ahmed et al. is a RCT of 30 patients with abdominal wall hernias of whom a 15 patient SG underwent a 30-min per session, three sessions per week, six week preoperative flexibility and abdominal wall muscle strengthening program [21]. The peak abdominal muscle torque of all 30 participants was measured at initial assessment and then again preoperatively and 6 months postoperatively [21]. Although the primary outcome is not explicitly stated, the SG was shown to have experienced a significantly greater change in abdominal wall muscle strength postoperatively compared to the CG (45.89 ± 9.53 Nm preoperative to 41.3 ± 0.89 Nm postoperative (p = 0.0001) versus 33.97 ± 6.78 Nm preoperative to 30.05 ± 8.94 Nm postoperative (p = 0.002)), respectively [21].
Pezeshk et al. is a retrospective cohort study of 275 abdominal wall hernia patients of whom 137 were prescribed a regimen of abdominal wall flexibility and strengthening exercises to be done both preoperatively as well as postoperatively [16]. The exact nature of the outcome measures and follow-up protocol was inadequately described however patients were followed up longitudinally and the duration from surgery until recurrence was recorded [16]. Significantly fewer recurrences were recorded in the SG (9% vs 22% (p < 0.01)) and their median time to recurrence was significantly longer than the CG (13 months vs 6 months (p < 0.05)) [16]. However, each of these findings may have resulted from differences in the surgical techniques used [16].
None of the three included studies reported any adverse events resulting from their interventions.
Owing to the heterogeneity and low number of yielded studies no pooling of data or meta-analysis was feasible. Liang et al. and Ahmed et al. were each found to have moderate risk of bias (Fig. 2), whereas Pezeshk et al. showed a critical risk of bias (Fig. 3) [11, 12, 22].
Discussion
The literature regarding physical activity in relation to AWR is indeed limited as only three papers examining physical exercise before or after AWR were found. Each of the three studies had significant methodological issues preventing confident conclusions and there was no consistent message which could be used to guide patient care. The paucity of studies on physical exercise in the context of AWR raises important questions. First and foremost, we must conclude that any current recommendations are based on assumptions or expert opinions.
The concern regarding increased physical activity prior to AWR is that it may result in the aggravation of symptoms or enlargement or incarceration of the hernia. The studies included in the current review reported no adverse events related to the preoperative physical activity which is consistent with other previously published work on abdominal wall function before and after AWR [23]. There is no evidence that physical activity prior to AWR is harmful. The main argument for encouraging physical activity prior to AWR is that it hypothetically improves the postoperative outcomes. A recent multinational Delphi consensus statement outlined a variety of preoperative recommendations for AWR patients [24]. One of the strong recommendations listed was specialist prehabilitative/physiotherapeutic treatment to patients with poor exercise tolerance although whether this treatment pertains to general fitness or the abdominal wall specifically is unclear [24]. There is evidence indicating improved patient-reported recovery after different surgical procedures albeit with varying results as regards complications and length of stay [25, 26]. Preoperative physical therapy prior to cardiac surgery reduces the risk of postoperative pulmonary complications, which are also common after AWR [27, 28]. Patient-reported physical activity quality of life (QOL) scores suggest that AWR improves abdominal wall function [23].
Another hypothetical advantage of preoperative physical exercise may be the hypertrophy of abdominal wall musculature resulting in easier identification of surgical planes when performing retromuscular dissection and transversus abdominis release [29, 30]. Theoretically, it could be argued that the optimal preoperative prehabilitation program prior to AWR should include both cardiopulmonary exercise and core strength training, enhancing both the pulmonary reserve and the abdominal wall function.
Preoperative exercise programs also need to take into consideration the increasingly common adjunct of preoperative administration of botulinum toxin A into the abdominal oblique muscles prior to AWR. This temporary chemo-denervation facilitates midline fascial reapproximation with reconstruction of the linea alba and permits a greater number of patients to avoid permanent anatomical division of functionally important muscles due to either anterior or posterior components separation. Whilst several studies have reported this technique to be safe and without serious adverse events, it is not without its issues [31, 32]. The paralysis of the oblique muscles impacts the patient by limiting their respiratory capacity and some patients have reported reduced muscular function when trying to utilize the lateral abdominal wall [33]. It has been suggested that the pharmacological properties of botulinum toxin are not purely due to its local action at the site of muscular injection but also that a heteronymous effect is seen at the spinal level [34]. Little research has been done to show how paralysing the lateral abdominal wall impacts those core and trunk stabilizing muscles which are not injected and how this may impact a preoperative prehabilitation program remains unknown and fully undescribed in the literature.
We must acknowledge that we do not actually have meaningful evidence-based advice on how best to physically rehabilitate after AWR. The natural concern regarding physical activity for patient and surgeon alike is damage to the repair and a subsequent recurrence of the hernia. However, the concern that too much physical activity increases the risk of fascial dehiscence may be overestimated considering that simple coughing has been shown to generate significantly higher intraabdominal pressures (100 mmHg) and tensile forces (25 N/cm) than any other non-resistance activity aside from jumping (170 mmHg and 50 N/cm, respectively) [35,36,37]. Conversely, cadaveric studies have shown that the maximum tensile strength of the abdominal wall is 15 N/cm and that this force is achieved when the intraabdominal pressure reaches 55 mmHg [38,39,40]. These figures correspond with those experienced when lifting as little as five kilograms from a squatting position [37, 40]. Considering the wide range of physiological stresses imposed on the abdominal wall by different physical activities, and the supposed implications to the hernia and its subsequent repair, it is notable that none of the three included studies detailed the underlying reasons for how or why they chose the specific components of the exercise regimen used in their methods [36, 37, 41]. The exercise regimen used are described in broad terms in the studies by Ahmed et al. and Pezeshk et al. but no specifics were provided in the paper by Liang et al. [15, 16, 21]. A detailed exercise prescription as described in the 2011 position stand by the American College of Sports Medicine, in which the frequency, intensity, timing, type, volume or repetitions, pattern and progression of each prescribed exercise is clearly documented, would enable investigators to predict the expected physiological stresses on the abdominal wall or hernia repair and thus determine whether patients are liable to exceed safe limits [42]. Such an exercise prescription would also enable the replication of a study’s method thus allowing other investigative teams to assess reproducibility.
The previous considerations are related to preventing exercise-related damage to a hernia repair in the postoperative period; however, modern AWR techniques are about return of abdominal wall function as well as correcting a fascial defect. In this regard there is little known on how a postoperative exercise program might expedite or enhance this return of function. If this is so in general terms there is even less sense of how different surgical techniques, with or without preoperative chemo-denervation or components separation, might differ in their postoperative exercise program. A major MSK operation without a prescribed postoperative physical therapy regimen is an anathema, yet in AWR surgery there is no identifiable prescribed postoperative rehabilitation program evident in the published literature to enhance functional recovery.
The current study has both strengths and limitations. The primary strength is the robustness of the search performed. By utilizing an intentionally broad strategy for the database search yet yielding only two papers from this process it has been demonstrated that there is little evidence to support current clinical advice. By then responding to this low yield by widening the scope of the study to include the results of the additional snowballing search, a further dimension has been added to the process of examining the literature that is entirely separate to the traditional database search and thus we have been able to fully expose the lack of applicable literature on this topic. Including allied health professionals in the investigative team has made it possible to highlight some of the more kinesiological implications of prehabilitation and rehabilitation. Arguably the primary weakness of the study is the lack of literature found.
Conclusion
In conclusion, the current literature review found that the evidence behind perioperative physical activity in relation to AWR is simply too sparse and too weak to justify making any confident recommendations at all.
References
Gignoux B et al (2021) Incidence and risk factors for incisional hernia and recurrence: retrospective analysis of the French national database. Colorectal Dis 23(6):1515–1523
Cherla DV, Poulose B, Prabhu AS (2018) Epidemiology and disparities in care: the impact of socioeconomic status, gender, and race on the presentation, management, and outcomes of patients undergoing ventral hernia repair. Surg Clin North Am 98(3):431–440
Poulose BK et al (2012) Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia 16(2):179–183
Hadeed JG et al (2011) Complex abdominal wall hernias: a new classification system and approach to management based on review of 133 consecutive patients. Ann Plast Surg 66(5):497–503
Slater NJ et al (2014) Criteria for definition of a complex abdominal wall hernia. Hernia 18(1):7–17
Holihan JL et al (2015) Adverse events after ventral hernia repair: the vicious cycle of complications. J Am Coll Surg 221(2):478–485
Lode L et al (2021) Enhanced recovery after abdominal wall reconstruction: a systematic review and meta-analysis. Surg Endosc 35(2):514–523
Slim K, Standaert D (2020) Enhanced recovery after surgical repair of incisional hernias. Hernia 24(1):3–8
Bhargava A et al (2021) Physical activity pre and post complex abdominal wall reconstruction: where is the evidence? PROSPERO CRD42021236745 2021; 15th March. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021236745
Chapter 5: Collecting data. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) Cochrane handbook for systematic reviews of interventions version 6.1 September 2020 [cited 2021 11th January]. Available from: www.training.cochrane.org/handbook
Sterne JA et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
Sterne JAC et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898
Page MJ et al (2021) Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol 134:103–112
Wohlin C (2014) Guidelines for snowballing in systematic literature studies and a replication in software engineering. In: Proceedings of the 18th international conference on evaluation and assessment in software engineering. Association for Computing Machinery, London, England, United Kingdom, p Article 38
Liang MK et al (2018) Modifying risks in ventral hernia patients with prehabilitation: a randomized controlled trial. Ann Surg 268(4):674–680
Pezeshk RA et al (2015) Complex abdominal wall reconstruction: a novel approach to postoperative care using physical medicine and rehabilitation. Plast Reconstr Surg 136(3):362e–369e
Jensen KK, Kjaer M, Jorgensen LN (2016) Isometric abdominal wall muscle strength assessment in individuals with incisional hernia: a prospective reliability study. Hernia 20(6):831–837
Jensen KK, Backer V, Jorgensen LN (2017) Abdominal wall reconstruction for large incisional hernia restores expiratory lung function. Surgery 161(2):517–524
Jensen KK et al (2016) Enhanced recovery after giant ventral hernia repair. Hernia 20(2):249–256
Jensen KK, Kjaer M, Jorgensen LN (2014) Abdominal muscle function and incisional hernia: a systematic review. Hernia 18(4):481–486
Ahmed MG et al (2018) Effect of preoperative abdominal training on abdominal muscles strength outcomes after ventral hernia repair. Med J Cairo Univ 86:4495–4501
McGuinness LA, Higgins JPT (2021) Risk-of-bias visualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 12(1):55–61
Jensen KK et al (2017) Abdominal wall reconstruction for incisional hernia optimizes truncal function and quality of life: a prospective controlled study. Ann Surg 265(6):1235–1240
Grove TN et al (2021) Perioperative optimization in complex abdominal wall hernias: Delphi consensus statement. BJS Open 5(5)
Onerup A et al (2021) Effect of short-term homebased pre- and postoperative exercise on recovery after colorectal cancer surgery (PHYSSURG-C): a randomized clinical trial. Ann Surg
Schwartz CE et al (2021) Moving toward better health: exercise practice is associated with improved outcomes after spine surgery in people with degenerative lumbar conditions. Can J Surg 64(4):E419–E427
Hulzebos EH et al (2012) Preoperative physical therapy for elective cardiac surgery patients. Cochrane Database Syst Rev 11:CD010118
