Abstract
Purpose
Studies in humans suggest that consumption of low-carbohydrate, high-fat diets (LC–HF) could be detrimental for growth and bone health. In young male rats, LC–HF diets negatively affect bone health by impairing the growth hormone/insulin-like growth factor axis (GH/IGF axis), while the effects in female rats remain unknown. Therefore, we investigated whether sex-specific effects of LC–HF diets on bone health exist.
Methods
Twelve-week-old male and female Wistar rats were isoenergetically pair-fed either a control diet (CD), “Atkins-style” protein-matched diet (LC–HF-1), or ketogenic low-protein diet (LC–HF-2) for 4 weeks. In females, microcomputed tomography and histomorphometry analyses were performed on the distal femur. Sex hormones were analysed with liquid chromatography–tandem mass spectrometry, and endocrine parameters including GH and IGF-I were measured by immunoassay.
Results
Trabecular bone volume, serum IGF-I and the bone formation marker P1NP were lower in male rats fed both LC–HF diets versus CD. LC–HF diets did not impair bone health in female rats, with no change in trabecular or cortical bone volume nor in serum markers of bone turnover between CD versus both LC–HF diet groups. Pituitary GH secretion was lower in female rats fed LC–HF diet, with no difference in circulating IGF-I. Circulating sex hormone concentrations remained unchanged in male and female rats fed LC–HF diets.
Conclusion
A 4-week consumption of LC–HF diets has sex-specific effects on bone health—with no effects in adult female rats yet negative effects in adult male rats. This response seems to be driven by a sex-specific effect of LC–HF diets on the GH/IGF system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low-carbohydrate, high-fat (LC–HF) diets are commonly promoted for weight loss around the globe, particularly “Atkins-style” diets which encourage the consumption of fat and protein and limit carbohydrate intake [1]. Further restriction of protein content of LC–HF diets results in a ketogenic diet, which is an established treatment option for epilepsy in children [2]. Despite the established weight loss effect of moderate “Atkins-style” LC–HF diets in several human and animal studies [3–5], we and others have previously reported a number of unfavourable metabolic effects including an increase in adiposity and glucose intolerance [6–8]. In male rats, we have shown that the consumption of LC–HF diets—independent of caloric intake—leads to significantly higher visceral and subcutaneous fat mass together with higher serum leptin concentrations and a concurrent loss of lean body mass [9, 10]. While this unfavourable effect of LC–HF diets has been very consistent in male rats, little is known about the effects of LC–HF diets on body composition in female rats and whether or not hormonal circuits are modified by these changes.
With respect to bone health, lifestyle factors such as nutrition and physical activity together with genetic predisposition act synergistically with hormones to regulate bone homoeostasis. Dietary micronutrients, in particular calcium, phosphate and magnesium, are established to play a key role in the regulation of bone homoeostasis [11]. In contrast, the role of macronutrients in bone health and especially how diets with an altered relative abundance of fat, protein and carbohydrates affect bone metabolism are not yet fully understood. Adding to the complexity of the matter, it is not well understood whether the skeletal system of males and females reacts similar to diets with an altered relative abundance of macronutrients. Despite studies in humans which have shown the negative effects of LC–HF diets on growth and bone health, ketogenic LC–HF diets are commonly used as a therapeutic approach for children suffering from epilepsy [12, 13]. Given the wide use of well-defined, ketogenic LC–HF diets as a treatment option for epilepsy in children and the frequent consumption of less strictly controlled LC–HF diets for weight loss purposes in the general population, an enhanced understanding on the potential side effects of LC–HF diets as well as additional insights into potential sex-specific effects of LC–HF diets is required.
Hormonal circuits particularly oestrogen and androgen involving pathways are fundamental to the aetiology of osteoporosis in both women and men with sex-specific differences in the susceptibility and response to osteoporotic fractures [14, 15]. Parallel to the actions of sex hormones in the regulation of bone, growth hormone (GH) and insulin-like growth factor-I (IGF-I) are essential for bone growth and skeletal microarchitecture maintenance [16]. We have previously reported that young male rats fed an “Atkins-style” LC–HF diet which is matched in its protein content to the control diet (CD) or a ketogenic LC–HF diet have impaired skeletal growth and less BMD due to lower bone formation compared to CD [17]. These skeletal changes in young male rats fed the protein-matched LC–HF and ketogenic LC–HF diets were consistent with lower circulating GH and IGF-I concentrations. Furthermore, male rats fed with the LC–HF diets displayed GH resistance due to a reduced hepatic expression of GH receptors (GHR) compared to CD [9, 17].
Most studies using diets with altered macronutrient composition in rodents have investigated the metabolic and endocrine consequences of LC–HF diets only in male or in female rodents, but not in both sexes. However, there is a clear rationale to speculate that the effects of diets with altered macronutrient composition are also modulated through the “endocrine environment” of the subject consuming the diet, that is, diets may have different effects in male versus female subjects.
Considering the importance of sex hormones on metabolism and the skeletal system and in light of our previous findings in young male rats, this study investigated in adult male and female rats whether the LC–HF diets used in previous studies would have sex-specific effects on body composition, bone homoeostasis, sex hormones and the GH/IGF system.
Methods
Animal husbandry
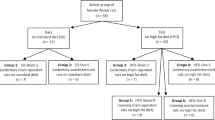
Ten-week-old female Wistar Han rats (n = 10–12/diet group; Charles River, Sulzfeld, Germany) were housed in individual cages in a controlled environment (21.3 ± 0.6 °C, humidity 60 ± 15 %) and maintained on a 12-h light–dark cycle. All animals received ad libitum access to water and a standard laboratory rodent diet for 2 weeks to ensure acclimatisation. Body weight and 24-h food intake were measured daily (Sartorius Competence CP2201, Goettingen, Germany) before the onset of the dark period. Following acclimatisation, rats were divided into diet groups and were matched for body weight. Rats were then pair-fed daily with LC–HF diets (detailed below) on an isoenergetic basis to the CD for 4 weeks, as previously described [6] and culled at 16 weeks of age. For the pair-feeding procedure, daily (ad libitum) food intake of the CD group was measured, which allowed subsequent calculation of daily energy intake (metabolisable energy, ME) and allocation of the individual amounts of the respective LC–HF diets to the two other diet groups. This procedure ensured that all effects observed are solely due to differences in macronutrient composition and not to differences in energy intake. A second cohort of 10-week-old male Wistar Han rats (n = 5–8/diet group, Charles River, Sulzfeld, Germany) was kept under the same conditions to investigate the effects of the same LC–HF diets on sex hormones and BMD using identical methods also in age-matched male rats. Experiments with male and female rats were not conducted in parallel to avoid a potential interference of mating behaviour. All procedures were in accordance with the European animal welfare act and have been approved by the Upper Bavarian Government’s ethical committee for animal experiments.
Diet composition
All diets were custom-made and purchased from Provimi Kliba Nafag (Kaiseraugst, Switzerland). The detailed composition of each diet is provided in Table 1. The macronutrient composition of each diet was independently controlled after production by Weende analysis (AGROLAB group/LUFA ITL, Kiel, Germany). As shown previously [6], the LC–HF-1 is an example of a very-low-carbohydrate, protein-matched, high-fat diet, whereas LC–HF-2 represents a truly ketogenic diet. Diets were semi-purified, and only a single, identical source was used for each macronutrient (protein source: sodium casein; fat source: beef tallow; carbohydrate source: starch). The LC–HF diets contained a minimum amount of carbohydrates (~2 % of ME content), which is necessary to deliver minerals and vitamins. The CD corresponds to the standard rodent diet used by the American Institute of Nutrition (AIN-93G diet). The amounts of micronutrients (minerals and vitamins) added to the diets were based on recommendations from the AIN-93G reference diet and have been adapted to the respective ME content of each diet. Importantly, the calcium and phosphorous contents of the diets were adjusted to the energetic density of the respective diet, ensuring that all rats consume equal amounts of calcium and phosphorous also in a pair-feeding setting [18].
Tissue collection
Female rats were subcutaneously injected with the fluorescent compound calcein (20 mg/kg; Sigma-Aldrich, St Louis, MO, USA) 3 and 10 days prior to tissue collection to enable subsequent calculation of mineral apposition rate (MAR) as previously described [19]. After 4 weeks on the respective diets, rats were given access to food for 1 h after lights out and then fasted for 6 h (to standardise gastrointestinal filling) before decapitation under isoflurane anaesthesia. Body length (nose to rump) was measured before decapitation while anesthetised rats were in ventral position. Trunk blood was collected, centrifuged and stored at −80 °C until analysis. The brown, gonadal, inguinal, retroperitoneal fat pads (only one side of each fat pad) and the liver were excised, carefully freed from adherent tissues and weighed (Scaltec Instruments, Goettingen, Germany). Following decapitation and bleeding, all organs and the skin/fur were excised from the rats. The remaining carcass was then weighed to measure lean body mass. Femurs were excised, and a calliper was used to determine femoral length. The femurs were then fixed overnight in 4 % paraformaldehyde (PFA) at 4 °C and then stored in 70 % ethanol at 4 °C before undergoing processing.
Microcomputed tomography
Microcomputed tomography (micro-CT) was carried out at an isometric resolution of 11 µm (µCT 20, Scanco, Brüttisellen, Switzerland), and analyses were performed using ImageJ [20] with the BoneJ plugin, as previously described [21]. Following fixation, the femur was packed into an enclosed rigid plastic tube filled with 70 % ethanol and scanned. The following parameters were generated for cortical bone analyses: cortical thickness and cortical bone volume. The trabecular bone parameters generated were: trabecular bone volume (BV/TV), trabecular thickness, trabecular bone connective density, trabecular separation, degree of anisotropy and structure model index (determines the plate- or rod-like geometry of trabecular structures with 0 = plates and 3 = rods). For this analysis, we excluded scans that had too much motion which distorted the image. As such, there were 5–10 female femurs and 4–5 male femurs per diet group which were included in the final analysis.
Bone histomorphometry
The right femur was bisected transversely at the midpoint of the shaft and processed as previously described [22]. Samples were embedded in resin made up of methyl methacrylate (Sigma-Aldrich, Munich, Germany) and dibutyl phthalate (Sigma-Aldrich, Munich, Germany), and 5-µm sagittal sections were analysed as previously described [22]. Endocortical MAR was estimated from calcein-labelled images [22] (obtained with a Leica DMI 600B microscope, Leica, Wetzlar, Germany) using ImageJ analysis software.
Serum analysis
All blood samples were handled, processed and stored as previously described [23]. Circulating serum concentrations of total procollagen type 1 N-terminal propeptide (P1NP), C-terminal telopeptide type I (CTX-I, Ratlaps), IGF-I (all assays from IDS, Boldon, UK), leptin (Mediagnost, Reutlingen, Germany) and estradiol (Calbiotech, Spring Valley, CA, USA) were measured in duplicate by commercially available manual immunoassays following the manufactures instructions. Serum albumin, aspartate aminotransaminase (ASAT), alanine aminotransaminase (ALAT), cholesterol (total and HDL cholesterol) and triglycerides were measured by an automated system (Cobas 8000, C702, Roche diagnostics, Mannheim, Germany). Fasting blood glucose was measured using the semi-automated glucose oxidase method (EcoSolo; Care Diagnostica, Voerde, Germany).
Liquid chromatography–tandem mass spectrometry analysis of sex hormones
Corticosterone, 11deoxycorticosterone (DOC), androstenedione, testosterone, 17OH-progesterone (17OHP) and progesterone were measured by isotopic dilution (IS) liquid chromatography–tandem mass spectrometry (LC–MS/MS) as described previously [24] using an API4000 QTrap mass spectrometer (AB-Sciex, Toronto, Canada). Intra-assay coefficient of variation (CV) ranged between 1.0 and 11.9 % for all analytes. The functional sensitivity was 0.62 ng/ml for corticosterone, 0.078 ng/ml for androstenedione and testosterone, 0.313 ng/ml for DOC and 17OHP and 0.391 ng/ml for progesterone.
Analysis of GH secretion in female rats
After 3 weeks on the respective diets, multiple blood samplings (10 samples during 5 h, sampling every 30 min) from the tail vein were collected via bleeding to analyse GH secretion profiles in a subset of six female rats from each group as described previously [9]. Circulating GH concentrations were measured by immunoassay (Merck Millipore, Billerica, MA, USA).
Statistical analysis
Statistical analyses were performed using GraphPad Prism (GraphPad Prism®, version 5, La Jolla, CA, USA). For the statistical comparison of data between the three dietary groups (e.g. plasma hormone concentrations, bone parameters etc.), nonparametric Kruskal–Wallis with subsequent Dunn’s post hoc tests for multiple comparisons was performed. Final body weights after 28 days of feeding were also compared using the Kruskal–Wallis test (with Dunn’s post hoc tests). GH secretion was analysed by the rank plot method as previously described [9, 25]. The data are shown as mean ± standard error of the mean (SEM), and significance was considered at p < 0.05.
Results
Effects of LC–HF diets on body weight and longitudinal growth in female rats
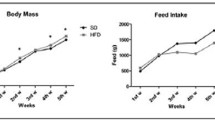
After an initial decline in body weight following the commencement of diets, all female and male rats continuously gained weight throughout the experimental period (Fig. 1a). In female rats, isoenergetic consumption of either LC–HF diet led to a lower final body weight after the 4-week feeding period; however, this difference only reached statistical significance (p < 0.05) when comparing CD versus LC–HF-2 (CD: 213.6 ± 2.0 g; LC–HF-1: 209.7 ± 2.7 g; LC–HF-2: 203.6 ± 2.7 g). Consistent with our previous studies, male rats fed the protein-matched LC–HF-1 or the ketogenic LC–HF-2 diet weighed significantly less compared to CD (p < 0.0001). Lean body mass (carcass weight) was also lower in female rats fed the protein-matched LC–HF-1 and the ketogenic LC–HF-2 diets, reaching statistical significance when comparing CD with LC–HF-2 (p < 0.05; Fig. 1b). No significant differences between groups were detected in nose-to-rump length (CD: 18.56 ± 0.37 cm; LC–HF-1: 17.76 ± 0.16 cm; LC–HF-2: 17.90 ± 0.16 cm, p = 0.09) or femur length (CD: 30 ± 0.18 mm; LC–HF-1: 29.92 ± 0.15 mm; LC–HF-2: 30.00 ± 0.17 mm, p = 0.57).
Body weight and lean body mass in adult rats fed CD, LC–HF-1 and LC–HF-2 for 4 weeks. a Cumulative body weight gain in male (dashed) and female (solid) rats fed with the protein-matched LC–HF-1 (green lines) and the ketogenic LC–HF-2 (red lines) diets (CD: control diet, black lines). b Lean body mass in female rats fed the protein-matched and ketogenic diets. Data represent means ± SEMs, n = 8–12 per group. Means not sharing a common letter differ significantly, p < 0.05. CD control diet, LC–HF-1 protein-matched low-carbohydrate, high-fat diet, LC–HF-2 ketogenic low-carbohydrate, high-fat diet
Increased visceral adiposity in LC–HF diet-fed female rats
Brown adipose tissue (both absolute and normalised to body weight) was not significantly different between the groups. In comparison to CD, female rats fed the ketogenic LC–HF-2 had greater liver weight when normalised to body weight (Table 2). Subcutaneous adiposity was not significantly changed in female rats in both protein-matched and ketogenic LC–HF diet groups, as there were no significant differences between CD and LC–HF-1 and LC–HF-2 in the absolute inguinal adipose tissue weight; however, this was attenuated when normalised to body weight in both LC–HF-1 and LC–HF-2 diet groups (Table 2). In contrast, central adiposity (gonadal and retroperitoneal depots) was significantly greater in female rats fed the protein-matched and the ketogenic LC–HF diets compared to CD (Table 2).
Lower trabecular bone volume and P1NP in adult male rats on the LC–HF diets
In adult 16-week-old male rats, micro-CT revealed lower trabecular bone volume (BV/TV) in the protein-matched and ketogenic LC–HF diet groups when compared to CD reaching statistical significance with the ketogenic LC–HF-2 diet (Fig. 2a), with representative reconstructions illustrating the trabecular effects of LC–HF diet consumption (Fig. 2b). No significant effects were found in the cortical compartment of the femur in adult male rats (cortical thickness: CD: 0.74 ± 0.02 mm, LC–HF-1: 0.74 ± 0.01 mm, LC–HF-2: 0.75 ± 0.03 mm). Similarly, cortical bone volume was not affected by the diets in adult male rats (CD: 12.1 ± 0.18 mm3, LC–HF-1: 11.7 ± 0.28 mm3, LC–HF-2: 11.8 ± 0.31 mm3). Consistent with lower trabecular bone volume in adult male rats, the bone formation marker P1NP was also lower in male rats fed LC–HF diets (CD: 3.6 ± 0.4 ng/ml, LC–HF-1: 2.4 ± 0.5 ng/ml, LC–HF-2: 2.1 ± 0.1 ng/ml; CD vs. LC–HF-1 and LC–HF-2: p < 0.05).
Trabecular bone volume in male rats fed CD, LC–HF-1 and LC–HF-2 for 4 weeks. a Trabecular bone volume. b Representative micro-CT images of femoral trabecular bone volume in male rats fed CD, LC–HF-1 and LC–HF-2 for 4 weeks. Data represent means ± SEMs, n = 4–5 per group. Means not sharing a common letter differ significantly, p < 0.05. CD control diet, LC–HF-1 protein-matched low-carbohydrate, high-fat diet, LC–HF-2 ketogenic low-carbohydrate, high-fat diet
No femoral trabecular bone loss in female rats fed LC–HF diets
In females, BV/TV was significantly greater in ketogenic LC–HF-2-fed female rats compared to CD (Fig. 3a), but unchanged in rats fed the protein-matched LC–HF-1. This was consistent with thicker trabeculae in LC–HF-2 rats compared to CD, with no difference in LC–HF-1 rats (Fig. 3b). There were no significant differences in trabecular separation (Fig. 3c) or trabecular connectivity (Fig. 3d) between the diet groups. Consistent with thicker trabeculae, the structure model index of LC–HF-2 rats suggests that there were more “plate-like” trabeculae compared to CD (Fig. 3e), indicating better bone quality. The degree of anisotropy was similar between all female rats (Fig. 3f). Representative 3D reconstructions of the bone scans demonstrate that there were no differences between the groups (Fig. 3g).
Trabecular bone parameters in female rats fed LC–HF diets CD, LC–HF-1 and LC–HF-2 for 4 weeks. a Trabecular bone volume, b trabecular thickness, c trabecular separation, d trabecular connectivity, e structure model index and f degree of anisotropy in female rats. g Representative reconstructed images of femoral trabecular bone. Data represent means ± SEMs, n = 5–10 per group. Means not sharing a common letter differ significantly, p < 0.05. CD control diet, LC–HF-1 protein-matched low-carbohydrate, high-fat diet, LC–HF-2 ketogenic low-carbohydrate, high-fat diet
LC–HF diets do not affect cortical bone in female rats
In females, there were no significant differences between groups in cortical bone volume (Fig. 4a) or cortical thickness (Fig. 4b). Representative 3D images clearly show that female rats in protein-matched and ketogenic LC–HF diets groups have similar cortical bone outcomes to CD (Fig. 4c). To ensure that these were not only static observations, we also investigated bone formation. Endocortical MAR was not different between groups (Fig. 4d), illustrated by the similar distance between the two calcein bands across all groups (Fig. 4e).
Cortical bone parameters in female rats fed LC–HF diets CD, LC–HF-1 and LC–HF-2 for 4 weeks. a Cortical bone volume, b cortical thickness and c Representative reconstructed images of femoral cortical bone, d endocortical mineral apposition rate in female rats, e fluorescence micrographs showing similar distance between the calcein bands on CD, LC–HF–1 and LC–HF–2 fed female rats. Data represent means ± SEMs, n = 5–10 per group. Means not sharing a common letter differ significantly, p < 0.05. CD control diet, LC–HF-1 protein-matched low-carbohydrate, high-fat diet, LC–HF-2 ketogenic low-carbohydrate, high-fat diet, micro-CT, microcomputed tomography
Serum clinical chemistry, leptin and biomarkers of bone formation and resorption in female rats
Serum albumin was not changed by protein-matched or ketogenic LC–HF diets (Table 3). ALAT was significantly greater in LC–HF-2, with no differences in LC–HF-1-fed rats compared to CD (Table 3). There were no significant group differences in serum ASAT, total cholesterol and triglycerides. However, HDL cholesterol concentrations were significantly higher in female rats fed LC–HF-2 compared to CD (Table 3). Fasting glucose measured at cull was significantly lower in both LC–HF-1- and LC–HF-2-fed female rats compared to CD (Table 3). Serum leptin was higher in rats fed with protein-matched or ketogenic LC–HF diets compared to CD, but this difference failed to reach statistical significance. Furthermore, protein-matched or ketogenic LC–HF diets did not significantly affect the serum biomarkers of bone formation—P1NP—or bone resorption, CTX-I (Table 3).
Effects of LC–HF diets on steroid hormone concentrations in male and female rats
As expected, the absolute concentrations of the sex hormone testosterone differed by an order of magnitude between male and female rats. However, within each sex, protein-matched and ketogenic LC–HF diets did not significantly affect the concentrations of serum estradiol, testosterone, androstenedione and progesterone (Table 4). Concentrations of 17OH-progesterone were not statistically evaluated because most samples displayed concentrations which were below the detection limit of the method. Of note, testosterone trended to be higher (not significant) and the adrenal hormones corticosterone and 11deoxycorticosterone were significantly lower in male rats fed with LC–HF-1 and LC–HF-2 compared to CD. This difference was not observed in female rats fed with the LC–HF diets (Table 4).
Effects on the GH/IGF system in male and female rats
As expected and observed multiple times in our previous studies [9, 10, 17], the adult male rats from the current investigation also displayed significantly lower serum IGF-I concentrations when fed with either of the LC–HF diets (CD: 1169 ± 45 ng/ml, LC–HF-1: 936 ± 40 ng/ml, LC–HF-2: 873 ± 48 ng/ml; CD vs. LC–HF-1 p < 0.05, CD vs. LC–HF-2: p < 0.01). In contrast, both LC–HF-1 and LC–HF-2 did not significantly affect circulating IGF-I concentrations in serum of female rats (Table 3). In order to gain a comprehensive understanding of GH secretion in female rats, we compared the three diet groups by ranking the GH concentrations of each group by magnitude (Fig. 5). This rank plot analysis demonstrates the nadir-to-peak distribution of GH values. Female rats fed either LC–HF-1 or LC–HF-2 displayed significantly lower GH secretion compared to CD (p < 0.01; Fig. 5).
Rank plots of growth hormone concentrations in female rats fed CD, LC–HF-1 and LC–HF-2 for 4 weeks. Data points are derived from GH measurements from multiple blood samplings (10 samples during 5 h, sampling every 30 min) in six female rats from each dietary group. GH growth hormone, CD control diet, LC–HF-1 protein-matched low-carbohydrate, high-fat diet, LC–HF-2 ketogenic low-carbohydrate, high-fat diet
Discussion
In this study, we demonstrate for the first time that the isoenergetic consumption of LC–HF diets exerts sex-specific effects on bone health in rats. The current investigation extended our previous finding, that is, LC–HF diets induce lower trabecular BV/TV and lower serum concentrations of the bone formation marker P1NP in young male rats [17], now also in adult male Wistar rats fed with LC–HF diets. In sharp contrast, both LC–HF diets did not impair bone health in female rats, as there were no effects of diet on trabecular or cortical bone compartments. Furthermore, there were no changes in cortical bone formation as assessed by histomorphometry. Also unchanged serum markers of bone turnover (P1NP and CTX-I) in female rats fed with LC–HF diets compared to CD are in the same direction, that is, no negative effects on bone health during a 4-week feeding period. Interestingly, this study shows that LC–HF diets impair pituitary GH secretion similarly in adult male [9] and adult female rats. In contrast to male rats, there were no significant effects of both LC–HF diets on circulating IGF-I concentrations in female rats.
Accumulating evidence shows that males and females can differ in their response to medical treatments [26–28]. Tailoring certain medical interventions to the sex of the patient could improve both safety and efficacy. While it is not surprising that the physiological differences in sex hormones can affect many aspects of growth, metabolism and bone health [26, 29], the vast number of animal studies focus on either male or female animals without directly examining both sexes in a single study. Investigations that study only a single sex cannot determine whether an observed phenotype or mechanism is equally true for both males and females. In this context, a recent study has clearly shown that mice display a sexually dimorphic response to a high-fat diet with respect to hypothalamic inflammation and several metabolic parameters such as glucose tolerance [30]. Owing to the important sex-specific differences, authors have highlighted the need to correct for the sex bias in both human and animal research, and journal editors and reviewers should state the sex of subjects used already in the title of publications [31, 32]. With respect to LC–HF diets and their effects on body weight gain, body composition, growth and bone metabolism, our current study together with our previous findings now closes this specific gap and shows data for male and female rats. The knowledge that LC–HF diets can have sex-specific effects may also help to better interpret conflicting data from the literature.
In the present study, female rats fed LC–HF diets do not appear to have any defects in growth as there were no differences in nose-to-rump length or femur length compared to CD. In contrast, we have previously demonstrated that young male rats fed LC–HF diets displayed stunted growth demonstrated by decreased nose-to-rump length, femur length and lean body mass when compared to CD [17]. Growth retardation and lower BMD have been described to be major drawbacks of treatment with ketogenic LC–HF diets in children suffering from intractable epilepsy [12, 13, 33–35]. In these studies, the authors did not specifically compare the effects of ketogenic diets on bone between boys and girls which might also be of less importance due to similar hormonal conditions in pre-pubertal children. In adults, very little information on the effects of ketogenic diets on growth and bone health is available. Ketogenic diets are the only known treatment option for glucose transporter 1 deficiency syndrome (GLUT-1 DS) which also requires treatment in adults [36]. In adult patients with GLUT-1 DS, a recent case series has shown that treatment with ketogenic diets has no adverse effects on bone health—interestingly all three subjects studied were women [37]. Given the obvious sex-specific differences in circulating sex hormones between males and females, we hypothesised a strong effect of LC–HF diets on circulating sex hormone concentrations. To our surprise, LC–MS/MS analysis did not reveal significant differences in sex hormone concentrations between the diet groups for either sex.
In our previous studies in male rats [9, 17], both LC–HF diets impaired the GH/IGF system. Secretion of GH per se is known to be sex-specific, with male rats showing a higher pulsatility of GH, while female rats have less GH peaks, but display a higher basal secretion [38, 39]. IGF-I concentrations were significantly lower in male rats fed with both LC–HF diets—confirming our previous findings [9, 17]. In contrast to males, serum IGF-I was not different in female rats on either of the LC–HF diets. However, pituitary GH secretion was lower in female rats in both LC–HF diet groups, as shown by the rank plot method from serial blood samplings. These findings indicate that the effects of LC–HF diets on circulating GH levels are similar in male and female rats; however, the effects on circulating IGF-I are sex-specific. We therefore hypothesise that unchanged IGF-I in female rats could be a result of less peripheral GH resistance (that is, lower hepatic GHR expression) which has been previously demonstrated in male rats fed with the LC–HF diets [9]. Our data suggest that the unchanged IGF-I concentrations in female rats fed with LC–HF diets may, in part, explain the absence of a LC–HF diet-induced impairment in bone health.
The current study aimed to investigate how a short-term, 4-week feeding intervention with LC–HF diets affects bone health in adult rats. While young [17] and adult male rats showed marked effects of LC–HF diet consumption after 4-weeks, this was not observed in female rats. However, the current study cannot exclude the possibility that a longer feeding period or feeding the LC–HF diets to younger female rats would have led to similar findings in female rats compared to male rats, that is, the detrimental effects on bone health. The amount of micronutrients, including phosphorous, calcium, vitamin D and vitamin K, were matched to the energetic density of each diet, so that pair-fed animals consumed identical quantities of micronutrients as well as calories. We did not investigate intestinal calcium uptake and serum parathyroid hormone (PTH) in female rats fed with the different diets. Therefore, it remains speculative whether calcium and phosphorus uptake with LC–HF diets is also regulated in a sex-specific manner and whether in relation to lean body mass, the higher uptake of calcium and phosphorus in rats fed with LC–HF diets could have had a protective or compensatory effect on bone measures in females. In male rats, a 4-week isoenergetic consumption of mineral-balanced LC–HF diets—which also resulted in a lower lean body mass—did not significantly affect circulating serum concentrations of calcium, phosphorous and PTH despite markedly reduced bone mineral density [17, 18]. Therefore, it may be assumed that at least the ‘higher calcium and phosphorous intake relative to lean body mass’ did not significantly affect bone measures in females. In addition to vitamin D, vitamin K—especially vitamin K2, has been demonstrated to play an important role in bone formation [40]. It has been shown that vitamin K2 affects osteoblastic cells through a protein kinase A-dependent mechanism [41]. All diets in the current study were formulated as non-natural, semi-purified diets, and vitamins were added via a defined synthetic vitamin pre-mix. In this pre-mix, vitamin K1 and vitamin K2 were not present at all, and all of the vitamin K was derived from vitamin K3 only. Therefore, vitamin K2 intake can be excluded as an influencing variable in this study.
While the effects of LC–HF diets on bone are sex-specific, other undesirable side effects of LC–HF diets, especially increased adiposity, were also detected in female rats. Female rats fed LC–HF diets showed lower body weight after the 4-week feeding period; however, this was not to the same magnitude as in male rats fed LC–HF diets. Studies in humans have shown a clear association between BMD and weight, with a simultaneous loss in body weight and decrease in BMD [15]—consistent with our previous findings in male rats [17]. Bone is a load-bearing tissue, and mechanical forces such as body weight affect the development and maintenance of its structure [42]. Given that female rats fed LC–HF diets had a less pronounced loss of body weight compared to male rats in our previous studies [6], female rats may have had greater physical forces exerted upon the skeleton compared to male rats, relative to control. Thus, body mass may have potentially impacted the bone outcomes in this study, with osteocytes responding to greater physical forces [42]. Further studies involving mechanical hindlimb unloading in rats are required to investigate the role of osteocytes in response to LC–HF diets.
In conclusion, these data demonstrate sex-specific effects of LC–HF diets on bone health. In female rats fed LC–HF diets, body weight gain was affected to a lesser degree when compared to males. In contrast to male rats, there were no detrimental effects of the protein-matched or the ketogenic LC–HF diets on bone health, as measured by serum markers of bone turnover, bone cellular activity and cortical and trabecular bone compartments in female rats. While the effects of LC–HF diets on bone are sex-specific, other undesirable side effects of LC–HF diets, especially increased adiposity and loss of lean body mass, were also detected in female rats. Importantly, the effects of LC–HF diets on fat accumulation in females were independent of energy intake due to the pair-feeding procedure. This study is a clear example of sex-specific effects of LC–HF diets and highlights the need for sex-specific investigations of pathologies related to diet, bone and metabolic abnormalities.
References
Sharma S, Jain P (2014) The modified Atkins diet in refractory epilepsy. Epilepsy Res Treat 2014:404202. doi:10.1155/2014/404202
Mackay MT, Bicknell-Royle J, Nation J, Humphrey M, Harvey AS (2005) The ketogenic diet in refractory childhood epilepsy. J Paediatr Child Health 41(7):353–357. doi:10.1111/j.1440-1754.2005.00630.x
Atallah R, Filion KB, Wakil SM, Genest J, Joseph L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ (2014) Long-term effects of 4 popular diets on weight loss and cardiovascular risk factors: a systematic review of randomized controlled trials. Circ Cardiovasc Qual Outcomes 7(6):815–827. doi:10.1161/CIRCOUTCOMES.113.000723
Bielohuby M, Sisley S, Sandoval D, Herbach N, Zengin A, Fischereder M, Menhofer D, Stoehr BJ, Stemmer K, Wanke R, Tschop MH, Seeley RJ, Bidlingmaier M (2013) Impaired glucose tolerance in rats fed low-carbohydrate, high-fat diets. Am J Physiol Endocrinol Metab 305(9):E1059–E1070. doi:10.1152/ajpendo.00208.2013
Pissios P, Hong S, Kennedy AR, Prasad D, Liu FF, Maratos-Flier E (2013) Methionine and choline regulate the metabolic phenotype of a ketogenic diet. Mol Metab 2(3):306–313. doi:10.1016/j.molmet.2013.07.003
Bielohuby M, Menhofer D, Kirchner H, Stoehr BJ, Muller TD, Stock P, Hempel M, Stemmer K, Pfluger PT, Kienzle E, Christ B, Tschop MH, Bidlingmaier M (2011) Induction of ketosis in rats fed low-carbohydrate, high-fat diets depends on the relative abundance of dietary fat and protein. Am J Physiol Endocrinol Metab 300(1):E65–E76. doi:10.1152/ajpendo.00478.2010
Ellenbroek JH, van Dijck L, Tons HA, Rabelink TJ, Carlotti F, Ballieux BE, de Koning EJ (2014) Long-term ketogenic diet causes glucose intolerance and reduced beta- and alpha-cell mass but no weight loss in mice. Am J Physiol Endocrinol Metab 306(5):E552–E558. doi:10.1152/ajpendo.00453.2013
Jornayvaz FR, Jurczak MJ, Lee H-Y, Birkenfeld AL, Frederick DW, Zhang D, Zhang X-M, Samuel VT, Shulman GI (2010) A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. Am J Physiol Endocrinol Metab 299(5):E808–E815. doi:10.1152/ajpendo.00361.2010
Bielohuby M, Sawitzky M, Stoehr BJ, Stock P, Menhofer D, Ebensing S, Bjerre M, Frystyk J, Binder G, Strasburger C, Wu Z, Christ B, Hoeflich A, Bidlingmaier M (2011) Lack of dietary carbohydrates induces hepatic growth hormone (GH) resistance in rats. Endocrinology 152(5):1948–1960. doi:10.1210/en.2010-1423
Caton SJ, Bielohuby M, Bai Y, Spangler LJ, Burget L, Pfluger P, Reinel C, Czisch M, Reincke M, Obici S, Kienzle E, Tschop MH, Bidlingmaier M (2012) Low-carbohydrate high-fat diets in combination with daily exercise in rats: effects on body weight regulation, body composition and exercise capacity. Physiol Behav 106(2):185–192. doi:10.1016/j.physbeh.2012.02.003
Aaseth J, Boivin G, Andersen O (2012) Osteoporosis and trace elements—an overview. J Trace Elem Med Biol 26(2–3):149–152. doi:10.1016/j.jtemb.2012.03.017
Peterson SJ, Tangney CC, Pimentel-Zablah EM, Hjelmgren B, Booth G, Berry-Kravis E (2005) Changes in growth and seizure reduction in children on the ketogenic diet as a treatment for intractable epilepsy. J Am Diet Assoc 105(5):718–725. doi:10.1016/j.jada.2005.02.009
Williams S, Basualdo-Hammond C, Curtis R, Schuller R (2002) Growth retardation in children with epilepsy on the ketogenic diet: a retrospective chart review. J Am Diet Assoc 102(3):405–407
Blouin K, Despres JP, Couillard C, Tremblay A, Prud’homme D, Bouchard C, Tchernof A (2005) Contribution of age and declining androgen levels to features of the metabolic syndrome in men. Metab Clin Exp 54(8):1034–1040. doi:10.1016/j.metabol.2005.03.006
Nguyen TV, Sambrook PN, Eisman JA (1998) Bone loss, physical activity, and weight change in elderly women: the Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res 13(9):1458–1467. doi:10.1359/jbmr.1998.13.9.1458
Giustina A, Mazziotti G, Canalis E (2008) Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev 29(5):535–559. doi:10.1210/er.2007-0036
Bielohuby M, Matsuura M, Herbach N, Kienzle E, Slawik M, Hoeflich A, Bidlingmaier M (2010) Short-term exposure to low-carbohydrate, high-fat diets induces low bone mineral density and reduces bone formation in rats. J Bone Miner Res 25(2):275–284. doi:10.1359/jbmr.090813
Frommelt L, Bielohuby M, Stoehr BJ, Menhofer D, Bidlingmaier M, Kienzle E (2014) Effects of low-carbohydrate, high-fat diets on apparent digestibility of minerals and trace elements in rats. Nutrition 30(7–8):869–875. doi:10.1016/j.nut.2013.11.017
Suzuki HK, Mathews A (1966) Two-color fluorescent labeling of mineralizing tissues with tetracycline and 2,4-bis[N,N′-di-(carbomethyl)aminomethyl] fluorescein. Stain Technol 41(1):57–60
Abramoff M, Magalhaes P, Ram S (2004) Image processing with ImageJ. Biophotonics Int 11:36–42
Doube M, Klosowski MM, Arganda-Carreras I, Cordelieres FP, Dougherty RP, Jackson JS, Schmid B, Hutchinson JR, Shefelbine SJ (2010) BoneJ: free and extensible bone image analysis in ImageJ. Bone 47(6):1076–1079. doi:10.1016/j.bone.2010.08.023
Baldock PA, Sainsbury A, Couzens M, Enriquez RF, Thomas GP, Gardiner EM, Herzog H (2002) Hypothalamic Y2 receptors regulate bone formation. J Clin Investig 109(7):915–921. doi:10.1172/JCI14588
Bielohuby M, Popp S, Bidlingmaier M (2012) A guide for measurement of circulating metabolic hormones in rodents: pitfalls during the pre-analytical phase. Mol Metab 1(1–2):47–60. doi:10.1016/j.molmet.2012.07.004
Fanelli F, Belluomo I, Di Lallo VD, Cuomo G, De Iasio R, Baccini M, Casadio E, Casetta B, Vicennati V, Gambineri A, Grossi G, Pasquali R, Pagotto U (2011) Serum steroid profiling by isotopic dilution-liquid chromatography-mass spectrometry: comparison with current immunoassays and reference intervals in healthy adults. Steroids 76(3):244–253. doi:10.1016/j.steroids.2010.11.005
Xu J, Bekaert AJ, Dupont J, Rouve S, Annesi-Maesano I, De Magalhaes Filho CD, Kappeler L, Holzenberger M (2011) Exploring endocrine GH pattern in mice using rank plot analysis and random blood samples. J Endocrinol 208(2):119–129. doi:10.1677/JOE-10-0317
Arnetz L, Ekberg NR, Alvarsson M (2014) Sex differences in type 2 diabetes: focus on disease course and outcomes. Diabetes Metab Syndr Obes 7:409–420. doi:10.2147/DMSO.S51301
Kautzky-Willer A, Handisurya A (2009) Metabolic diseases and associated complications: sex and gender matter! Eur J Clin Investig 39(8):631–648. doi:10.1111/j.1365-2362.2009.02161.x
Leitner MK, Kautzky-Willer A (2013) Gender-specific differences in age-associated endocrinology. Z Gerontol Geriatr 46(6):505–510. doi:10.1007/s00391-013-0512-x
Rasul S, Ilhan A, Wagner L, Luger A, Kautzky-Willer A (2012) Diabetic polyneuropathy relates to bone metabolism and markers of bone turnover in elderly patients with type 2 diabetes: greater effects in male patients. Gend Med 9(3):187–196. doi:10.1016/j.genm.2012.03.004
Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, Garcia-Caceres C, Navas CR, Gordillo R, Neinast M, Kalainayakan SP, Li DL, Gao Y, Yi CX, Hahner L, Palmer BF, Tschop MH, Clegg DJ (2014) Hypothalamic PGC-1alpha protects against high-fat diet exposure by regulating ERalpha. Cell Rep 9(2):633–645. doi:10.1016/j.celrep.2014.09.025
Kim AM, Tingen CM, Woodruff TK (2010) Sex bias in trials and treatment must end. Nature 465(7299):688–689. doi:10.1038/465688a
Zucker I, Beery AK (2010) Males still dominate animal studies. Nature 465(7299):690. doi:10.1038/465690a
Bergqvist AG, Schall JI, Stallings VA, Zemel BS (2008) Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. Am J Clin Nutr 88(6):1678–1684. doi:10.3945/ajcn.2008.26099
Hahn TJ, Halstead LR, DeVivo DC (1979) Disordered mineral metabolism produced by ketogenic diet therapy. Calcif Tissue Int 28(1):17–22
Kim JT, Kang HC, Song JE, Lee MJ, Lee YJ, Lee EJ, Lee JS, Kim HD (2013) Catch-up growth after long-term implementation and weaning from ketogenic diet in pediatric epileptic patients. Clin Nutr 32(1):98–103. doi:10.1016/j.clnu.2012.05.019
Gras D, Roze E, Caillet S, Meneret A, Doummar D, Billette de Villemeur T, Vidailhet M, Mochel F (2014) GLUT1 deficiency syndrome: an update. Rev Neurol 170(2):91–99. doi:10.1016/j.neurol.2013.09.005
Bertoli S, Trentani C, Ferraris C, De Giorgis V, Veggiotti P, Tagliabue A (2014) Long-term effects of a ketogenic diet on body composition and bone mineralization in GLUT-1 deficiency syndrome: a case series. Nutrition 30(6):726–728. doi:10.1016/j.nut.2014.01.005
Ho KY, Leong DA, Sinha YN, Johnson ML, Evans WS, Thorner MO (1986) Sex-related differences in GH secretion in rat using reverse hemolytic plaque assay. Am J Physiol 250(6 Pt 1):E650–E654
Jansson JO, Eden S, Isaksson O (1985) