Abstract
A high-fat diet (HFD) can have a negative effect on bone quality in young and old people. Although bone healing in children is normally efficient, there is no evidence that children who have a diet rich in fat have compromised bone fracture regeneration compared with children with recommended dietary fat levels. The purpose of the present study was to evaluate the effects of an HFD on bone healing in growing female rats. Twenty-six postweaning female Wistar rats were divided into two groups (13 animals per group): a standard diet (SD) group and an HFD (with 60% of energy from fat) group. The rats received the assigned diets for 5 weeks, and in the third week they were submitted to an osteotomy procedure of the left tibia. Body mass and feed intake were recorded during the experiment. One day before euthanasia, an insulin tolerance test was performed. After euthanasia, the tibiae were removed and analyzed by densitometry, mechanical testing, histomorphometry, stereology and immunohistochemistry. An HFD caused an adaptive response to maintain energetic balance by decreasing feed intake and causing insulin insensitivity. There was no change in bone mineral density, collagen amount and immunostaining for bone formation, but maximal load and stiffness were decreased in the HFD group. In addition, bone volume had a tendency to be higher in the SD group than in the HFD group. Compared with rats receiving an SD, growing rats receiving an HFD for 5 weeks had similar bone mineral density but altered mechanical properties at the osteotomy defect site.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite advances in bone studies, normal bone healing is a challenge in approximately 10% of fractures [1]. The process of bone remodeling involves local and systemic regulation, and is affected by various factors, such as age, previous disease, smoking, pharmacological agents, diabetes mellitus, and diet [2, 3].
Fat cells can undergo hypertrophy, with accumulation of fat in the body as a result of a high-fat diet (HFD) [4]. Foods rich in fat are increasingly common in the Western world, and some of its effects on bone healing have been studied [5, 6]. Dietary fat has been reported to negatively affect bone quality [7], specifically concerning calcium excretion, mechanical properties, mineral content, and osteoblast formation in adults, including elderly adults [8]. The relationship between body fat and bone is mediated by adipokines, which modulate bone remodeling and adipogenesis [9]. In addition, homeostatic feedback between bone and fat plays an important role in this association. The accumulation of body fat also increases the risk of developing insulin resistance, leading to type 2 diabetes, which can be related to delayed bone healing [10, 11].
Bone healing in children is efficient in general. However, body fat accumulation influences bone formation during childhood [12]. Moreover, child obesity is a risk factor for fractures [13, 14]. This relationship might be explained by the extra weight that bones of obese children have to endure [15] and by their higher risk of falling [13, 14]. The World Health Organization [16] reported that less than one quarter of children aged between 6 and 23 months old meet the nutritional recommendation for their age. Proper nutrition, obtained by a diet with a diversity of nutrients, is necessary for the healthy maintenance of the organism, and represents a protective measure against age-related osteometabolic disorders [17].
However, there is no evidence that children with HFDs have a worse bone healing response compared with children whose dietary fat levels are within the recommendations. Therefore, the purpose of the present study was to evaluate the effects of an HFD on bone healing in an experimental model of young female rats. With this study design we hope to contribute to knowledge on potential alternative measures to prevent future bone metabolic problems in women, who are at increased risk of developing osteoporosis and obesity. We hypothesized that consumption of an HFD impairs bone healing.
Materials and methods
This experimental study was performed according to the National Institutes of Health guidelines for the use of experimental animals, and was approved by the local Ethics Committee for Animal Experimentation, under process number 15/2015.
Twenty-six female postweaning Wistar rats aged 21 days were used. They were maintained under standard laboratory conditions at a temperature of 22 ± 2 °C and humidity of (55 ± 5)% with a 12-h light and dark cycle and with free access to water and food.
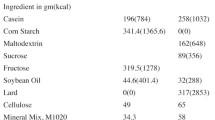
The rats were equally assigned to two groups (n = 13 in each): an HFD group and a standard diet (SD) group. The SD was produced according to recommendations by the American Institute of Nutrition (AIN-93G) [18], and the HFD was a modified AIN-93G diet containing 60% of the caloric content from saturated fat. The body weight of the rats and the feed intake of each group were measured weekly.
Surgical procedure
After 3 weeks on their assigned diets, the groups were submitted to an osteotomy procedure. For standardization purposes and to avoid impaired healing and stabilization, an osteotomy model was adopted in which a surgical partial transection was performed in the left midshaft tibia as described by Paiva et al. [19] and Sartori et al. [20].