Fischer JP et al (2014) Analysis of risk factors, morbidity, and cost associated with respiratory complications following abdominal wall reconstruction. Plast Reconstr Surg 133(1):147–156
Novitsky YW et al (2012) Transversus abdominis muscle release: a novel approach to posterior component separation during complex abdominal wall reconstruction. Am J Surg 204(5):709–716
Stoppa RE (1989) The treatment of complicated groin and incisional hernias. World J Surg 13(5):545–554
Nielsen MO et al (2020) Short-term safety of preoperative administration of botulinum toxin A for the treatment of large ventral hernia with loss of domain. Hernia 24(2):295–299
Wegdam JA et al (2020) Prehabilitation of complex ventral hernia patients with Botulinum: a systematic review of the quantifiable effects of Botulinum. Hernia
Elstner KE et al (2020) Selective muscle botulinum toxin A component paralysis in complex ventral hernia repair. Hernia 24(2):287–293
Weise D, Weise CM, Naumann M (2019) Central effects of botulinum neurotoxin-evidence from human studies. Toxins (Basel) 11(1):21
Guttormson R et al (2008) Are postoperative activity restrictions evidence-based. Am J Surg 195(3):401–403; discussion 403–404
Cobb WS et al (2005) Normal intraabdominal pressure in healthy adults. J Surg Res 129(2):231–235
Gerten KA et al (2008) Intraabdominal pressure changes associated with lifting: implications for postoperative activity restrictions. Am J Obstet Gynecol 198(3):306e1-5
Junge K et al (2001) Elasticity of the anterior abdominal wall and impact for reparation of incisional hernias using mesh implants. Hernia 5(3):113–118
Konerding MA et al (2011) Maximum forces acting on the abdominal wall: experimental validation of a theoretical modeling in a human cadaver study. Med Eng Phys 33(6):789–792
Forbes J et al (2012) Timing of return to work after hernia repair: recommendations based on a literature review. Brit Columbia Med J 54(7):341–345
Blazek D et al (2019) Systematic review of intra-abdominal and intrathoracic pressures initiated by the Valsalva manoeuvre during high-intensity resistance exercises. Biol Sport 36(4):373–386
Garber CE et al (2011) American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43(7):1334–1359
Gunnarsson U, Johansson M, Strigard K (2011) Assessment of abdominal muscle function using the Biodex System-4. Validity and reliability in healthy volunteers and patients with giant ventral hernia. Hernia 15(4):417–421
Stark B et al (2012) Validation of Biodex system 4 for measuring the strength of muscles in patients with rectus diastasis. J Plast Surg Hand Surg 46(2):102–105
Parker M et al (2011) Pilot study on objective measurement of abdominal wall strength in patients with ventral incisional hernia. Surg Endosc 25(11):3503–3508
Krpata DM et al (2012) Design and initial implementation of HerQLes: a hernia-related quality-of-life survey to assess abdominal wall function. J Am Coll Surg 215(5):635–642
Bigolin AV et al (2020) What is the best method to assess the abdominal wall? Restoring strength does not mean functional recovery. Arq Bras Cir Dig 33(1):e1487
Strigard K et al (2016) Giant ventral hernia-relationship between abdominal wall muscle strength and hernia area. BMC Surg 16(1):50
Kato S et al (2020) Reliability of the muscle strength measurement and effects of the strengthening by an innovative exercise device for the abdominal trunk muscles. J Back Musculoskelet Rehabil 33(4):677–684
Grabiner MD, Jeziorowski JJ, Divekar AD (1990) Isokinetic measurements of trunk extension and flexion performance collected with the biodex clinical data station. J Orthop Sports Phys Ther 11(12):590–598
Estrazulas JA et al (2020) Evaluation isometric and isokinetic of trunk flexor and extensor muscles with isokinetic dynamometer: a systematic review. Phys Ther Sport 45:93–102
Guilhem G et al (2014) Validity of trunk extensor and flexor torque measurements using isokinetic dynamometry. J Electromyogr Kinesiol 24(6):986–993
Criss CN et al (2014) Functional abdominal wall reconstruction improves core physiology and quality-of-life. Surgery 156(1):176–182
den Hartog D et al (2010) Isokinetic strength of the trunk flexor muscles after surgical repair for incisional hernia. Hernia 14(3):243–247
