Abstract
Background
Enhanced recovery after surgery (ERAS) are evidence-based protocols associated with improved patient outcomes. The use of ERAS pathways is well documented in various surgical specialties. The aim of this systematic review and meta-analysis was to examine the efficacy of ERAS protocols in patients undergoing abdominal wall reconstruction (AWR).
Methods
This systematic review and meta-analysis were reported according to PRISMA and MOOSE guidelines. The databases PubMed, EMBASE, CINAHL, Web of Science and Cochrane Library were searched for original studies comparing ERAS with standard care in patients undergoing AWR. The primary outcome was length of stay (LOS) and secondary outcomes were readmission and surgical site infection (SSI) and/or surgical site occurrences (SSO).
Results
Five studies were included in the meta-analysis. All were retrospective cohort studies including 453 patients treated according to ERAS protocols, and 494 patients treated according to standard care. The meta-analysis demonstrated that patients undergoing AWR managed with ERAS had a mean 0.89 days reduction in LOS compared with patients treated with standard care (95% CI − 1.70 to − 0.07 days, p = 0.03). There was no statistically significant difference in readmission rate (OR 1.00, 95% CI 0.53 to 1.87, p = 1.00) or SSI/SSO (OR 1.19, 95% CI 0.67 to 2.11, p = 0.56) between groups.
Conclusions
The use of ERAS in patients undergoing AWR was found to significantly reduce LOS without increasing the readmission rate or SSI/SSO. Based on the existing literature, ERAS protocols should be implemented for patients undergoing AWR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Enhanced recovery after surgery (ERAS) represents multimodal protocols to improve surgical outcomes and enhance the value of care [1]. The concept of fast-track surgery pathways was introduced in the mid-1990s as a care-bundle of evidence-based interventions to improve postoperative outcomes after surgery [2, 3]. In addition to enhanced patient outcomes, ERAS pathways are intended to provide a more economical and efficient utilization of the health care system [4]. ERAS include evidence-based protocols for preoperative, perioperative and postoperative measures with the goal of minimizing surgical stress and thus improve recovery and decrease the risk of organ dysfunction and postoperative complications [1]. The approach is well documented in several surgical specialties and especially in colorectal surgery, where the implementation of ERAS has shown a reduction in overall postoperative complications and a significant reduction in length of stay (LOS) [5].
Abdominal wall reconstruction (AWR) for the repair of large ventral hernias is often requiring the use of component separation techniques and placement of large mesh materials. Patients undergoing AWR often have significant comorbidities and a high risk of postoperative complications, and therefore may benefit greatly from enrollment in an ERAS protocol [6].
The aim of this systematic review and meta-analysis was to examine the efficacy of ERAS protocols in patients undergoing AWR. The primary outcome was LOS and secondary outcomes were readmission and surgical site infection (SSI) and/or surgical site occurrences (SSO) requiring intervention.
Materials and methods
The study is reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) and Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines [7, 8]. The institutional review board (IRB) approval and written consent were not required for this systematic review. The protocol was not registered online before starting on this systematic review and meta-analysis.
Literature search strategy
A literature search was performed in the databases PubMed, EMBASE, CINAHL, Web of Science and Cochrane Library from initiation of the databases until 22 November 2019. The following search string was used in PubMed without language restrictions: ((incisional hernia repair) OR (ventral hernia repair) OR (abdominal wall reconstruction) OR (AWR)) AND ((fast-track) OR (enhanced recovery) OR (ERAS)). A translated search string was subsequently used in the other databases. Furthermore, the references of the included studies and the 100 first hits in Google Scholar were scrutinized for any additional eligible studies.
Study outcomes
The primary outcome of the study was LOS, defined as number of days of hospitalization after AWR. Secondary outcomes were 30-day readmission rate and SSI and/or SSO requiring intervention. SSI was defined as an infection occurring in the incision, deep tissue or organ space at the operation site, developing up to 30 days after surgery. SSO included SSI, seroma, wound dehiscence, enterocutaneous fistula, wound cellulitis, non-healing incisional wound, fascial disruption, skin or soft tissue ischemia or necrosis, wound serous or purulent drainage, stitch abscess, hematoma and infected or exposed mesh [9].