The rats were anesthetized with an intramuscular injection of xylazine (10 mg/kg) and ketamine (50 mg/kg) and positioned with an external rotation of the hip and triple flexion. A skin and muscle incision was made in the medial aspect of the left lower leg, exposing the tibia. With a 2-cm diameter disc coupled to an electric motor for oral implants (Strong 210/105L micro motor, Saeshin Korea), set to 3000 rpm, an approximately 1-mm-deep cut was produced in the left midshaft tibia under saline irrigation. The cut depth was controlled and measured with a periodontal probe. Subsequently, the tissues were sutured, and the bone defect was confirmed by radiography.
At 14 days after surgery the rats were euthanized with an overdose of anesthetic (xylazine at 30 mg/kg and ketamine at 150 mg/kg), and their left tibiae were removed and cleaned of soft tissue. Thirteen tibiae per group were obtained, of which eight were stored at −20 °C and later used for densitometry and mechanical analyses, and five were prepared for histological analysis.
Insulin tolerance test
One day before euthanasia, a 1 mL/kg solution of insulin (1 U/kg, Novolin) was administered by intraperitoneal injection. Blood samples were collected via small tail incisions at 0, 20, 40, 60, 100, 120, and 150 min after insulin injection. Glucose levels were measured by a glucometer (Abbott Optium mini). The area under the glycemic curve was assessed to compare insulin sensitivity between the groups.
Geometry analysis
Tibiae were individually weighed with a precision balance (AC-2000, Marte) with 0.01-g precision. Tibial length was measured with a digital caliper with 0.01-mm precision (Mitutoyo).
Bone mineral density analysis
Eight tibiae were placed in a plastic vessel containing saline at a depth of 2.0 cm and scanned by dual-energy X-ray absorptiometry with a DPX-IQ densitometer (Lunar, USA). The region of interest (4 mm2) was selected manually in the midshaft tibia, on the basis of a constant area around the surgery site, by a specific protocol using radiologic images. The osteotomy site was measured on radiographic images, and the same site was assessed on densitometry images. DPX (version 4.7E, Lunar, USA) designed for small animals set at high resolution was used to determine bone mineral density (BMD) (g/cm2).
Biomechanical analysis
After densitometry analysis the tibiae were tested by the three-point bending test with a universal testing machine (DL10000, Emic, São José dos Pinhais, Brazil) coupled to a load cell with 500-N capacity. The tibiae were placed in supports 25 mm apart, and a force was applied at a speed of 1.0 mm/min in the posterior–anterior direction of the tibia to generate a tension load on the osteotomy site. The software program Tesc (version 13.0, Emic, Brazil) created a load versus deformation graph, providing maximal load (N) and stiffness (N/mm) data.
Histological protocol
Five tibiae were fixed in 4% formaldehyde for 24 h, decalcified in 10% ethylenediaminetetraacetic acid, dehydrated in an ascending series of alcohols, and diaphonized in xylene. Sequentially, the tibiae were embedded in paraffin. Serial 5-µm-thick sagittal sections were obtained from the defect region with use of an RM 2165 microtome (Leica, Houston, USA). Twenty-four of these sections were stained with trichrome by the Masson method, 12 were stained with the picrosirius red method, and four were submitted to immunohistochemistry.
Stereology analysis
The volume of newly formed bone at the osteotomy site was estimated in the sections stained with Masson’s trichrome with use of an Axio Imager Z2 optical microscope (Zeiss, Göttingen, Germany) at ×100 magnification and stereological Stereo Investigator software (MBF Bioscience, USA). The bone volume to total volume (BV/TV) ratio [21] of the defect site was calculated by the Cavalieri method, by our counting the points of a grid applied over the newly formed bone, on the basis of the known thickness (5 µm).
Histomorphometry analysis
The collagen area at the osteotomy site was evaluated on the sections stained with picrosirius red. Analysis was performed with polarized light and birefringence, which allows the observation of collagen fibers. The percentage of collagen area in total area evaluated was measured with use of an Axio Imager Z2 optical microscope (Zeiss, Göttingen, Germany) at ×100 magnification and an Axiovision 4.8 software grid system (Zeiss, Göttingen, Germany).
