Abstract
Chronic obstructive pulmonary disease (COPD) is a disease characterized by chronic respiratory symptoms due to inflammatory and destructive changes of the lung leading to progressive airflow obstruction. Fragility fractures associated with osteoporosis are among major comorbidities and have significant impacts on quality of life and prognosis of patients with COPD. Evidence suggests that both decreased bone mineral density (BMD) and impaired bone quality contribute to bone fragility and resultant fractures in COPD. Although various clinical risk factors of osteoporosis have been described, mechanisms of COPD-associated osteoporosis are still largely unknown. In addition, its specific treatment has not been established, either. Previous studies have suggested involvement of low BMI and sarcopenia in the pathogenesis of COPD-associated osteoporosis. In this narrative review, we will propose critical roles of vitamin D deficiency and inflammation, both of which are often present in COPD and may underlie the development of osteosarcopenia and impaired bone quality, ultimately causing fractures in COPD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous lung condition characterized by chronic respiratory symptoms (dyspnea, cough, sputum production and/or exacerbations) due to abnormalities of the airways (bronchitis, bronchiolitis) and/or alveoli (emphysema) that cause persistent, often progressive, airflow obstruction [1]. COPD is diagnosed by the criteria based on airflow limitation: post-bronchodilation forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) being less than 0.7. The previous GOLD (Global Initiative for Chronic Obstructive Lung Disease) stages were defined according to %FEV1.0, the ratio of actual FEV1 to the predicted value by age and height [2]. These classifications have been applied to most studies of COPD-associated osteoporosis and thus used also in this review.
COPD is now recognized as a systemic disease complicated with various comorbidities such as lung cancer, atherosclerosis, diabetes, depression, muscle wasting and osteoporosis. These comorbidities have significant impact on hospitalization, mortality and quality of life of COPD subjects [3,4,5,6,7]. Osteoporosis is one of the major systemic comorbidities of COPD. Epidemiological studies have indicated that osteoporosis is highly prevalent in COPD patients [8,9,10,11].
Moreover, osteoporosis-associated fractures may further deteriorate pulmonary function and impair activities of daily life (ADL) of COPD patients. COPD patients with vertebral fracture had more admissions, longer hospital stays, and higher mortality than those without [12]. Every single vertebral fracture has been shown to be associated with a 9% decline in vital capacity (VC) [13].
Conversely, COPD is common among subjects suffering hip fracture and is associated with increased risk of death after fracture [14,15,16]. Regan and colleagues [16] investigated the impact of comorbid COPD in subjects with hip fracture and showed that 47% had COPD. Among them 26% had severe COPD and showed 1-year mortality of 40.2%, which was higher than 31.0% in mild COPD and 28.8% in non-COPD subjects, demonstrating association of mortality with COPD and its severity after hip fracture. Another study demonstrated that COPD significantly increased 5- or 10-year mortality in subjects with surgically treated hip fracture [17]. Thus, COPD and osteoporosis may form a vicious cycle, potentially further deteriorating the prognosis of each disease.
Despite such clinical impacts, osteoporosis in COPD patients is extremely undertreated [9, 18]. And specific treatment of COPD-associated osteoporosis is yet to be established, as neither its molecular mechanisms nor unique therapeutic targets have been identified. Clinically, a recent meta-analysis has identified low BMI and sarcopenia as significant predictors of comorbid osteoporosis in patients with COPD [11]. We have reported that 25-hydroxyvitamin D, which reflects the vitamin D status, is a major determinant of bone mineral density (BMD), while high sensitivity (hs)-CRP predicts trabecular bone score (TBS), an indicator of microarchitecture of the trabecular bone in vertebrae, in male patients with COPD [19]. Because both vitamin D deficiency and systemic inflammation are common in COPD patients and play a general role in osteoporosis and in sarcopenia as well, they are likely to be a significant contributor to the pathogenesis and potentially a good therapeutic target of COPD-associated osteoporosis.
In this narrative review, after briefly summarizing epidemiology and clinical risk factors of COPD-associated osteoporosis, we would like to propose that vitamin D and systemic inflammation are among key mechanisms of osteoporosis in subjects with COPD.
Epidemiology
BMD
In a previous systematic review [8] including a total of 775 COPD patients from 13 studies, the reported prevalence of osteoporosis ranged from 8.7 to 69% with overall prevalence being 35.1%. An updated systematic review and meta-analysis of 58 studies [11] showed that the pooled global prevalence of osteoporosis was 38% (95% CI 34–43). Pooled odds ratio (OR) of having osteoporosis in COPD compared with controls was 2.99 (95% CI 2.09–4.27), and OR adjusted for negative publication bias was 2.83 (95% CI 2.00–4.03). It is of note that in most of the studies a diagnosis of osteoporosis was made by WHO definition [20] based on BMD values measured by dual-energy X-ray absorptiometry (DXA): BMD T score (standard deviations from the young adult mean) equal to or less than − 2.5. Because the contribution of BMD-independent risk or “bone quality” to fracture risk in COPD-associated osteoporosis may be larger than that in primary osteoporosis, the number of patients already having fragility fractures as well as those with high fracture risk may have been underestimated in those studies relying on BMD for the diagnosis of osteoporosis [9, 10, 19, 21].
Prevalent vertebral fractures in COPD
Previous reports indicated that prevalence of morphometric vertebral fractures was 24–79% in subjects with COPD [21]. The values widely varied because the characteristics of study subjects such as age, sex and COPD severity were substantially different between studies. Two studies comparing COPD patients with controls reported that OR for morphometric vertebral fracture in each study was 3.75 [22] and 1.72 [23]. Limitations of these studies include that the data was obtained through medical records dependent on the attending physicians and that identification of morphological deformity of the vertebrae was not always consistent among radiologists [21].
Clinical fracture
Clinical fractures are those with symptoms, bringing a patient to clinical attention. In a large observational cohort study called GLOW (Global Longitudinal Study of Osteoporosis Women) [24], 6.2% of 52,960 subjects suffered an incident clinical fracture over two years, and hazard ratio (HR) for fracture in subjects with COPD was 1.5 (95% CI 1.3–1.7). A retro-prospective cohort study of 87,360 men aged 50 and older (66.8 on the average) diagnosed with COPD in USA reported that absolute hip fracture rate was 3.99 per 1000 person-years, which was several times higher than the general population [25]. Thus, COPD increases the risk of clinical osteoporotic fractures including hip fracture.
Bone quality
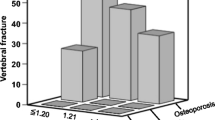
Bone strength is determined by BMD and bone quality. Several studies have suggested that COPD confers BMD-independent fracture risk, which is explained largely by deteriorated bone quality and in part by extra-skeletal factors. A study in Netherlands [10] indicated that 36.5% had vertebral fractures while 23.6% showed BMD-defined osteoporosis (T ≤ 2.5). A Japanese study of 136 COPD males [9] also found prevalent vertebral fractures in 79.4% and low BMD (T ≤ 2.5) only in 38.8%. A recent study comparing 91 COPD patients with 81 age- and sex-matched controls demonstrated that OR for total fractures were 4.7 times greater in COPD [26]. In this study, OR for fractures was consistently higher in COPD patients than in controls across all the BMD categories, which is reminiscent of the relationship between BMD and fracture risk in subjects with type 2 diabetes [27]. Moreover, the odds of fracture increased exponentially with a T score less than − 0.6 in the COPD group while this threshold was − 2.7 in the controls (Fig. 1). It is also of note that fracture prevalence in COPD far exceeds that predicted by FRAX [10, 24, 28], which calculates a 10 year probability of fracture based on clinical risk factors such as age, sex, BMI or BMD, a prior fragility fracture, parental history of hip fracture, current smoking, and ever use of oral glucocorticoids, and so on [29]. FRAX-independent risk of fracture is also a feature of COPD shared by type 2 diabetes [27].

(adapted from Adas-Okuma, et al. [26])
Impaired bone quality in COPD. Odds of fractures according to the values of femoral neck T scores are shown. The overall odds of fracture in the COPD group were 4.7 times greater than those in the control group
Impaired bone microstructure has been histologically demonstrated by histomorphometric analysis of biopsied bone specimens obtained from postmenopausal women with COPD who had never been treated with systemic glucocorticoid [30]. COPD was associated with decreased trabecular bone volume and connective density, and decreased cortical width and increased cortical porosity, compared with age-matched postmortem control samples.
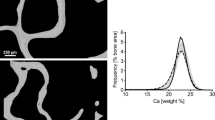
The trabecular bone score (TBS) is a gray‐level textural metric that can be extracted from the two‐dimensional lumbar spine DXA image, and reflects microarchitecture of trabecular bone [31]. The study in Manitoba using a large retro-prospective cohort of 29,407 women aged 50 years or more investigated clinical factors associated with TBS and demonstrated that the presence of COPD was associated with lower TBS, even after adjustment for age and BMD [32]. Another study of 145 active or former smokers showed that subjects with COPD had lower TBS compared to those without COPD [33]. Independent association of BMD and TBS with grade 2 or 3 vertebral fracture has also been reported in Japanese male patients with COPD [19] (Fig. 2). Thus, lower TBS seems to make a substantial contribution to bone fragility in COPD.
Independent association of bone mineral density (BMD) and trabecular bone score (TBS) with grade 2 or 3 vertebral fractures [19]. Prevalence of grade 2 or 3 vertebral fractures in TBS tertile groups within normal, osteopenic, and osteoporotic BMD strata is shown in 3D columns
Collectively, these results indicate that impaired bone quality, particularly structural deterioration, confers fracture risk independent of BMD in COPD patients. Consequently, in the clinical practice guide on fracture risk in lifestyle diseases in Japan [34], we have proposed a tentative algorithm of starting medical treatment of osteoporosis: the criteria for medication have been extended to osteopenic subjects with BMD of 70–80% YAM who have lifestyle diseases such as COPD, type 2 diabetes and chronic kidney disease (CKD).
Clinical risk factors
The mechanisms of COPD-associated osteoporosis are not completely understood.
Clinical studies have, however, identified various general and disease-specific risk factors of osteoporosis in COPD subjects, which include aging, smoking, low BMI and reduced physical activity as general risk factors; and pulmonary dysfunction, glucocorticoid use, vitamin D deficiency and systemic inflammation as disease-specific factors [11, 21, 35, 36]. We will focus on the disease-specific factors, particularly on vitamin D deficiency and inflammation, which are potentially involved in the pathogenesis of osteoporosis as well as sarcopenia in COPD.
Pulmonary dysfunction
Association between osteoporosis and pulmonary dysfunction has been reported. Decreased FEV1 and/or advanced GOLD stages correlated with lower BMD [9, 10, 23, 28]. Correlation between BMD and FEV1 has also been demonstrated in the general population in some reports [37,38,39], but not in others [40]. Relationship between pulmonary function and fractures in cross-sectional studies should be interpreted with caution, because they can mutually affect each other. Vertebral fracture has been shown to be associated with decreased vital capacity (VC) [9, 13], as already discussed earlier. And in COPD patients, correlation of GOLD stages determined by %FEV1 with fracture prevalence was not always clear [9, 10, 41]. COPD subjects seem already susceptible to osteoporotic fractures at early stages well before overt deterioration of pulmonary function. Thus, respiratory dysfunction may contribute to abnormal bone metabolism largely at later stages of COPD and that other mechanisms such as inflammation may predominate at earlier stages of COPD. This point will be discussed later in detail.
Glucocorticoid
Glucocorticoid (GC) excess is a common and established secondary cause of osteoporosis [42,43,44]. Many studies of COPD-associated osteoporosis have however demonstrated increased fracture incidence in subjects without systemic GC use [21, 45]. The standard management of COPD does not include oral GC treatment. Nonetheless, oral GC is still prescribed for the treatment of acute COPD exacerbations, often for a short period of time. A recent study using UK primary care data compared 53,299 matched pairs of COPD and non-COPD subjects followed up for 6.9 years and 5.4 years, respectively, for long-term adverse outcomes [46]. Oral GC use was associated with increased fracture risk with adjusted hazard ratio of 1.80 (95% CI 1.70–1.92).
More relevant to the COPD-associated fracture risk is the use of inhaled GC or corticosteroid (ICS). In a meta-analysis of 44 RCTs in 87,954 patients, inhaled therapies containing ICS, especially ICS/LABA: long-acting β2-agonists (RR = 1.30; 95% CI 1.10–1.53; P = 0.002) and triple therapy (ICS/LABA/LAMA: long-acting muscarinic receptor antagonist) (RR = 1.49; 95% CI 1.03–2.17; P = 0.04), were associated with increased risk of fracture [47]. Another meta-analysis of 26 RCTs including 61,380 subjects, 59.3% of whom received ICS therapy, however, did not find significant fracture risk by ICS treatment [14]. In a study of 251 COPD males, ICS reduced annual BMD loss in bronchitic patients [48], most likely due to ameliorated inflammation. These results are consistent with the idea that inflammation itself contributes to fracture.
Thus, overall effect of ICS seems to depend on the balance between the benefit from its local anti-inflammatory effect and the fracture risk caused by its systemic effect.
Collectively, although detrimental effect of GC to bone should always be considered in COPD patients, GC is not likely a major cause of osteoporosis in most cases of COPD [45].
Role for vitamin D in COPD as a respiratory disease
Serum levels of 25-hydroxyvitamin D (25D), the most abundant circulating metabolite of vitamin D, reflect the whole-body store of vitamin D and is used to clinically evaluate vitamin D status. Vitamin D deficiency and insufficiency are defined as 25D levels below 20 ng/ml and 20–30 ng/ml, respectively [49]. Vitamin D deficiency is common across all ages in virtually all over the world. Vitamin D deficiency causes reduced calcium absorption from the intestine, impaired skeletal calcification, and secondary hyperparathyroidism with high bone turnover, leading to osteomalacia and osteoporosis with increased fracture risk as well as blunted response to anti-resorptive treatment in osteoporotic patients. It may also be associated with sarcopenia and falling, which will also increase the risk of fracture, although considerable controversies still exist [49,50,51,52,53].
In COPD, vitamin D seems to play a role in deteriorated pulmonary function, severity and acute exacerbations. A meta-analysis of 21 studies including 4818 COPD patients and 7175 controls [54] demonstrated that 25D levels were lower in COPD patients than in controls (standardized mean difference: − 0.69, 95% CI − 1.00, − 0.38) and that the rate of vitamin D deficiency was associated with COPD severity (OR: 2.83, 95% CI 2.00–4.00). The exact reason for such a link is unknown, although vitamin D deficiency may be caused by various reasons including unbalanced diet, limited outdoor activity causing decreased sun exposure, and reduced capacity for vitamin D synthesis due to premature aging of the skin and smoking, increased vitamin D catabolism and lower vitamin D storage capacity [54,55,56]. It is of note that COPD patients not only had lower baseline levels of 25D but also showed blunted 25D response to oral supplementation of vitamin D3 with lower molar ratios of 25D- to-vitamin D than controls, suggesting disturbed vitamin D metabolism such as suppressed 25D production through 25-hydroxylation and/or promoted metabolism of 25D [57].
Vitamin D is also associated with respiratory function in the general population [21, 39, 58]. Serum 25D levels have been shown to positively correlate with pulmonary function parameters such as VC and FEV1. Such correlations have also been reported in COPD, but inconsistently [55]. Although causal relationship between respiratory dysfunction and vitamin D deficiency remains to be established, the associations with pulmonary parameters, together with its well-known immunomodulatory function [59, 60], has led to a postulation that the vitamin D status may affect COPD development and exacerbations [55]. Cross-sectional studies suggested association of acute exacerbations with low 25D levels, leaving some controversy [54, 55]. And attempts to improve pulmonary function and prevent acute exacerbations by vitamin D supplementation have been not at all or only partially effective [61,62,63,64,65]. A meta-analysis of 4 RCTs with 560 total participants indicated that vitamin D supplementation had no overall significant effect: it only reduced the rate of moderate/severe COPD exacerbations in patients with baseline 25D levels less than 10 ng/ml but not in those with higher levels [66]. Thus, pathogenic roles of vitamin D and its involvement in acute exacerbations in COPD are yet to be elucidated. It seems, however, worthy of attention that acute exacerbations are associated with hip fracture in COPD patients (adjusted OR, 2.25; 95% CI 1.66–3.05) [67] in a nested, case-control study in Korea, which may partly explain potential contribution of vitamin D status to COPD-associated osteoporosis.
Role for vitamin D in COPD-associated osteoporosis
Several studies showed that serum 25D levels were correlated with BMD in COPD patients [9, 19, 68, 69]. Association between the vitamin D status and the risk for osteoporosis has also been demonstrated in a longitudinal cohort study [70], in which vitamin D deficiency at baseline increased incidence of osteoporosis by 7.5 fold in 100 stable COPD patients during the 3-year follow-up period.
In our previous study of Japanese men with COPD [9, 19], both the presence of grade 2 or 3 [71] vertebral fracture and low BMD were associated with lower 25D levels. Multivariable linear regression analysis further indicated that the only independent determinants of BMD were body mass index (BMI) and 25D levels. In these studies, both BMI and BMD declined as the COPD stage defined by %FEV1 advanced, consistent with previous reports [10, 23]. Thus, as the COPD stage advances, BMI and 25D levels become lower, both of which may cause a stage-dependent decline in BMD. It is also notable that vitamin D deficiency is also associated with sarcopenia as discussed later.
Systemic inflammation
The pathophysiological process of COPD includes inflammatory cell infiltration to the mucosa, submucosa and glandular tissues of the lung. In addition to the basic innate immune response, chronic inflammatory responses of the lung to smoking and other noxious gas stimuli involve activation of Th1, 2, 17 systems, although the pathophysiology of COPD is quite heterogenous. Increased production of various cytokines has been demonstrated, and targeted cytokine therapies for COPD have been tested in clinical trials, although with limited success [72]. “Spill-over” of such local inflammatory factors has been proposed to cause various systemic comorbidities of COPD [4, 21, 73, 74].
Systemic inflammatory response is known to play a role in osteoporosis in the general population [75, 76]. A meta-analysis [76] of eight prospective cohort studies involving 34, 840 participants and 3, 407 incident fracture events has demonstrated association between fracture risk and high sensitivity C-reactive protein (hs-CRP) levels. Subjects in the top quartile of baseline hs-CRP levels showed a higher risk of overall fracture than those in the bottom quartile (RR = 2.14; 95% CI 1.51–3.05). Association between hs-CRP and BMD seemed less consistent, suggesting contribution of deteriorated bone quality. Another meta-analysis of ten studies (n = 29,741) including more recent studies with larger sizes reported similar results (RR = 1.60; 95% CI 1.07–2.49), confirming the association between hs-CRP levels and osteoporotic fracture [77]. Although a direct causal role for CRP is unknown, inflammation itself seems to be a key player in bone fragility in patients with various inflammatory diseases [75]. Roles for inflammation in osteoporosis is consistent with the fact that many inflammation-associated factors such as interleukin (IL)-6, IL-17, IL-1, matrix metalloproteases (MMPs) and RANKL are associated with enhanced osteoclastogenesis, leading to bone loss and fracture [75, 78, 79].
Consistent with a potential role of inflammation in COPD-associated osteoporosis, COPD patients with lower BMD have been shown to exhibit higher levels of CRP and inflammatory cytokines such as TNF-α, Interleukin (IL)-1 and IL-6 [80, 81]. Inflammation seems to contribute to bone fragility from early stages in COPD. As mentioned earlier, we previously found that both low BMD and TBS were independently associated with vertebral fracture in Japanese male patients with COPD [19] (Fig. 2). In this cohort, prevalence of vertebral fracture was quite high from early stages with mild pulmonary dysfunction, whereas the degree of BMD decrease was stage-dependent and relatively mild, suggesting a greater contribution of deteriorated bone quality to bone fragility. We further identified hs-CRP as the only independent determinant of TBS besides age and BMD by multivariate linear regression analysis [19]. These results suggest that persistent systemic inflammation in COPD contributes to the development of osteoporosis predominantly through deteriorating bone quality. So far, however, there have been no successful anti-inflammatory interventions that prevented fracture in COPD patients [72, 82, 83]. Etiological roles for inflammation in COPD-associated osteoporosis is still yet to be established.
Osteosarcopenia in COPD
Osteoporosis and sarcopenia often coincide and together promote frailty particularly in the aged [84, 85]. It is also the case in COPD [74, 86,87,88,89]. A meta-analysis reported that prevalence of sarcopenia in COPD varied from 15.5 to 34% [86] depending on the definition of sarcopenia. The presence of sarcopenia is an apparent risk of osteoporosis in COPD, as demonstrated by the meta-analysis [11]. Sarcopenia and osteopenia share common etiological factors such as IGF-1, sclerostin, myostatin, irisin, mitochondrial dysfunction, and inflammatory cytokines [88]. COPD patients have increased risk of frailty [90], which correlates with the degree of airflow limitation, and falls [91]. They also exhibit balance impairment, which will also lead to increased risk of falls [92]. Body weight loss is frequently found in COPD, particularly at advanced stages, and is associated with a poor prognosis [93]. Low BMI is a well-known risk factors of bone loss and fracture as well as sarcopenia. Consistently, low BMI has also been shown to predict osteoporosis defined by decreased BMD among COPD patients [9, 18].
It is of note here that both osteoporosis and sarcopenia in COPD are associated with body weight loss or cachexia, systemic inflammation, and vitamin D deficiency. Cachexia in severe COPD has been attributed to systemic inflammation with increased levels of cytokines such as TNF-α [94]. And this is inevitably accompanied by sarcopenia as well as osteopenia [88, 95]. Although detrimental effect of persistent inflammation should be continuously affecting both bone and muscle tissues throughout the clinical courses of COPD, evidence suggests that quantitative loss of bone and muscle mass becomes overt only in later stages in which body weight loss is manifest. This is probably because other factors such as malnutrition, oxidative stress, pulmonary dysfunction and decreased ADL also contribute to the development of cachexia in advanced stages.
Vitamin D is critical to not only bone but also muscle metabolism. Histologically, vitamin D deficiency is associated with muscle abnormalities characterized by type II fiber atrophy, muscle infiltration with fat cells and glycogen granules, enlargement of the inter-fibrillary gaps and increased fibrosis [96]. Loss of vitamin D signaling in a mouse model has indeed been shown to cause abnormalities in muscle fiber development [97]. Substantial evidence has also suggested epidemiological link between vitamin D deficiency and muscle function [96, 98,99,100]. However, effect of interventions by either native or active form of vitamin D was only partially effective [101, 102], leaving controversies about causal relationship between vitamin D and muscle. Similarly, there is currently little evidence that vitamin D supplementation ameliorates osteosarcopenia in COPD patients. Further studies will be necessary to elucidate the role of vitamin D in osteosarcopenia of COPD patients.
Mechanisms of COPD-associated osteoporosis: a proposal
As discussed above, current evidence suggests a critical role of systemic inflammation, cachexia and vitamin D deficiency in the pathogenesis of osteoporosis in COPD patients. Figure 3 illustrates our hypothetical view of the mechanisms of COPD-associated osteoporosis. COPD is a local inflammatory disease characterized by destruction of the lung tissue, leading to pulmonary dysfunction. Local inflammation somehow evokes systemic inflammatory responses (inflammation “spill-over”), causing various comorbidities in COPD patients. Persistent inflammation impairs quality of the bone. On the other hand, despite potential contribution of inflammation, cachexia usually does not occur until pulmonary dysfunction progresses, probably because it involves other mechanism such as hypoxia, oxidative stress, restricted physical activity, and so forth. Vitamin D deficiency is common in COPD patients but also correlates with the disease severity determined by the extent of pulmonary dysfunction. Both decreased BMI and vitamin D deficiency promote bone and muscle loss, deteriorating osteosarcopenia. Osteosarcopenia impairs ADL and causes frailty. It will increase falls, ultimately resulting in fracture. In this scenario, deterioration of bone quality may predominate over loss of bone mass in COPD patients with long persistent inflammation but without advanced respiratory failure. Large-scale longitudinal studies will be necessary to elucidate the relative contribution of BMD and bone quality to fracture and their relationship with inflammation and pulmonary dysfunction in COPD patients.
Conclusion
Osteoporosis is among major comorbidities of COPD, which profoundly affects the prognosis and mortality of the patients. However, neither precise mechanisms nor specific therapeutic maneuvers of COPD-associated osteoporosis are known. We would like to put an emphasis again on the potential roles of vitamin D deficiency and inflammation in the pathogenesis of COPD-associated osteoporosis. We propose to screen every COPD subject for osteoporosis, to replete vitamin D if possible, and to initiate appropriate treatment of osteoporosis when applicable, even though specific treatment guidelines for COPD-associated osteoporosis are unavailable at this point. Other than vitamin D supplementation, at present, only supportive care including rehabilitation and nutritional care can be done to prevent cachexia. We hope that future studies will establish specific treatment regimen for COPD-associated osteoporosis and anti-inflammatory therapy targeting COPD itself and its comorbid conditions including osteoporosis.
References
Celli B, Fabbri L, Criner G, Martinez FJ, Mannino D, Vogelmeier C, Montes de Oca M, Papi A, Sin DD, Han MK, Agusti A (2022) Definition and nomenclature of chronic obstructive pulmonary disease: time for its revision. Am J Respir Crit Care Med 206:1317–1325
Brat K, Svoboda M, Zatloukal J, Plutinsky M, Volakova E, Popelkova P, Novotna B, Dvorak T, Koblizek V (2023) Prognostic properties of the GOLD 2023 classification system. Int J Chronic Obstr Pulmon Dis 18:661–667
Skajaa N, Laugesen K, Horváth-Puhó E, Sørensen HT (2023) Comorbidities and mortality among patients with chronic obstructive pulmonary disease. BMJ Open Respir Res. https://doi.org/10.1136/bmjresp-2023-001798
Decramer M, Janssens W, Miravitlles M (2012) Chronic obstructive pulmonary disease. Lancet 379:1341–1351
Frei A, Muggensturm P, Putcha N, Siebeling L, Zoller M, Boyd CM, ter Riet G, Puhan MA (2014) Five comorbidities reflected the health status in patients with chronic obstructive pulmonary disease: the newly developed COMCOLD index. J Clin Epidemiol 67:904–911
Burgel PR, Escamilla R, Perez T, Carre P, Caillaud D, Chanez P, Pinet C, Jebrak G, Brinchault G, Court-Fortune I, Paillasseur JL, Roche N, Committee IBS (2013) Impact of comorbidities on COPD-specific health-related quality of life. Respir Med 107:233–241
Recio Iglesias J, Díez-Manglano J, López García F, Díaz Peromingo JA, Almagro P, Varela Aguilar JM (2020) Management of the COPD patient with comorbidities: an experts recommendation document. Int J Chronic Obstr Pulmon Dis 15:1015–1037
Graat-Verboom L, Wouters EF, Smeenk FW, van den Borne BE, Lunde R, Spruit MA (2009) Current status of research on osteoporosis in COPD: a systematic review. Eur Respir J 34:209–218
Watanabe R, Tanaka T, Aita K, Hagiya M, Homma T, Yokosuka K, Yamakawa H, Yarita T, Tai N, Hirano J, Inoue D, Okazaki R (2015) Osteoporosis is highly prevalent in Japanese males with chronic obstructive pulmonary disease and is associated with deteriorated pulmonary function. J Bone Miner Metab 33:392–400
Graat-Verboom L, van den Borne BE, Smeenk FW, Spruit MA, Wouters EF (2011) Osteoporosis in COPD outpatients based on bone mineral density and vertebral fractures. J Bone Miner Res 26:561–568
Chen YW, Ramsook AH, Coxson HO, Bon J, Reid WD (2019) Prevalence and risk factors for osteoporosis in individuals with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Chest. https://doi.org/10.1016/j.chest.2019.06.036
Pascual-Guardia S, Badenes-Bonet D, Martin-Ontiyuelo C, Zuccarino F, Marin-Corral J, Rodriguez A, Barreiro E, Gea J (2017) Hospital admissions and mortality in patients with COPD exacerbations and vertebral body compression fractures. Int J Chronic Obstruct Pulmon Dis 12:1837–1845
Leech JA, Dulberg C, Kellie S, Pattee L, Gay J (1990) Relationship of lung function to severity of osteoporosis in women. Am Rev Respir Dis 141:68–71
Zhang N, Fan X, Zhang Y, Xu N, Li L (2023) Risk of fracture and osteoporosis in patients With COPD and inhaled corticosteroids treatment. Respir Care 68:1719–1727
Ozel M, Altintas M, Tatliparmak AC (2023) Predictors of one year mortality following hip fracture surgery in elderly. PeerJ 11:e16008
Regan EA, Radcliff TA, Henderson WG, Cowper Ripley DC, Maciejewski ML, Vogel WB, Hutt E (2013) Improving hip fractures outcomes for COPD patients. COPD 10:11–19
Loh B, Jiang L, Timing L, Kong N, Ganesan G, Tan KB, Bee S, Koh JSB, Howe TS, Huei NY (2023) Predictors of 10 year mortality after hip fracture surgery in a pre-pandemic cohort. Geriatr Orthop Surg Rehabil 14:21514593231216560
Graat-Verboom L, Spruit MA, van den Borne BE, Smeenk FW, Martens EJ, Lunde R, Wouters EF (2009) Correlates of osteoporosis in chronic obstructive pulmonary disease: an underestimated systemic component. Respir Med 103:1143–1151
Watanabe R, Tai N, Hirano J, Ban Y, Inoue D, Okazaki R (2018) Independent association of bone mineral density and trabecular bone score to vertebral fracture in male subjects with chronic obstructive pulmonary disease. Osteoporos Int 29:615–623
Kanis JA (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group Osteoporos Int 4:368–381
Inoue D, Watanabe R, Okazaki R (2016) COPD and osteoporosis: links, risks, and treatment challenges. Int J Chronic Obstr Pulmon Dis 11:637–648
Papaioannou A, Parkinson W, Ferko N, Probyn L, Ioannidis G, Jurriaans E, Cox G, Cook RJ, Kumbhare D, Adachi JD (2003) Prevalence of vertebral fractures among patients with chronic obstructive pulmonary disease in Canada. Osteoporos Int 14:913–917
Kjensli A, Falch JA, Ryg M, Blenk T, Armbrecht G, Diep LM, Ellingsen I (2009) High prevalence of vertebral deformities in COPD patients: relationship to disease severity. Eur Respir J 33:1018–1024
Dennison EM, Compston JE, Flahive J, Siris ES, Gehlbach SH et al (2012) Effect of co-morbidities on fracture risk: findings from the global longitudinal study of osteoporosis in women (GLOW). Bone 50:1288–1293
Morden NE, Sullivan SD, Bartle B, Lee TA (2011) Skeletal health in men with chronic lung disease: rates of testing, treatment, and fractures. Osteoporos Int 22:1855–1862
Adas-Okuma MG, Maeda SS, Gazzotti MR, Roco CM, Pradella CO, Nascimento OA, Porto EF, Vieira JGH, Jardim JR, Lazaretti-Castro M (2020) COPD as an independent risk factor for osteoporosis and fractures. Osteoporos Int 31:687–697
Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Strotmeyer ES, Ensrud KE, Donaldson MG, Cauley JA, Harris TB, Koster A, Womack CR, Palermo L, Black DM (2011) Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA 305:2184–2192
Ogura-Tomomatsu H, Asano K, Tomomatsu K, Miyata J, Ohmori N, Kodama M, Ueda S, Takihara T, Tanaka K, Kamiishi N, Suzuki Y, Fukunaga K, Oguma T, Sayama K, Betsuyaku T (2012) Predictors of osteoporosis and vertebral fractures in patients presenting with moderate-to-severe chronic obstructive lung disease. COPD 9:332–337
Kanis JA, Oden A, Johansson H, Borgstrom F, Strom O, McCloskey E (2009) FRAX and its applications to clinical practice. Bone 44:734–743
Kulak CA, Borba VC, Jorgetti V, Dos Reis LM, Liu XS, Kimmel DB, Kulak J Jr, Rabelo LM, Zhou H, Guo XE, Bilezikian JP, Boguszewski CL, Dempster DW (2010) Skeletal microstructural abnormalities in postmenopausal women with chronic obstructive pulmonary disease. J Bone Miner Res 25:1931–1940
Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, McCloskey EV, Kanis JA, Bilezikian JP (2014) Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res 29:518–530
Leslie WD, Krieg MA, Hans D (2013) Clinical factors associated with trabecular bone score. J Clin Densitom 16:374–379
Gonzalez J, Rodriguez-Fraile M, Rivera P, Restituto P, Colina I, Calleja MLD, Alcaide AB, Campo A, Berto J, Seijo LM, Perez T, Zulueta J, de VaroTorres NJP (2019) Trabecular bone score in active or former smokers with and without COPD. PLoS One 14:e0209777
Kanazawa I, Inaba M, Inoue D, Uenishi K, Saito M, Shiraki M, Suzuki A, Takeuchi Y, Hagino H, Fujiwara S, Sugimoto T, from the Japan Osteoporosis Society Lifestyle diseases-related Fracture Risk Investigation C (2020) Executive summary of clinical practice guide on fracture risk in lifestyle diseases. J Bone Miner Metab 38:746–758
Sarkar M, Bhardwaj R, Madabhavi I, Khatana J (2015) Osteoporosis in chronic obstructive pulmonary disease. Clin Med Insights Circ Respir Pulm Med 9:5–21
Romme EA, Smeenk FW, Rutten EP, Wouters EF (2013) Osteoporosis in chronic obstructive pulmonary disease. Expert Rev Respir Med 7:397–410
Choi JW, Pai SH (2004) Association between respiratory function and osteoporosis in pre- and postmenopausal women. Maturitas 48:253–258
Jeon YK, Shin MJ, Kim WJ, Kim SS, Kim BH, Kim SJ, Kim YK, Shin YB, Kim IJ (2014) The relationship between pulmonary function and bone mineral density in healthy nonsmoking women: the Korean national health and nutrition examination survey (KNHANES) 2010. Osteoporos Int 25:1571–1576
Lin Z, Shi G, Liao X, Liu W, Luo X, Zhan H, Cai X (2023) Effect of pulmonary function on bone mineral density in the united states: results from the NHANES 2007–2010 study. Osteoporos Int 34:955–963
Dennison EM, Dhanwal DK, Shaheen SO, Azagra R, Reading I, Jameson KA, Sayer AA, Cooper C (2013) Is lung function associated with bone mineral density? results from the hertfordshire cohort study. Arch Osteoporos 8:115
Jorgensen NR, Schwarz P, Holme I, Henriksen BM, Petersen LJ, Backer V (2007) The prevalence of osteoporosis in patients with chronic obstructive pulmonary disease: a cross sectional study. Respir Med 101:177–185
Tanaka Y, Soen S, Hirata S, Okada Y, Fujiwara S et al (2024) The 2023 Guidelines for the management and treatment of glucocorticoid-induced osteoporosis. J Bone Miner Metab 42:143–154
Bankole A, Greear EL (2023) Glucocorticoid-induced osteoporosis: increased awareness as a management strategy for prevention of this complication in patients with systemic autoimmune rheumatic disease. Eur J Rheumatol 10:97–100
Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J et al (2017) American college of rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol 69:1521–1537
Adami G, Gatti D, Rossini M, Giollo A, Gatti M, Bertoldo F, Bertoldo E, Mudano AS, Saag KG, Viapiana O, Fassio A (2023) Risk of fracture in women with glucocorticoid requiring diseases is independent from glucocorticoid use: an analysis on a nation-wide database. Bone 179:116958
Tse G, Emmanuel B, Ariti C, Bafadhel M, Papi A, Carter V, Zhou J, Skinner D, Xu X, Müllerová H, Price D (2023) A long-term study of adverse outcomes associated with oral corticosteroid use in COPD. Int J Chronic Obstr Pulmon Dis 18:2565–2580
Peng S, Tan C, Du L, Niu Y, Liu X, Wang R (2023) Effect of fracture risk in inhaled corticosteroids in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. BMC Pulm Med 23:304
Mathioudakis AG, Amanetopoulou SG, Gialmanidis IP, Chatzimavridou-Grigoriadou V, Siasos G, Evangelopoulou E, Mathioudakis GA (2013) Impact of long-term treatment with low-dose inhaled corticosteroids on the bone mineral density of chronic obstructive pulmonary disease patients: aggravating or beneficial? Respirology 18:147–153
Okazaki R, Ozono K, Fukumoto S, Inoue D, Yamauchi M, Minagawa M, Michigami T, Takeuchi Y, Matsumoto T, Sugimoto T (2017) Assessment criteria for vitamin D deficiency/insufficiency in Japan: proposal by an expert panel supported by the research program of intractable diseases, ministry of health, labour and welfare, Japan, the Japanese society for bone and mineral research and the Japan endocrine society [opinion]. J Bone Miner Metab 35:1–5
Tan L, He R, Zheng X (2024) Effect of vitamin D, calcium, or combined supplementation on fall prevention: a systematic review and updated network meta-analysis. BMC Geriatr 24:390