Ueland W et al (2020) The contribution of specific enhanced recovery after surgery (ERAS) protocol elements to reduced length of hospital stay after ventral hernia repair. Surg Endosc 34(10):4638–4644
Stearns E et al (2018) Early outcomes of an enhanced recovery protocol for open repair of ventral hernia. Surg Endosc 32(6):2914–2922
Mohapatra S, Balaji M, Ganapathi R (2019) Application of enhanced recovery pathway in abdominal wall reconstruction surgery in a tertiary care hospital in Andhra Pradesh. Int J Surg Sci 3(4):141–143
Majumder A et al (2016) Benefits of multimodal enhanced recovery pathway in patients undergoing open ventral hernia repair. J Am Coll Surg 222(6):1106–1115
Harryman C et al (2020) Enhanced value with implementation of an ERAS protocol for ventral hernia repair. Surg Endosc 34(9):3949–3955
Fayezizadeh M et al (2014) Enhanced recovery after surgery pathway for abdominal wall reconstruction: pilot study and preliminary outcomes. Plast Reconstr Surg 134(4 Suppl 2):151S-159S
Colvin J et al (2019) Enhanced recovery after surgery pathway for patients undergoing abdominal wall reconstruction. Surgery 166(5):849–853
Crocetti D et al (2020) Dietary protein supplementation helps in muscle thickness regain after abdominal wall reconstruction for incisional hernia. Am Surg 86(3):232–236
Gormley J et al (2020) Impact of rectus diastasis repair on abdominal strength and function: a systematic review. Cureus 12(12):e12358
Emanuelsson P et al (2016) Operative correction of abdominal rectus diastasis (ARD) reduces pain and improves abdominal wall muscle strength: a randomized, prospective trial comparing retromuscular mesh repair to double-row, self-retaining sutures. Surgery 160(5):1367–1375
Olsson A et al (2019) Cohort study of the effect of surgical repair of symptomatic diastasis recti abdominis on abdominal trunk function and quality of life. BJS Open 3(6):750–758
Jensen KK et al (2019) Enhanced recovery after abdominal wall reconstruction reduces length of postoperative stay: an observational cohort study. Surgery 165(2):393–397
DuBay DA et al (2007) Incisional herniation induces decreased abdominal wall compliance via oblique muscle atrophy and fibrosis. Ann Surg 245(1):140–146
Culbertson EJ et al (2013) Reversibility of abdominal wall atrophy and fibrosis after primary or mesh herniorrhaphy. Ann Surg 257(1):142–149
Mazzocchi M et al (2014) A study of postural changes after abdominal rectus plication abdominoplasty. Hernia 18(4):473–480
Wilhelmsson S et al (2017) Abdominal plasty with and without plication-effects on trunk muscles, lung function, and self-rated physical function. J Plast Surg Hand Surg 51(3):199–204
Staalesen T, Olsen MF, Elander A (2015) The effect of abdominoplasty and outcome of rectus fascia plication on health-related quality of life in post-bariatric surgery patients. Plast Reconstr Surg 136(6):750e–761e
Temel M, Turkmen A, Berberoglu O (2016) Improvements in vertebral-column angles and psychological metrics after abdominoplasty with rectus plication. Aesthet Surg J 36(5):577–587
Paiuk I, Wasserman I, Dvir Z (2014) Effects of abdominal surgery through a midline incision on postoperative trunk flexion strength in patients with colorectal cancer. Hernia 18(4):487–493
Khan OA et al (2012) Impact of training on outcomes following incisional hernia repair. Acta Chir Belg 112(6):432–435
Pommergaard HC et al (2014) No consensus on restrictions on physical activity to prevent incisional hernias after surgery. Hernia 18(4):495–500
Rodrigues MA et al (2018) Preoperative respiratory physiotherapy in abdominoplasty patients. Aesthet Surg J 38(3):291–299
Funding
No funding sources were used for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that they have no competing interests in the conduction or publication of this study. Individual conflict of interest forms have been provided for each author.
Ethical approval
This article did not require ethical approval of any kind.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Adams, S.T., Bedwani, N.H., Massey, L.H. et al. Physical activity recommendations pre and post abdominal wall reconstruction: a scoping review of the evidence. Hernia 26, 701–714 (2022). https://doi.org/10.1007/s10029-022-02562-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-022-02562-5