Immunohistochemistry analysis
The sections were deparaffinized in xylene and hydrated in a descending ethanol series. Antigen retrieval was performed in Diva Decloaker buffer (Biocare Medical, Pacheco, CA, USA) in a pressurized decloaking chamber (Biocare Medical, CA, Pacheco, USA) at 95 °C for 10 min. Phosphate-buffered saline (PBS) was used to wash the sections. Sequentially, the endogenous peroxidase was blocked by immersion of the sections in 3% hydrogen peroxide for 1 h. Then sections were treated in 3% bovine albumin serum for 12 h. The incubation with primary antibodies was done with anti-osteocalcin (Santa Cruz Biotechnology, Santa Cruz CA, USA) and anti-osteopontin (Millipore, Temecula, CA, USA). A streptavidin–biotin kit (Dako Laboratories, Burlington, CA, USA) was used in the subsequent steps. The sections were incubated in biotinylated secondary antibody for 2 h, washed, and treated with streptavidin conjugated with horseradish peroxidase for 1 h. After three washes in PBS plus 0.1% Triton X-100, the sections were developed with use of the chromogen 3.3′-diaminobenzidine tetrahydrochloride (DAB chromogen kit, Dako Laboratories, Burlington, CA, USA) and washed again with PBS. The sections were stained with Harris hematoxylin. As a negative control, the specimens were submitted to the procedures described above but without use of primary antibodies.
Immunolabeling analysis
Positive immunolabeling (osteocalcin-positive and osteopontin-positive) areas had a brownish appearance. The areas restricted to osteotomy sites were assessed with an Axio Imager Z2 optical microscope (Zeiss, Göttingen, Germany) at ×100 magnification. With use of AxioVision 4.8 (Zeiss), these areas were recognized by the color and quantified. The ratio of positive immunolabeling areas to the total area of interest was evaluated.
Statistical analysis
Statistical analyses were performed with IBM SPSS Statistics 20 (IBM, Amonk, NY, USA). Comparisons among the groups were statistically assessed by Student’s t test and the Mann–Whitney test according to data distribution. ANOVA was performed with application of a Bonferroni adjustment for multiple comparisons for feed intake and body weight data. The level of statistical significance was set at p ≤ 0.05.
Results
Body weight and feed intake
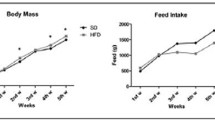
Figure 1 show the rats’ body weight and the weekly feed intake. The body weight of the groups was similar at the beginning of the experiment (p = 0.755). All rats showed weight gain over the weeks (p < 0.001). The HFD group was slightly heavier at the fifth week than the SD group; however, this difference was not statistically significant (p = 0.204).
a Comparison between standard diet (SD) and high-fat diet (HFD) groups with similar body weight at the beginning of the experiment (p = 0.755). At the fifth week the HFD group became slightly heavier than the SD group, without a significant difference (p = 0.204). b Feed intake increased for the first 3 weeks and decreased in the fourth week in both groups. The HFD group showed lower feed intake than the SD group in the fifth week (p = 0.034)
Feed intake increased over the weeks, except in week 4, in which the surgical procedure was performed and a decrease in feed intake was observed (Fig. 1b). The HFD group had a lower feed intake than the SD group in the fifth week (p = 0.034).
Insulin tolerance test
The area under the curve analysis showed an elevated blood glucose level in the HFD group when compared with the SD group (p = 0.01), implying that the HFD rats developed insensitivity to insulin (Fig. 2).
Geometry properties
The weight (p = 0.804) and length (p = 0.091) of the tibiae were similar between the groups; the results are shown in Table 1.
Bone mineral density
The HFD did not affect BMD at the osteotomy site. Comparison between the groups showed no significant difference (p = 0.302); the results are shown in Table 1.
Biomechanical properties
The three-point bending test showed that HFD rats had compromised healing when compared with SD rats. Lower maximal load (p = 0.040) and bending stiffness (p = 0.020) were observed in tibiae of HFD rats (Fig. 3).
Histological analysis
Newly formed bone was statistically similar between the groups. However, the BV/TV ratio showed a decreasing trend in the defect site of the HFD group (p = 0.054). The amount of collagen at the osteotomy site was similar between the groups (p = 0.293) (Table 1). Representative Masson and picrosirius red stained sections are shown in Fig. 4.
Histological photomicrographs of trabecular bone and collagen from the defect site in the experimental groups. The volume of newly formed bone at sections stained with Masson stain was slightly lower in the high-fat diet (HFD) group (a) than in the standard diet (SD) group (b). Collagen fibers observed under polarized light in sections stained with picrosirius red showed similar appearance in the HFD group (c) and the SD group (d). Magnification ×100
Immunohistochemistry analysis
No significant difference was observed in osteocalcin (p = 0.496) and osteopontin (p = 0.743) immunolabeling at the osteotomy site between the groups (Table 1, Fig. 5).
Discussion
This study compared the effects of an HFD and an SD on bone healing in growing rats, and no difference in BMD was observed between the groups. However, an HFD caused a lower bone maximal load and stiffness, and a trend to decreased BV/TV. Expression of osteocalcin and osteopontin, as molecules related to bone mineralization and consequently to BMD, was unaltered. The same was found for the amount of collagen.
HFDs have been associated with obesity development [6, 22]. Previously, it was believed that obesity was beneficial to bone [23] because of the increase in mechanical load, which stimulates bone formation [24]. This hypothesis had been supported by the argument that bone adapts to maintain the optimal size and properties to support a particular load [25]. However, recent reports indicate that body weight increase could be negatively related to bone mass because of the possible competitive effect between adipocyte differentiation and osteoblast differentiation, as they share a common progenitor [26,27,28]. Cao and Gregoire [29] observed that increased body weight from an HFD did not mitigate bone loss. Although external load is considered a positive stimulus for bone formation [30], fat accumulation is not protective against bone loss. Adipose tissue is not an inert organ with a sole function of storing energy; it has metabolic functions, secreting proteins that are involved in bone metabolism [12, 30, 31]. Indeed, an HFD has a greater impact on bone metabolism by endocrine stimulus than by mechanical stimulus, regardless of weight.
There is evidence that an HFD can cause negative alterations in bone structure of growing mice [32], and the effect of an HFD on bone healing has been investigated by different methods [5, 6]. Histing et al. [6] used the traumatic fracture method, and found no effect of an HFD on the bone healing process in femur. Brown et al. [5] used the osteotomy procedure, but their experiment was performed in adult male mice. To study the effects on growing rats we opted to perform an osteotomy [5, 19], which consists of a cut on the bone surface, creating a clean defect. This model does not match real lesion conditions but confers high standardization in biology studies [33]. Moreover, as the rats receiving the HFD could have bone fragility, the traumatic fractures could yield different bone fragmentation between groups.
In our study, an HFD, because of bone and body fat interplay, delayed restoration of bone resistance after an injury indicated by the decreased strength and stiffness. The lower maximal load showed that tibiae of the HFD rats when compared with those of SD rats had compromised biomechanical integrity. Imbalanced remodeling can affect bone structure, mass, and strength [34], and therefore a morphologic study complemented with a bending test allows a broader view of bone quality aspects and its capacity to resist fractures [35].
An HFD increases bone quantity and the mineral content but decreases bone quality related to biomechanical properties [25]. This evidence indicates that bone quantity and bone quality play important compensatory roles in determining fracture risk. Brown et al. [5] observed that adult mice receiving a HFD had significantly weaker healed fractures, which was similar to our results in growing rats. Although some authors have described a change in BMD in animals receiving an HFD [35, 36], this was not found in our study, corroborating the results of Macedo et al. [37]. Lower maximal load with no alteration in BMD has been reported in bone of rats fed with an HFD [38]. In a study with postmenopausal women with low-trauma fractures, Premaor et al. [39] found that the women had a normal BMD score. Therefore, these results confirm that BMD alone cannot determine the quality of bone repair, although it is considered the gold standard in bone fragility evaluation.
A trend for a reduced BV/TV ratio was observed at the osteotomy site of our HFD rats. This corroborates the results obtained by Cao and Gregoire [29], who assessed tibia proximal microstructure of HFD mice and found no significant difference in the BV/TV ratio but a 13% reduction trend in comparison with the control group. In the study by Brown et al. [5], the HFD group had lower bone callus volume at the 21st day, and the percentage of newly formed bone area was lower only at the 28th day. However, no differences were found at the 7th and 14th days, which is similar to the differences in the present study in new bone volume, for which a decreasing trend was observed only by the 14th day. In addition, HFD animals can develop a larger adipocyte area and a smaller osteoblast area on the bone callus when compared with control animals [5]. This confirms that adipogenesis and osteoblastogenesis are interrelated and that an HFD can cause changes in the microenvironment, affecting their balance [40].
Collagen is one of the main bone matrix compounds, especially in the early stages of bone regeneration, and is important for maintaining bone resistance [41], and thus it was evaluated in our study, as described by other authors [42]. No difference was observed in collagen amount between the groups. In addition, the expression of osteocalcin and osteopontin, which are bone formation markers [40, 41], showed no difference between the HFD group and the SD group, which suggests that an HFD does not affect cell adhesion in bone matrix and mineralization. These results contrast with the BMD results on the bone defect region, which were similar between the groups. As cell adhesion and mineralization are inherent stages in bone tissue formation and consequently bone repair, these results suggest that the effects of an HFD may be involved in the osteoblast differentiation stage but do not affect matrix formation.
In the present study, HFD rats showed no difference in body mass at the end of the experiment, but a lower feed intake was observed. Postweaning rats receiving an HFD may develop obesity resistance [43], and body weight gain results from an imbalance between energy intake and energy expenditure [44]. Furthermore, studies have found elevated levels of the satiety hormone leptin [12] in the animals receiving an HFD [6, 43], which might be related to the lower feed intake of the HFD group. In addition, both groups in our study had a decrease of feed intake in the third week, without body weight being affected. Overall, despite not causing an increase in body weight, the HFD caused a negative effect on bone, confirming that feeding with a fat-rich diet can lead to metabolic disorders even in the absence of obesity [45, 46].
We found insulin insensitivity the HFD rats in comparison with SD rats. It is well known that lipids accumulated in tissues and used as an energy substrate can cause a reduction of glucose optimization, and consequent insulin resistance [4, 47]. An experimental study showed that HFD mice have an increased risk of developing insulin resistance [45], corroborating the results of this study. At the same time, insulin resistance has been described as a risk factor for bone fractures and a negative influence in bone healing [10]. Insulin insensitivity might be a possible mechanism by which the tibiae of HFD rats had a weaker healing bone.
In this experiment, an HFD with 60% of its energy from saturated fat was used. Food rich in saturated fat is common in daily diets and can have an adverse effect on bone quality [48]. Contrarily, improved microstructure parameters were found by other authors with use of an HFD composed of monounsaturated and unsaturated fat [36, 49]. Therefore, it can be inferred that bone is differently affected according to the fat type present in the diet.
In conclusion, growing rats receiving an HFD for 5 weeks had impaired bone healing after an osteotomy defect, on the basis of mechanical parameters. Healthy habits during childhood can prevent age-related health problems and can contribute to better repair of bone lesions. However, the relationship between an HFD and bone healing needs more detailed understanding. Further studies on metabolic and cellular mechanisms should help to clarify the complex relationship between osteoblastogenesis and osteogenesis and its impact on bone healing.
References
Azad V, Breitbart E, Al-Zube L, Azad Yeh S, O’Connor JP, Lin SS (2009) rhBMP-2 enhances the bone healing response in a diabetic rat segmental defect model. J Orthop Trauma 23:267–276
Diniz SF, Amorim FPLG, Cavalcante-Neto FF, Bocca AL, Batista AC, Simm GEPM, Silva TA (2008) Alloxan-induced diabetes delays repair in a rat model of closed tibial fracture. Braz J Med Biol Res 41:373–379
Ogasawara A, Nakajima A, Nakajima F, Goto K, Yamazaki M (2008) Molecular basis for affected cartilage formation and bone union in fracture healing of the streptozotocin-induced diabetic rat. Bone 43:832–839
Gustafson B, Hedjazifar S, Gogg S, Hammarstedt A, Smith U (2015) Insulin resistance and impaired adipogenesis. Trends Endocrinol Metab 26:193–200