Study selection criteria
All original studies comparing ERAS with traditional care in patients undergoing AWR were assessed for eligibility. After removal of duplicates, all identified records were screened by title and abstract. Full-text articles were assessed by all named authors. Included studies fulfilled the following criteria: the study cohort constituted patients undergoing AWR; patients enrolled in an ERAS protocol was compared to a control group assigned to standard care; and the abovementioned study outcomes were described. Authors of identified studies were contacted to retrieve any relevant unreported data. Exclusion criteria were conference abstracts, letters/comments and reviews. Furthermore, studies were excluded if a more recent publication with an overlapping patient population was identified.
Quality assessment of the included studies
The ROBINS-I tool was used to assess the risk of bias and the methodological quality of the included studies [10]. ROBINS-I is a tool for evaluating the risk of bias in non-randomized studies of interventions. According to ROBINS-I, each study is evaluated through “signaling questions” in seven different domains which then establish the basis for an overall risk of bias judgment for the outcome being assessed. Furthermore, the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology was used to assess the quality of evidence for outcomes reported in the included studies, and GRADEpro Guideline Development Tool software was used to develop an evidence table (Table 2) [11,12,13]. The included studies were evaluated on domains of study limitations (risk of bias), inconsistency, indirectness, imprecision and publications bias, and downgraded in case of important limitations. Additionally, according to GRADE, studies were upgraded to a higher level of evidence based on a strong magnitude of effect, in the presence of a dose–response gradient and residual confounding that would have reduced the demonstrated effect [13]. Finally, an overall rating of the quality of evidence across all outcomes was determined.
Statistics
Statistical analyses were performed with Review Manager, version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). For LOS, pooled data were analyzed with the inverse variance test using a random effects model to generate mean differences with 95% confidence intervals (CI). For dichotomous outcomes (readmission and SSI/SSO), pooled data were used to generate odds ratios (OR) with 95% CI using the Mantel–Haenszel test including a random effects model. The random effects model was used due to anticipated considerable heterogeneity among the included studies.
Results
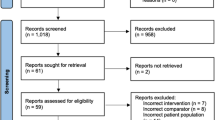
In total, 109 unique records were screened, 16 full-text articles assessed for eligibility, and six studies were included in the review (Fig. 1). One study was excluded due to critical risk of bias [14]. The studies included a total of 947 patients undergoing AWR, 453 patients subjected to ERAS protocols compared with 493 historical controls treated according to standard care, considered as “non-ERAS”. All studies were retrospective cohort studies.
Assessment of the included studies
The five studies included in the meta-analyses had a moderate overall risk of bias according to the ROBINS-I tool (Table 1) [15,16,17,18,19]. The overall GRADE quality of evidence was evaluated to be low across all three outcomes. Detailed reasons for upgrade and downgrade are shown in Table 2 [20]. A low overall quality of evidence grade demonstrates a limited confidence in the effect estimates and that the true effect may be markedly different from the estimate of effect [11].
ERAS elements in the included studies
As illustrated in Table 3, the elements of the ERAS protocols in the included studies were heterogeneous. Only two of the included studies outlined the use of µ-opioid receptor antagonist alvimopan to accelerate intestinal recovery [15, 19]. Early oral feeding was described in three of the studies [15, 17, 18] and judicious intravenous fluid administration was reported by only two of the studies [15, 18]. However, all studies implemented postoperative multimodal pain control.
TAP block versus epidural
A transversus abdominis plane (TAP) block with use of liposomal bupivacaine was administrated perioperative for all patients in two of the studies [15, 18]. Warren et al. [16] applied epidural catheters selectively for large VHR cases with expected LOS of at least 3 days. For patients not receiving an epidural, intraoperative ketamine infusion bolus was administered, followed by continuous infusion with the addition of intravenous lidocaine. Postoperative ketamine infusion was maintained at a subanesthetic dose, and epidural infusion was maintained at 8–12 ml/h of 0.125% bupivacaine. Jensen et al. [17] described placement of an epidural catheter in the thoracic vertebral interspace 8 to 10, according to the hernia location, preoperatively in all patients. The epidural anesthesia was discontinued on the evening of postoperative day (POD) 2, one hour after administration of oral morphine 10 mg, as analgesic bridging. The epidural catheter was then removed the morning after. In addition, the protocol by Jensen et al. utilized preoperative singe-shot high dose glucocorticoid. Only one of the included studies did not report use of either epidural nor TAP block as perioperative pain control [19].
Length of stay (LOS)
All five studies reported LOS, and two of the studies demonstrated a statistically significant reduction in LOS in the ERAS group compared with standard care [15, 17]. The meta-analysis demonstrated that patients undergoing AWR managed with ERAS protocols had a mean reduction in LOS of 0.89 days compared with patients managed with standard care (95% CI − 1.70 to − 0.07 days, p = 0.03, Fig. 2).
Readmission rate
One study demonstrated a significant reduction in readmission rate when implementing ERAS protocols compared to standard care [15]. Readmission rates ranged from 4 to 21% for the ERAS protocol groups, and from 4 to 19% for the traditional care groups. The estimated OR (ERAS vs. standard care) for readmission was 1.00 (95% CI 0.53 to 1.87, p = 1.00), indicating that readmission was equally likely to occur in both groups (Fig. 3).
Surgical site infection (SSI)/surgical site occurrence (SSO)
Four studies reported on SSI/SSO [16,17,18,19]. SSI/SSO rates ranged from 3 to 28% in the ERAS group and 3% to 40% in the standard care group. Ueland et al. [19] was the only study demonstrating a trend towards decreased SSI/SSO rates with the use of ERAS protocols. In the meta-analysis, there was no statistically significant difference in SSI/SSO (OR ERAS vs. standard care 1.19, 95% CI 0.67 to 2.11, p = 0.56) between groups (Fig. 4).
Other outcomes
Other outcomes relevant for evaluating the effect of ERAS were also reported. Majumder et al. [15] demonstrated that the use of ERAS resulted in significantly shorter times to liquid and regular diet (1.1 vs 2.7 and 3.0 vs 4.8 days, respectively), and significantly shorter times to flatus and bowel movement (3.1 vs 3.9 and 3.6 vs 5.2 days, respectively) compared with standard care. Warren et al. found that the use of ERAS nearly eliminated patient-controlled analgesia use and significantly reduced narcotic requirements on POD 0, 1, and 2 compared to standard care [16]. Colvin et al. showed a trend toward a decreased duration of epidurals or use of patient-controlled analgesia for ERAS compared with standard care [18].
Discussion
This systematic review and meta-analysis presented the current research on ERAS protocols in relation to AWR, focusing on LOS, readmission rate and SSI/SSO. The results demonstrated a significant association between the use of ERAS pathways and a reduction in LOS in patients undergoing AWR. There were no significant differences between the use of ERAS pathways and readmission rate or SSI/SSO in patients undergoing AWR.
This meta-analysis found a slight, yet significant reduction in LOS with the use of ERAS protocols for AWR compared with standard care. A small pilot study on ERAS, excluded from this review due to overlapping patient populations, also showed a significant reduction in LOS after the implementation of ERAS [21]. Even though the mean reduction in LOS was less than 1 day, these results strengthen the perception that ERAS pathways do have clinical implications to shorten LOS. However, the heterogeneity in our meta-analysis was rather high, indicating that the results from the included studies regarding LOS might not be comparable due to methodological variations in the included studies.
In colorectal surgery, extensive research has demonstrated a reduction in LOS with the use of ERAS protocols [5], and in 2018, Visioni et al. [22] reported that ERAS protocols reduced LOS without increasing complications or readmission rates in non-colorectal abdominal surgery.
There was a wide range in readmission rates among the included studies, and only one of the included studies demonstrated a significant reduction in readmission rate with the use of ERAS protocols compared with standard care [15]. These results are similar to those obtained from studies of ERAS in other surgical specialties [23, 24]. Importantly, the readmission rates were not increased despite an earlier discharge from the hospital in the ERAS patients compared with standard care.
Similarly, the results regarding SSI/SSO showed a great variety within the included studies. This variation in complication rates may reflect inter-study differences in patient populations and surgical techniques applied [25]. Reasons for reduced complication rates after ERAS implementation have been proposed to include reduced catabolism, reduced loss of muscle mass as well as muscle function, all due to the fast removal of drains, early oral feeding and mobilization [26]. However, in this study, we found no difference in rates of SSI/SSO comparing ERAS and standard care after AWR.
Patients undergoing AWR are heterogenous and often have several comorbidities that might affect the outcomes regarding postoperative complications and readmission rates. Lovecchio et al. [27] showed that patients undergoing ventral hernia repair had a 4.9% 30-day readmission rate. Although complications were the main reason for readmission, the authors underlined that surgeons must be aware of comorbidities that may increase the risk of readmission, even in the absence of complications. The most common postoperative complications in patients undergoing AWR are wound-related, such as SSI [28]. SSIs can lead to increased LOS, readmissions, reoperations as well as increase the risk of hernia recurrence [29].
In 2014, Fayezizadeh et al. [4], suggested a standard for ERAS pathways for patients undergoing AWR using evidence-based interventions. The studies included in this review represent various ERAS pathways in both the preoperative and postoperative care of the patients. This presumably reflects the patient population, which may differ significantly between institutions. Therefore, it has been suggested that the ERAS protocols for patients undergoing AWR should be individualized and flexible to improve patient outcomes [1].
The postoperative analgesic treatment of patients undergoing AWR remains subject to debate. Traditionally, epidural catheters have been used in accordance with the standard of care for patients undergoing traditional open surgery and has also been described as an important element of ERAS pathways to minimize the use of opioids [26]. Recently, however, the use of TAP blocks has gained support, as hypotensive episodes and risk of epidural headaches are avoided [30, 31]. The introduction of long-lasting liposomal bupivacaine (Exparel®) may further improve the outcomes of TAP blocks for patients undergoing AWR, however, no conclusions can be made based on the current literature [32].
ERAS protocols are, among other things, developed to provide a more efficient utilization of health care resources, with the goal of enhancing patient outcome and reducing costs [1, 33]. A recent study evaluating the clinical and financial impacts of ERAS protocols for patients undergoing AWR demonstrated that implementation of ERAS improved clinical outcomes without affecting total costs [33]. However, the study did not demonstrate a reduction in LOS, possibly due to the use of epidural analgesia. Nevertheless, a reduction in costs due to decreased LOS in non-colorectal abdominal surgical procedures has previously been described [22].
This is the first systematic review and meta-analysis on ERAS in patients undergoing AWR. The study is limited by the fact that the included studies are retrospective cohort studies, with historical control groups, and not randomized controlled trials. This imposes a risk of selection bias. Further, the heterogeneity of the studies might have biased the meta-analyses. Nevertheless, it seems likely that ERAS pathways have a place in complex AWR, as in other major abdominal surgery.
In conclusion, ERAS after AWR was found to significantly reduce the postoperative LOS by no more than one day, but without increasing the rate of postoperative complications or readmissions. However, due to heterogeneity the results should be treated with precautions and a more uniform approach is required. Based on the current literature ERAS should be implemented for patients undergoing AWR.
