Abstract
Summary
The aim of this study was to examine the association between pulmonary function and bone mineral density (BMD) in subjects who had never smoked. Pulmonary function was associated with BMD in premenopausal, but not postmenopausal, women.
Introduction
It has been reported that low bone mass is common in patients with pulmonary disorders such as chronic obstructive pulmonary disease. However, in healthy nonsmoking women, the relationship between bone mass and pulmonary function has yet to be clarified. The object of this study was to determine whether pulmonary function is related to BMD in healthy nonsmoking women based on menopausal status.
Methods
This study was a cross-sectional study based on data obtained from the Korean National Health and Nutrition Examination Survey (KNHANES), a nationwide representative survey conducted by the Korean Ministry of Health and Welfare in 2010. This study included 456 subjects who had never smoked and analyzed data concerning pulmonary function and BMD.
Results
Functional vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) were correlated with BMD at lumbar spine, femur neck (FN), and total hip in premenopausal women (p = 0.030, p = 0.003, p = 0.019, respectively, for FVC; p = 0.015, p = 0.006, p = 0.059, respectively, for FEV1). However, FVC and FEV1 were only correlated with BMD at FN in postmenopausal women (p = 0.003 for FVC; p = 0.006 for FEV1). Body mass index (BMI), FVC, and FEV1 were significantly related with BMD at FN, even after adjusting for age and other confounding factors (β = 0.334, p < 0.001; β = 0.145, p = 0.017; and β = 0.129, p = 0.037, respectively) in premenopausal women. However, only age and BMI were correlated with BMD at FN (β = −0.268, p = 0.001 and β = 0.384, p > 0.001) in postmenopausal women after adjusting for confounding factors.
Conclusions
Pulmonary function, including FVC and FEV1 are associated with BMD at FN in healthy nonsmoking premenopausal women but not in postmenopausal women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is an important public health problem worldwide because it can lead to fractures that may result in high morbidity and mortality. The bone mineral density (BMD) is a simple and common method for predicting fractures using risk assessment tools. BMD has been associated with pulmonary function in patients suffering from respiratory diseases such as cystic fibrosis and chronic obstructive pulmonary disease (COPD) [1–4]. Several studies have investigated the relationship between moderate-to-severe airflow obstruction and osteoporosis [5, 6] and found that subjects with obstructive airway disease have lower BMD even though they had not previously been exposed to steroids [7, 8]. However, few studies have investigated the relationship between pulmonary function and BMD in healthy people, and the results are not consistent [9–11]. Moreover, these studies utilized heterogeneous populations with regard to their smoking habits and included nonsmokers, ex-smokers, and current smokers. Cigarette smoking is a well-known risk factor that leads to a decline in lung function in adults and is a primary cause of COPD in conjunction with pathological changes in inflammation, fibrosis, and the destruction of alveolar attachments [12]. Even after the cessation of smoking, the negative effects of smoke on lung function are thought to continue [13]. Additionally, smoking is hypothesized to be a risk factor for bone loss and fractures [14]. In short, smoking is likely to represent an inevitable confounding factor for studies because it affects lung function and bone metabolism. Therefore, this study was undertaken to investigate the relationship between respiratory function and BMD in healthy nonsmoking women using data from a nationwide representative survey.
Patients and methods
Design and participants
This was a cross-sectional study based on the data obtained from the Korea National Health and Nutrition Examination (KNHANES), a nationwide representative survey conducted by the Korean Ministry of Health and Welfare in 2010. The KNHANES has been periodically conducted since 1998 and is composed of data from the civilian noninstitutionalized population of the Republic of Korea using a stratified multistage sampling with a probability proportional to size. KNHANES V was conducted between January 2010 and December 2010 and collected data via household interviews and direct standardized physical examinations performed in specially equipped mobile examination centers [15]. The ethnic makeup of the Korean population is Asian.
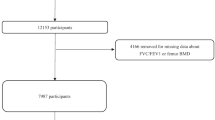
Women with BMD data and interpretable pulmonary function test data (n = 1,496) were included in this study. Participants with diseases such as cancer, arthritis, cardiovascular disease, or fractures or who were using medications for osteoporosis or pulmonary disease that could affect BMD or pulmonary function tests were excluded (n = 942). A history of smoking can affect pulmonary function; thus, subjects who had ever smoked during their lifetime were excluded (n = 48). The menopausal status was defined based on a questionnaire survey and was self-designated using the question of whether 1 year had passed since the time of last menstruation; women with premature surgical menopause were excluded. A total of 506 subjects (281 premenopausal women and 225 postmenopausal women) were included in this study, and all underwent a routine physical examination that recorded age, body weight, height, smoking history, drinking history, exercise levels, and calcium (Ca) intake. Dietary Ca intake was estimated using the 24 h dietary recall method. Exercise was recorded as “yes” when the subject exercised regularly at moderate levels, for more than 30 min at a time and more than five times a week. Blood samples for measuring serum 25-hydroxy vitamin D [25(OH)D] were obtained from all participants for biochemical analyses, immediately refrigerated, transported to the Central Testing Institute in Seoul, Korea, and analyzed within 24 h.
Bone mineral density
Subjects were in light clothing without shoes or jewelry that may have interfered with the BMD test. BMD levels were measured at the lumbar spine (L1–4) and proximal femur (total hip region) using dual-energy X-ray absorptiometry (DXA, Discovery-W model, Hologic, Bedford, MA, USA) in mobile examination centers. BMD is expressed in grams per square centimeter, and if the subject had prosthetic devices or implants, it was considered as missing data. For the purpose of DXA quality control, daily automatic calibration was performed according to the manufacturer’s instructions to maintain a precision standard of 1.5 % for total hip measurements [16].
Measurement of lung function
Lung function was measured using a dry rolling seal spirometer (model 2130; SensorMedics, Yorba Linda, CA, USA) according to the American Thoracic Society/European Respiratory Society criteria for standardization [17]. Spirometric data obtained on-site by clinical technicians were transferred to an internet review center for processing where the information was carefully examined and compared against criteria metrics for acceptability, reproducibility, and quality control. A principal investigator validated and stored the data in a Korea Centers for Disease Control and Prevention repository management system. Only interpretable data were included in this study.
Statistical analysis
All statistical analyses were performed with SPSS for Windows statistical package version 17.0 (SPSS Inc., Chicago, IL, USA). The baseline characteristics of the study groups were compared using independent Student’s t tests for continuous variables, and Pearson correlation coefficients were calculated in order to evaluate the correlations among femur neck (FN) BMD and age, body mass index (BMI), the pulmonary function test, serum 25(OH)D, and 24 h Ca intake. A partial correlation coefficient was used to measure the dependence of FN BMD on pulmonary function, and a multiple linear regression analysis was used to identify independent predictors of FN BMD in nonsmoking premenopausal and postmenopausal women. Results were considered to be significant when the p value was less than 0.5
Results
Baseline characteristics of participants
The baseline clinical and biochemical characteristics of subjects are presented (Table 1). The mean age of premenopausal and postmenopausal women was 44.9 ± 3.7 and 55.5 ± 6.5 years, respectively. BMI and the estimate of 24 h Ca intake were not significantly different between the two groups, but 25(OH)D levels were slightly higher in postmenopausal women. The BMDs at the lumbar spine, FN, and total hip were lower in postmenopausal women, and most of the lung function parameters were higher in premenopausal women.
The BMD at all three sites showed significant positive correlations with functional vital capacity (FVC, L; p < 0.05 for the lumbar spine and total hip, p < 0.01 for FN), forced expiratory volume in 1 s (FEV1, L; p < 0.01 for the lumbar spine and FN, p < 0.05 for total hip), forced expiratory volume in 6 s (FEV6, L; p < 0.01 the for lumbar spine and FN, p < 0.05 for total hip), and forced expiratory flow rate of 25–75 % (FEF25–27 (L/s); p < 0.01 for the lumbar spine and FN, p < 0.05 for total hip) in premenopausal women. However, in postmenopausal women, only the FN was positively correlated with these parameters (p < 0.05 for FVC(L), FEV1(L), FEV6(L), and FEF25–75 (L/s)). Age was not related with BMD at any site in premenopausal women, but these factors were positively related in postmenopausal women. BMI was positively related with BMD in both premenopausal and postmenopausal women, and 25(OH)D was negatively correlated with BMD in postmenopausal women (Table 2).
Multiple linear regression analyses of pulmonary function and FN BMD are shown (Table 3). In model 1, FN BMD showed a positive relationship with FVC and FEV1 in premenopausal women. In model 2, FVC and FEV1 remained the most significant independent variables among age, BMI, and pulmonary function. In model 3, after adding hormone therapy and physical activity as independent parameters, FVC and FEV1 were identified as significant factors that predicted FN BMD. Serum 25(OH)D and 24 h Ca intake were additionally adjusted in model 4. In premenopausal women, FVC and FEV1 showed significant positive correlations with FN BMD, even after adjusting for confounding factors. However, the same kind of relationship was not observed between pulmonary function and FN BMD in postmenopausal women (Table 3). In postmenopausal women, age and BMI showed negative and positive associations with FN BMD, respectively (p = −0.268 and p = 0.001 for age, p = 0.384 and p < 0.001 for BMI).
Discussion
This study demonstrated that FN BMD is closely associated with pulmonary functions such as FVC and FEV1 in nonsmoking healthy women. However, after adjusting for possible confounding factors including age, BMI, physical activity, and hormone replacement, FN BMD was significantly correlated with pulmonary function in premenopausal, but not postmenopausal, women. In postmenopausal women, only age and BMI were the significant factors associated with FN BMD.
Several studies have investigated the relationship between respiratory function and BMD in healthy people, but the results are inconsistent. In contrast to the current study, Lekamwasam et al. [9] found a positive relationship between FEV1 and BMD at the hip and lumbar spine even though the number of subjects was large, and the subject characteristics were heterogeneous. In that study, subjects were 45–76 years old, their menopausal statuses were not evaluated, and subjects with pulmonary disease such as COPD and those having used cancer or steroid medications that could affect pulmonary function and bone metabolism were included. Choi et al. [10] also found a positive correlation between pulmonary function and BMD, but there was a small population of subjects and they were from a single center. Both of these studies also included subjects with a history of smoking, which could affect pulmonary function and BMD, even after cessation. Moreover, these authors did not consider age, BMI, or behavioral habits; all of these factors are implicated in bone metabolism.
On the other hand, the current findings are consistent with the Hertfordshire Cohort study [11]. The subjects were males and females over 60 years of age and did not show any relationship between pulmonary function and BMD. These authors speculated that the different results were due a comparatively healthy cohort and that differences between countries regarding factors such as smoking, hypoxia, physical activity, water hardness, and fluoride concentration may have played a role. Similarly, the current study did not find a relationship between pulmonary function and BMD in postmenopausal women but did observe a positive relationship between these factors in premenopausal women. The unique difference in this study is that there was an attempt to minimize the effects of confounding factors such as cigarette smoking, pulmonary disease, and medications, including steroids. Smoking is a prominent factor that could affect pulmonary function, including FEV1. In a large study from the Netherlands that followed up with subjects over 24 years [13], pulmonary function changed according to smoking status, which was categorized as formal smokers, recidivist smokers, brief smokers, or new starters. All smoking subjects exhibited a more rapid decrease of FEV1, as compared to lifetime nonsmokers. It was suggested that the rapid decrease of FEV1 is related to hormonal fluctuations based on inflammation, disturbances in airway responsiveness, and hypersensitivity.
Smoking is a known risk factor for bone loss and fractures. In one cross-sectional study, BMD was lower by approximately 4–5 % at the FN, lumbar spine, and total body in smokers [18]. One follow-up study over 2 years found that smokers had lower spinal BMD [19], while another showed that the annual rate of bone loss at the radius was greater in smokers and proportionally related to pack years of smoking exposure, even after controlling for BMI and postmenopausal years [20]. Additionally, a prospective study found that current smokers had an increased risk for hip fracture compared with never-smokers [21]. The primary underlying mechanisms are not yet fully understood, although they are thought to involve impaired Ca absorption [22], reduced vitamin D levels [23], reduced estrogenic activity [24], and increased concentration of free radicals, which may interfere with bone resorption [25]. In vitro studies have found that nicotine decreases proliferation and collagen synthesis in osteoblast-like cells [26, 27]. In summary, smoking can interfere not only with bone metabolism, including BMD and fractures, but also with pulmonary function. Thus, this study excluded subjects with a history of smoking because smoking is an important confounding factor that needs to be controlled in order to accurately evaluate the relationship between pulmonary function and BMD.