Brown ML, Yukata K, Farnsworth CW, Chen DG, Awad H, Hilton MJ, O’Keefe RJ, Xing L, Mooney RA, Zuscik MJ (2014) Delayed fracture healing and increased callus adiposity in a C57BL/6J murine model of obesity-associated type 2 diabetes mellitus. PLoS One 9:1–11
Histing T, Andonyan A, Klein M, Scheuer C, Stenger D, Holstein JH, Veith NT, Pohlemann T, Menger MD (2016) Obesity does not affect the healing of femur fractures in mice. Injury 47:1435–1444
Woo DG, Lee BY, Lim D, Kim HS (2009) Relationship between nutrition factors and osteopenia: effects of experimental diets on immature bone quality. J Biomech 42:1102–1107
Corwin RL (2003) Effects of dietary fats on bone health in advanced age. Prostaglandins Leukot Essent Fat Acids 68:379–386
Greco EA, Lenzi A, Migliaccio S (2015) The obesity of bone. Ther Adv Endocrinol Metab 6:273–286
Kayal RA, Alblowi J, McKenzie E, Krothapalli N, Silkman L, Gerstenfeld L, Einhorn TA, Graves DT (2009) Diabetes causes the accelerated loss of cartilage during fracture repair which is reversed by insulin treatment. Bone 44:357–363
Kawai M, Paula FJA, Rosen CJ (2012) New insights into osteoporosis: the bone-fat connection. J Intern Med 272:317–329
Chaplais E, Thivel D, Greene D, Dutheil F, Duche P, Naughton G, Courteix D (2015) Bone-adiposity cross-talk: implications for pediatric obesity: a narrative review of literature. J Bone Miner Metab 33:592–602
Fornari ED, Suszter M, Roocroft J, Bastrom T, Edmonds EW, Schlechter J (2013) Childhood obesity as a risk factor for lateral condyle fractures over supracondylar humerus fractures pediatrics. Clin Orthop Relat Res 471:1193–1198
Taylor ED, Theim KR, Mirch MC, Ghorbani S, Tanofsky-kraff M, Adler-Wailes DC, Brady S, Reynolds JC, Calis KA, Pharma D, Yanovisk JA (2006) Orthopedic complications of overweight in children and adolescents. Pediatrics 117:2167–2174
Goulding A, Taylor RW, Jones IE, McAuley KA, Manning PJ, Williams SM (2000) Overweight and obese children have low bone mass and area for their weight. Int J Obes Relat Metab Disord 24:627–632
World Health Organization (2014) Obesity and overweight. http://www.who.int/gho/ncd/risk_factors/overweight/en/index1.html. Accessed 29 Jun 2016
Kin CFW, Shan WSY, Shun LJC, Chung LP, Jean W (2007) Experience of famine and bone health in post-menopausal women. Int J Epidemiol 36:1143–1150
Reeves PG, Nielsen FH, Fahey GC Jr (1939) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Paiva AG, Yanagihara GR, Macedo AP, Ramos J, Issa JPM, Shimano AC (2016) Analysis of fracture healing in osteopenic bone caused by disuse: experimental study. Braz J Med Biol Res 49:3–9
Sartori AR, Moreira JA, Santos AMM, Cintra DEC, Sartori LR, Baraúna MA, Canto RST (2008) Bone repair process in normal and osteopenic female rats’ tibiae: a comparative study. Acta Ortop Bras 16:37–40
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Bone Miner Res 2:595–610
Gautam J, Choudhary D, Khedgikar V, Kushwaha P, Singh RS, Singh D, Tiwari S, Trivedi R (2014) Micro-architectural changes in cancellous bone differ in female and male C57BL/6 mice with high-fat diet-induced low bone mineral density. Br J Nutr 111:1811–1821
Silva HGV, Mendonca LMC, Conceicao FL, Zahar SEV, Farias MLF (2007) Influence of obesity on bone density in postmenopausal women. Arq Bras Endocrinol Metab 51:943–949
Frost HM (1997) Obesity, and bone strength and “mass”: a tutorial based on insights from a new paradigm. Bone 21:211–214
Ionova-Martin SS, Do SH, Barth HD, Szadkowska M, Porter AE, Iii JWA, Ager JW Jr, Alliston T, Vaisse C, Ritchie RO (2010) Reduced size-independent mechanical properties of cortical bone in high-fat diet-induced obesity. Bone 46:217–225
Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME (1992) Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci 102:341–351
Nuttall ME, Patton AJ, Olivera DL, Nadeau DP, Gowen M (1998) Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: implications for osteopenic disorders. J Bone Miner Res 13:371–382
Rosen ED, MacDougald O (2006) Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7:885–896
Cao JJ, Gregoire BR (2016) A high-fat diet increases body weight and circulating estradiol concentrations but does not improve bone structural properties in ovariectomized mice. Nutr Res 36:320–327
Hammer A (2015) The paradox of Wolff’s theories. Ir J Med Sci 184:13–22
Vendrell J, Broch M, Vilarrasa N, Molina A, Gómez JM, Gutiérrez C, Simón I, Soler J, Richart C (2004) Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: relationships in obesity. Obes Res 12:962–971
Rendina-Ruedy E, Graef JL, Davis MR, Hembree KD, Gimble JM, Clarke SL, Lucas EA, Smith BJ (2016) Strain differences in the attenuation of bone accrual in a young growing mouse model of insulin resistance. J Bone Miner Metab 34:380–394
Jones E, Yang X (2011) Mesenchymal stem cells and bone regeneration: current status. Injury 42:562–568
Peel N (2009) Bone remodelling and disorders of bone metabolism. Surgery 27:70–74
Sharir A, Barak MM, Shahar R (2008) Whole bone mechanics and mechanical testing. Vet J 177:8–17
Wang Y, Dellatore P, Douard V, Qin L, Watford M, Ferraris RP, Lin T, Shapses SA (2016) High fat diet enriched with saturated, but not monounsaturated fatty acids adversely affects femur, and both diets increase calcium absorption in older female mice. Nutr Res 36:742–750
Macedo AP, Shimano RC, Ferrari DT, Issa JPM, Jordão AA, Shimano AC (2016) Influence of treadmill training on bone structure under osteometabolic alteration in rats subjected to high-fat diet. Scand J Med Sci Sport. doi:10.1111/sms.12650
Shen CL, Cao JJ, Dagda RY, Chanjaplammootil S, Lu C, Chyu MC, Gao W, Wang JS, Yeh JK (2012) Green tea polyphenols benefits body composition and improves bone quality in long-term high-fat diet-induced obese rats. Nutr Res 32:448–457
Premaor MO, Lesley P, Carol T, Parker RA, Compston J (2010) Obesity and fractures in postmenopausal women. Bone Miner Res 25:292–297
Tang QQ, Lane MD (2012) Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem 81:715–736
Ralstson S (2005) Structure and metabolism of bone. Calcium Bone 33:58–60
Issa JPM, Defino HLA, Sebald W, Coutinho-Netto J, Iyomasa MM, Shimano AC, Bentley MVLB, Pitol DL (2012) Biological evaluation of the bone healing process after application of two potentially osteogenic proteins: an animal experimental model. Gerodontology 29:258–264
Fiorino P, Américo ALV, Muller CR, Evangelista FS, Santos F, Leite APO, Farah V (2016) Exposure to high-fat diet since post-weaning induces cardiometabolic damage in adult rats. Life Sci 160:12–17
Jackman MR, MacLean PS, Bessesen DH (2010) Energy expenditure in obesity-prone and obesity-resistant rats before and after the introduction of a high-fat diet. Am J Physiol Regul Integr Comp Physiol 299:1097–1105
Higa TS, Spinola AV, Fonseca-Alaniz MH, Anna Evangelista FS (2014) Comparison between cafeteria and high-fat diets in the induction of metabolic dysfunction in mice. Int J Physiol Pathophysiol Pharmacol 6:47–54
Zhu K, Hunter M, James A, Lim EM, Cooke BR, Walsh JP (2016) Discordance between fat mass index and body mass index is associated with reduced bone mineral density in women but not in men: the Busselton Healthy Ageing Study. Osteoporos Int 28:259–268
Rutkowski JM, Stern JH, Scherer PE (2015) The cell biology of fat expansion. J Cell Biol 208:501–512
Cao JJ, Gregoire BR, Gao H (2009) High-fat diet decreases cancellous bone mass but has no effect on cortical bone mass in the tibia in mice. Bone 44:1097–1104
Malvi P, Piprode V, Chaube B, Pote ST, Mittal M, Chattopadhyay N, Wani MR, Bhat MK (2014) High fat diet promotes achievement of peak bone mass in young rats. Biochem Biophys Res Commun 455:133–138
Acknowledgements
The authors gratefully acknowledge financial support from the São Paulo Research Foundation (reference 2014/10733-8) and a scholarship from the Coordination for the Improvement of Higher Education Personnel.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Yamanaka, J.S., Yanagihara, G.R., Carlos, B.L. et al. A high-fat diet can affect bone healing in growing rats. J Bone Miner Metab 36, 255–263 (2018). https://doi.org/10.1007/s00774-017-0837-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-017-0837-4