Giustina A, Bouillon R, Dawson-Hughes B, Ebeling PR, Lazaretti-Castro M, Lips P, Marcocci C, Bilezikian JP (2023) Vitamin D in the older population: a consensus statement. Endocrine 79:31–44
Gallagher JC, Rosen CJ (2023) Vitamin D: 100 years of discoveries, yet controversy continues. Lancet Diabetes Endocrinol 11:362–374
Brincat M, Gambin J, Brincat M, Calleja-Agius J (2015) The role of vitamin D in osteoporosis. Maturitas 80:329–332
Zhu M, Wang T, Wang C, Ji Y (2016) The association between vitamin D and COPD risk, severity, and exacerbation: an updated systematic review and meta-analysis. Int J Chronic Obstr Pulmon Dis 11:2597–2607
Maes K, Serre J, Mathyssen C, Janssens W, Gayan-Ramirez G (2019) Targeting Vitamin D deficiency to limit exacerbations in respiratory diseases: utopia or strategy with potential? Calcif Tissue Int. https://doi.org/10.1007/s00223-019-00591-4
Janssens W, Mathieu C, Boonen S, Decramer M (2011) Vitamin D deficiency and chronic obstructive pulmonary disease: a vicious circle. Vitam Horm 86:379–399
Jolliffe DA, Stefanidis C, Wang Z, Kermani NZ, Dimitrov V, White JH, McDonough JE, Janssens W, Pfeffer P, Griffiths CJ, Bush A, Guo Y, Christenson S, Adcock IM, Chung KF, Thummel KE, Martineau AR (2020) Vitamin D metabolism is dysregulated in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 202:371–382
Park CY, Kwak SY, Jo G, Shin MJ (2018) Genetic association between serum 25-hydroxyvitamin D levels and lung function in korean men and women: data from KNHANES 2011(-)2012. Nutrients. https://doi.org/10.3390/nu10101362
Hamza FN, Daher S, Fakhoury HMA, Grant WB, Kvietys PR, Al-Kattan K (2023) Immunomodulatory properties of vitamin D in the intestinal and respiratory systems. Nutrients. https://doi.org/10.3390/nu15071696
Schrumpf JA, van der Does AM, Hiemstra PS (2020) Impact of the local inflammatory environment on mucosal vitamin D metabolism and signaling in chronic inflammatory lung diseases. Front Immunol 11:1433
Rafiq R, Aleva FE, Schrumpf JA, Daniels JM, Bet PM, Boersma WG, Bresser P, Spanbroek M, Lips P, van den Broek TJ, Keijser BJF, van der Ven A, Hiemstra PS, den Heijer M, de Jongh RT (2022) Vitamin D supplementation in chronic obstructive pulmonary disease patients with low serum vitamin D: a randomized controlled trial. Am J Clin Nutr 116:491–499
Chen FY, Xiao M, Ling B, Liu L, Chen L (2019) Vitamin D does not improve lung function decline in COPD: a meta-analysis. Eur Rev Med Pharmacol Sci 23:8637–8644
Sluyter JD, Camargo CA, Waayer D, Lawes CMM, Toop L, Khaw KT, Scragg R (2017) Effect of monthly high-dose long-term vitamin d on lung function: a randomized controlled trial. Nutrients. https://doi.org/10.3390/nu9121353
Janssens W, Decramer M, Mathieu C, Korf H (2013) Vitamin D and chronic obstructive pulmonary disease: hype or reality? Lancet Respir Med 1:804–812
Martineau AR, James WY, Hooper RL, Barnes NC, Jolliffe DA et al (2015) Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med 3:120–130
Jolliffe DA, Greenberg L, Hooper RL, Mathyssen C, Rafiq R, de Jongh RT, Camargo CA, Griffiths CJ, Janssens W, Martineau AR (2019) Vitamin D to prevent exacerbations of COPD: systematic review and meta-analysis of individual participant data from randomised controlled trials. Thorax 74:337–345
Gu KM, Yoon SW, Jung SY, Baek MS, Kim WY, Jung JW, Choi JC, Shin JW, Kim JY, Choi BW, Park IW (2022) Acute exacerbation of COPD increases the risk of hip fractures: a nested case-control study from the korea national health insurance service. Korean J Intern Med 37:631–638
Forli L, Halse J, Haug E, Bjortuft O, Vatn M, Kofstad J, Boe J (2004) Vitamin D deficiency, bone mineral density and weight in patients with advanced pulmonary disease. J Intern Med 256:56–62
Romme EA, Rutten EP, Smeenk FW, Spruit MA, Menheere PP, Wouters EF (2013) Vitamin D status is associated with bone mineral density and functional exercise capacity in patients with chronic obstructive pulmonary disease. Ann Med 45:91–96
Graat-Verboom L, Smeenk FW, van den Borne BE, Spruit MA, Jansen FH, van Enschot JW, Wouters EF (2012) Progression of osteoporosis in patients with COPD: a 3-year follow up study. Respir Med 106:861–870
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Schleich F, Bougard N, Moermans C, Sabbe M, Louis R (2023) Cytokine-targeted therapies for asthma and COPD. Eur Respir Rev. https://doi.org/10.1183/16000617.0193-2022
Mariniello DF, D’Agnano V, Cennamo D, Conte S, Quarcio G, Notizia L, Pagliaro R, Schiattarella A, Salvi R, Bianco A, Perrotta F (2024) Comorbidities in COPD: current and future treatment challenges. J Clin Med. https://doi.org/10.3390/jcm13030743
Zhang L, Sun Y (2021) Muscle-bone crosstalk in chronic obstructive pulmonary disease. Front Endocrinol (Lausanne) 12:724911
Briot K, Geusens P, Em Bultink I, Lems WF, Roux C (2017) Inflammatory diseases and bone fragility. Osteoporos Int 28:3301–3314
Wu ZJ, He JL, Wei RQ, Liu B, Lin X, Guan J, Lan YB (2015) C-reactive protein and risk of fracture: a systematic review and dose-response meta-analysis of prospective cohort studies. Osteoporos Int 26:49–57
Mun H, Liu B, Pham THA, Wu Q (2021) C-reactive protein and fracture risk: an updated systematic review and meta-analysis of cohort studies through the use of both frequentist and Bayesian approaches. Osteoporos Int 32:425–435
Li Y, Gao H, Zhao L, Wang J (2022) Osteoporosis in COPD patients: risk factors and pulmonary rehabilitation. Clin Respir J 16:487–496