In lifetime nonsmoking women, BMI is the most predictive factor for BMD at the FN for both premenopausal and postmenopausal women. In the multiple linear regression analysis, FVC and FEV1 were also factors that could predict FN BMD in premenopausal women, but only age was a predictive factor in postmenopausal women, even after adjustment for confounding factors such as age, BMI, hormonal replacement, physical activity, 24 h Ca intake, and serum 25(OH)D levels. This discrepancy is not sufficiently comprehensive to provide a clear understanding because hormonal differences could also result in differences; this deserves further investigation. Physical activity needs to be adjusted as a confounding factor. Physical activity is not only related to levels of lung function [28] but is also reported to have positive effects on lung function [29]. In addition, physical activity has been suggested to be a favorable factor affecting BMD [30]. Recently, increasing daily activity has been reported to help prevent decrease of BMD [31]. It was not hypothesized here that serum 25(OH)D levels would show a negative correlation with FN BMD based on the assumption that people who are concerned about bone health or who are at a high risk for osteoporosis tend to take more vitamin D, which is beneficial for bone health.
This study has some limitations. First, this is a retrospective study and includes an inherent selection bias because it was based on KNHANES data with interpretable lung function data and BMD information. Second, physical activity was not classified as strength training or aerobic exercise. Third, serum 25(OH)D levels were not adjusted for vitamin D supplementation. Finally, 24 h Ca intake was recorded based on remembered food estimates. Nevertheless, this is the first clinical study demonstrating a positive relationship between FN BMD and lung function in healthy nonsmoking premenopausal women. FN BMD did not show a relationship with pulmonary function, but there was a negative relationship with age in postmenopausal women. It is proposed here that if subjects exhibit decreased pulmonary function, BMD levels should be evaluated, even though premenopausal women are not thought to be at risk for low bone mass.
Abbreviations
- BMD:
-
Bone mineral density
- BMI:
-
Body mass index
- Ca:
-
Calcium
- 25(OH)D:
-
25-Hydroxy vitamin D
- FVC:
-
Functional vital capacity
- FEV1 :
-
Forced expiratory volume in 1 s
- FEV6 :
-
Forced expiratory volume in 6 s
- FEF25–75 :
-
Forced expiratory flow rate of 25–75 %
- PEFR:
-
Peak expiratory flow rate
References
Elkin SL, Fairney A, Burnett S, Kemp M, Kyd P, Burgess J, Compston JE, Hodson ME (2001) Vertebral deformities and low bone mineral density in adults with cystic fibrosis: a cross-sectional study. Osteoporos Int 12:366–372
Graat-Verboom L, Spruit MA, van den Borne BE, Smeenk FW, Martens EJ, Lunde R, Wouters EF, Network CIRO (2009) Correlates of osteoporosis in chronic obstructive pulmonary disease: an underestimated systemic component. Respir Med 103:1143–1151
Jorgensen NR, Schwarz P, Holme I, Henriksen BM, Petersen LJ, Backer V (2007) The prevalence of osteoporosis in patients with chronic obstructive pulmonary disease: a cross sectional study. Respir Med 101:177–185
Ferguson GT, Calverley PM, Anderson JA, Jenkins CR, Jones PW, Willits LR, Yates JC, Vestbo J, Celli B (2009) Prevalence and progression of osteoporosis in patients with COPD: results from the towards a revolution in COPD health study. Chest 136:1456–1465
Sin DD, Man JP, Man SF (2003) The risk of osteoporosis in Caucasian men and women with obstructive airways disease. Am J Med 114:10–14
Incalzi RA, Caradonna P, Ranieri P, Basso S, Fuso L, Pagano F, Ciappi G, Pistelli R (2000) Correlates of osteoporosis in chronic obstructive pulmonary disease. Respir Med 94:1079–1084
Dimai HP, Domej W, Leb G, Lau KH (2001) Bone loss in patients with untreated chronic obstructive pulmonary disease is mediated by an increase in bone resorption associated with hypercapnia. J Bone Miner Res 16:2132–2141
Iqbal F, Michaelson J, Thaler L, Rubin J, Roman J, Nanes MS (1999) Declining bone mass in men with chronic pulmonary disease: contribution of glucocorticoid treatment, body mass index, and gonadal function. Chest 116:1616–1624
Lekamwasam S, Trivedi DP, Khaw KT (2002) An association between respiratory function and bone mineral density in women from the general community: a cross sectional study. Osteoporos Int 13:710–715
Choi JW, Pai SH (2004) Association between respiratory function and osteoporosis in pre- and postmenopausal women. Maturitas 48:253–258
Dennison EM, Dhanwal DK, Shaheen SO, Azagra R, Reading I, Jameson KA, Sayer AA, Cooper C (2013) Is lung function associated with bone mineral density? Results from the Hertfordshire Cohort Study. Arch Osteoporos 8:115, 012–0115-y
Niewoehner DE, Kleinerman J, Rice DB (1974) Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med 291:755–758
Xu X, Weiss ST, Rijcken B, Schouten JP (1994) Smoking, changes in smoking habits, and rate of decline in FEV1: new insight into gender differences. Eur Respir J 7:1056–1061
Law MR, Hackshaw AK (1997) A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ 315:841–846
Choi HS, Oh HJ, Choi H, Choi WH, Kim JG, Kim KM, Kim KJ, Rhee Y, Lim SK (2011) Vitamin D insufficiency in Korea—a greater threat to younger generation: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J Clin Endocrinol Metab 96:643–651
Soen S, Fukunaga M, Sugimoto T, Sone T, Fujiwara S, Endo N, Gorai I, Shiraki M, Hagino H, Hosoi T, Ohta H, Yoneda T, Tomomitsu T, Japanese Society for Bone and Mineral Research and Japan Osteoporosis Society Joint Review Committee for the Revision of the Diagnostic Criteria for Primary Osteoporosis (2013) Diagnostic criteria for primary osteoporosis: year 2012 revision. J Bone Miner Metab 31:247–257
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, ATS/ERS Task Force (2005) Standardisation of spirometry. Eur Respir J 26:319–338
Jones G, Scott FS (1999) A cross-sectional study of smoking and bone mineral density in premenopausal parous women: effect of body mass index, breastfeeding, and sports participation. J Bone Miner Res 14:1628–1633
Mazess RB, Barden HS (1991) Bone density in premenopausal women: effects of age, dietary intake, physical activity, smoking, and birth-control pills. Am J Clin Nutr 53:132–142
Krall EA, Dawson-Hughes B (1991) Smoking and bone loss among postmenopausal women. J Bone Miner Res 6:331–338
Paganini-Hill A, Chao A, Ross RK, Henderson BE (1991) Exercise and other factors in the prevention of hip fracture: the leisure world study. Epidemiology 2:16–25
Krall EA, Dawson-Hughes B (1999) Smoking increases bone loss and decreases intestinal calcium absorption. J Bone Miner Res 14:215–220
Brot C, Jorgensen NR, Sorensen OH (1999) The influence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr 53:920–926
Barbieri RL, Gochberg J, Ryan KJ (1986) Nicotine, cotinine, and anabasine inhibit aromatase in human trophoblast in vitro. J Clin Invest 77:1727–1733
Duthie GG, Arthur JR, James WP (1991) Effects of smoking and vitamin E on blood antioxidant status. Am J Clin Nutr 53:1061S–1063S
Fang MA, Frost PJ, Iida-Klein A, Hahn TJ (1991) Effects of nicotine on cellular function in UMR 106-01 osteoblast-like cells. Bone 12:283–286
Ramp WK, Lenz LG, Galvin RJ (1991) Nicotine inhibits collagen synthesis and alkaline phosphatase activity, but stimulates DNA synthesis in osteoblast-like cells. Proc Soc Exp Biol Med 197:36–43
Nystad W, Samuelsen SO, Nafstad P, Langhammer A (2006) Association between level of physical activity and lung function among Norwegian men and women: the hunt study. Int J Tuberc Lung Dis 10:1399–1405
Berntsen S, Wisloff T, Nafstad P, Nystad W (2008) Lung function increases with increasing level of physical activity in school children. Pediatr Exerc Sci 20:402–410
Welten DC, Kemper HC, Post GB, Van Mechelen W, Twisk J, Lips P, Teule GJ (1994) Weight-bearing activity during youth is a more important factor for peak bone mass than calcium intake. J Bone Miner Res 9:1089–1096
Muir JM, Ye C, Bhandari M, Adachi JD, Thabane L (2013) The effect of regular physical activity on bone mineral density in post-menopausal women aged 75 and over: a retrospective analysis from the Canadian multicentre osteoporosis study. BMC Musculoskeletal Disorder 14:253
Grant support
None
Conflicts of interest
The authors declare no conflicts of interest
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. K. Jeon and M. J. Shin contributed equally to this study.
Rights and permissions
About this article
Cite this article
Jeon, Y.K., Shin, M.J., Kim, W.J. et al. The relationship between pulmonary function and bone mineral density in healthy nonsmoking women: the Korean National Health and Nutrition Examination Survey (KNHANES) 2010. Osteoporos Int 25, 1571–1576 (2014). https://doi.org/10.1007/s00198-014-2627-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-014-2627-3