References
Kleppe KL, Greenberg JA (2018) Enhanced recovery after surgery protocols: rationale and components. Surg Clin North Am. https://doi.org/10.1016/j.suc.2018.01.006
Kehlet H (1997) Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 78:606
Scott MJ, Baldini G, Fearon KCH, Feldheiser A, Feldman LS, Gan TJ, Ljungqvist O, Lobo DN, Rockall TA, Schricker T, Carli F (2015) Enhanced recovery after surgery (ERAS) for gastrointestinal surgery, part 1: pathophysiological considerations. Acta Anaesthesiol Scand 59:1212
Fayezizadeh M, Petro CC, Rosen MJ, Novitsky YW (2014) Enhanced recovery after surgery pathway for abdominal wall reconstruction: pilot study and preliminary outcomes. Plast Reconstr Surg. https://doi.org/10.1097/PRS.0000000000000674
Spanjersberg WR, Reurings J, Keus F, van Laarhoven CJ (2011) Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.cd007635.pub2
Orenstein SB, Martindale RG (2018) Enhanced recovery pathway for complex abdominal wall reconstruction. Plast Reconstr Surg. https://doi.org/10.1097/PRS.0000000000004869
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, Atkins D, Barbour V, Barrowman N, Berlin JA, Clark J, Clarke M, Cook D, D’Amico R, Deeks JJ, Devereaux PJ, Dickersin K, Egger M, Ernst E, Gøtzsche PC, Grimshaw J, Guyatt G, Higgins J, Ioannidis JPA, Kleijnen J, Lang T, Magrini N, McNamee D, Moja L, Mulrow C, Napoli M, Oxman A, Pham B, Rennie D, Sampson M, Schulz KF, Shekelle PG, Tovey D, Tugwell P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. J Am Med Assoc 283:2008–2012. https://doi.org/10.1001/jama.283.15.2008
DeBord J, Novitsky Y, Fitzgibbons R, Miserez M, Montgomery A (2018) SSI, SSO, SSE, SSOPI: the elusive language of complications in hernia surgery. Hernia 22:737
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP (2016) ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. https://doi.org/10.1136/bmj.i4919
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH (2011) GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. https://doi.org/10.1016/j.jclinepi.2010.07.015
McMaster University (2015) GRADEpro GDT: GRADEpro guideline development tool [software]. Evid. Prime Inc., Springfield
Schünemann H, Brożek J, Guyatt G, Oxman A (2013) GRADE handbook for grading quality of evidence and strength of recommendations. BMJ 3328:1490
Mohapatra SK, Balaji M, Ganapathi R (2019) Application of enhanced recovery pathway in abdominal wall reconstruction surgery in a tertiary care hospital in Andhra Pradesh. Int J Surg Sci. https://doi.org/10.33545/surgery.2019.v3.i4c.231
Majumder A, Fayezizadeh M, Neupane R, Elliott HL, Novitsky YW (2016) Benefits of multimodal enhanced recovery pathway in patients undergoing open ventral hernia repair. J Am Coll Surg 222:1106
Warren JA, Stoddard C, Hunter AL, Horton AJ, Atwood C, Ewing JA, Pusker S, Cancellaro VA, Walker KB, Cobb WS, Carbonell AM, Morgan RR (2017) Effect of multimodal analgesia on opioid use after open ventral hernia repair. J Gastrointest Surg. https://doi.org/10.1007/s11605-017-3529-4
Jensen KK, Dressler J, Baastrup NN, Kehlet H, Jørgensen LN (2019) Enhanced recovery after abdominal wall reconstruction reduces length of postoperative stay: an observational cohort study. Surgery (United States). https://doi.org/10.1016/j.surg.2018.07.035
Colvin J, Rosen M, Prabhu A, Rosenblatt S, Petro C, Zolin S, Krpata D (2019) Enhanced recovery after surgery pathway for patients undergoing abdominal wall reconstruction. Surgery (United States). https://doi.org/10.1016/j.surg.2019.05.023
Ueland W, Walsh-Blackmore S, Nisiewicz M, Davenport DL, Plymale MA, Plymale M, Roth JS (2019) The contribution of specific enhanced recovery after surgery (ERAS) protocol elements to reduced length of hospital stay after ventral hernia repair. Surg Endosc. https://doi.org/10.1007/s00464-019-07233-8
Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, Akl EA, Djulbegovic B, Falck-Ytter Y, Norris SL, Williams JW, Atkins D, Meerpohl J, Schünemann HJ (2011) GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. https://doi.org/10.1016/j.jclinepi.2010.07.017
Jensen KK, Brondum TL, Harling H, Kehlet H, Jorgensen LN (2016) Enhanced recovery after giant ventral hernia repair. Hernia. https://doi.org/10.1007/s10029-016-1471-0
Visioni A, Shah R, Gabriel E, Attwood K, Kukar M, Nurkin S (2018) Enhanced recovery after surgery for noncolorectal surgery? A systematic review and meta-analysis of major abdominal surgery. Ann Surg. https://doi.org/10.1097/SLA.0000000000002267
Huang ZD, Gu HY, Zhu J, Luo J, Shen XF, Deng QF, Zhang C, Li YB (2020) The application of enhanced recovery after surgery for upper gastrointestinal surgery: meta-analysis. BMC Surg. https://doi.org/10.1186/s12893-019-0669-3
AlBalawi Z, Gramlich L, Nelson G, Senior P, Youngson E, McAlister FA (2018) The Impact of the Implementation of the enhanced recovery after surgery (ERAS®) program in an entire health system: a natural experiment in Alberta, Canada. World J Surg. https://doi.org/10.1007/s00268-018-4559-0
Jensen KK, Henriksen NA, Jorgensen LN (2014) Endoscopic component separation for ventral hernia causes fewer wound complications compared to open components separation: a systematic review and meta-analysis. Surg Endosc. https://doi.org/10.1007/s00464-014-3599-2
Kehlet H (2009) Multimodal approach to postoperative recovery. Curr Opin Crit Care 15:355
Lovecchio F, Farmer R, Souza J, Khavanin N, Dumanian GA, Kim JYS (2014) Risk factors for 30-day readmission in patients undergoing ventral hernia repair. Surgery (United States). https://doi.org/10.1016/j.surg.2013.12.021
Hawn MT, Gray SH, Snyder CW, Graham LA, Finan KR, Vick CC (2011) Predictors of mesh explantation after incisional hernia repair. Am J Surg. https://doi.org/10.1016/j.amjsurg.2010.10.011
Sanchez VM, Abi-Haidar YE, Itani KMF (2011) Mesh infection in ventral incisional hernia repair: Incidence, contributing factors, and treatment. Surg Infect 12:205
Warren JA, Carbonell AM, Jones LK, Mcguire A, Hand WR, Cancellaro VA, Ewing JA, Cobb WS (2019) Length of stay and opioid dose requirement with transversus abdominis plane block vs epidural analgesia for ventral hernia repair. J Am Coll Surg. https://doi.org/10.1016/j.jamcollsurg.2018.12.017
Prabhu AS, Krpata DM, Perez A, Phillips S, Huang LC, Haskins IN, Rosenblatt S, Poulose BK, Rosen MJ (2018) Is it time to reconsider postoperative epidural analgesia in patients undergoing elective ventral hernia repair? Ann Surg. https://doi.org/10.1097/SLA.0000000000002214
Jensen KK (2019) Comment on “is it time to reconsider postoperative epidural analgesia in patients undergoing elective ventral hernia repair?”. Ann Surg 267:971
Harryman C, Plymale MA, Stearns E, Davenport DL, Chang W, Roth JS (2019) Enhanced value with implementation of an ERAS protocol for ventral hernia repair. Surg Endosc. https://doi.org/10.1007/s00464-019-07166-2
Funding
No funds were received for the current study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Lise Lode, Erling Oma, Nadia A. Henriksen and Kristian K. Jensen have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lode, L., Oma, E., Henriksen, N.A. et al. Enhanced recovery after abdominal wall reconstruction: a systematic review and meta-analysis. Surg Endosc 35, 514–523 (2021). https://doi.org/10.1007/s00464-020-07995-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-07995-6