Mundy GR (2007) Osteoporosis and inflammation. Nutr Rev 65:S147–S151
Bai P, Sun Y, Jin J, Hou J, Li R, Zhang Q, Wang Y (2011) Disturbance of the OPG/RANK/RANKL pathway and systemic inflammation in COPD patients with emphysema and osteoporosis. Respir Res 12:157
Liang B, Feng Y (2012) The association of low bone mineral density with systemic inflammation in clinically stable COPD. Endocrine 42:190–195
Ramírez-Fuentes C, Mínguez-Blasco P, Ostiz F, Sánchez-Rodríguez D, Messaggi-Sartor M, Macías R, Muniesa JM, Rodríguez DA, Vila J, Perkisas S, Escalada F, Marco E (2019) Ultrasound assessment of rectus femoris muscle in rehabilitation patients with chronic obstructive pulmonary disease screened for sarcopenia: correlation of muscle size with quadriceps strength and fat-free mass. Eur Geriatr Med 10:89–97
Mou K, Chan SMH, Vlahos R (2024) Musculoskeletal crosstalk in chronic obstructive pulmonary disease and comorbidities: emerging roles and therapeutic potentials. Pharmacol Ther 257:108635
Chen S, Xu X, Gong H, Chen R, Guan L, Yan X, Zhou L, Yang Y, Wang J, Zhou J, Zou C, Huang P (2024) Global epidemiological features and impact of osteosarcopenia: a comprehensive meta-analysis and systematic review. J Cachexia Sarcopenia Muscle 15:8–20
Huang T, Li C, Chen F, Xie D, Yang C, Chen Y, Wang J, Li J, Zheng F (2023) Prevalence and risk factors of osteosarcopenia: a systematic review and meta-analysis. BMC Geriatr 23:369
Sepúlveda-Loyola W, Osadnik C, Phu S, Morita AA, Duque G, Probst VS (2020) Diagnosis, prevalence, and clinical impact of sarcopenia in COPD: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 11:1164–1176
Kim SH, Shin MJ, Shin YB, Kim KU (2019) Sarcopenia associated with chronic obstructive pulmonary disease. J Bone Metab 26:65–74
Lippi L, Folli A, Curci C, D’Abrosca F, Moalli S, Mezian K, de Sire A, Invernizzi M (2022) Osteosarcopenia in patients with chronic obstructive pulmonary diseases: which pathophysiologic implications for rehabilitation? Int J Environ Res Public Health. https://doi.org/10.3390/ijerph192114314
Chua JR, Tee ML (2020) Association of sarcopenia with osteoporosis in patients with chronic obstructive pulmonary disease. Osteoporos Sarcopenia 6:129–132
Lahousse L, Ziere G, Verlinden VJ, Zillikens MC, Uitterlinden AG, Rivadeneira F, Tiemeier H, Joos GF, Hofman A, Ikram MA, Franco OH, Brusselle GG, Stricker BH (2016) Risk of frailty in elderly with COPD: a population-based study. J Gerontol A Biol Sci Med Sci 71:689–695
Lawlor DA, Patel R, Ebrahim S (2003) Association between falls in elderly women and chronic diseases and drug use: cross sectional study. BMJ 327:712–717
Loughran KJ, Atkinson G, Beauchamp MK, Dixon J, Martin D, Rahim S, Harrison SL (2020) Balance impairment in individuals with COPD: a systematic review with meta-analysis. Thorax 75:539–546
Vestbo J, Prescott E, Almdal T, Dahl M, Nordestgaard BG, Andersen T, Sorensen TI, Lange P (2006) Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen city heart study. Am J Respir Crit Care Med 173:79–83
Remels AH, Gosker HR, Langen RC (1985) Schols AM (2013) the mechanisms of cachexia underlying muscle dysfunction in COPD. J Appl Physiol 114:1253–1262
Dalle S, Koppo K (2020) Is inflammatory signaling involved in disease-related muscle wasting? evidence from osteoarthritis, chronic obstructive pulmonary disease and type II diabetes. Exp Gerontol 137:110964
Remelli F, Vitali A, Zurlo A, Volpato S (2019) Vitamin D deficiency and sarcopenia in older persons. Nutrients. https://doi.org/10.3390/nu11122861
Endo I, Inoue D, Mitsui T, Umaki Y, Akaike M, Yoshizawa T, Kato S, Matsumoto T (2003) Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology 144:5138–5144
Halfon M, Phan O, Teta D (2015) Vitamin D: a review on its effects on muscle strength, the risk of fall, and frailty. Biomed Res Int 2015:953241
Hirani V, Cumming RG, Naganathan V, Blyth F, Le Couteur DG, Hsu B, Handelsman DJ, Waite LM, Seibel MJ (2017) Longitudinal associations between Vitamin D metabolites and sarcopenia in older Australian men: the concord health and aging in men project. J Gerontol A Biol Sci Med Sci 73:131–138
Kotlarczyk MP, Perera S, Ferchak MA, Nace DA, Resnick NM, Greenspan SL (2017) Vitamin D deficiency is associated with functional decline and falls in frail elderly women despite supplementation. Osteoporos Int 28:1347–1353
Xiong A, Li H, Lin M, Xu F, Xia X, Dai D, Sun R, Ling Y, Qiu L, Wang R, Ding Y, Xie Z (2024) Effects of active vitamin D analogues on muscle strength and falls in elderly people: an updated meta-analysis. Front Endocrinol (Lausanne) 15:1327623
Webster J, Dalla Via J, Langley C, Smith C, Sale C, Sim M (2023) Nutritional strategies to optimise musculoskeletal health for fall and fracture prevention: looking beyond calcium, vitamin D and protein. Bone Rep 19:101684
Author information
Authors and Affiliations
Contributions
DI and RI both contributed to literature search and writing.
Corresponding author
Ethics declarations
Conflict of interest
DI has received honoraria from Amgen, Asahi-Kasei Pharma, Astellas Pharma, Daiichi-Sankyo Co., Eisai, and Teijin Pharma.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Inoue, D., Inoue, R. Mechanisms of osteoporosis associated with chronic obstructive pulmonary disease. J Bone Miner Metab 42, 428–437 (2024). https://doi.org/10.1007/s00774-024-01527-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-024-01527-1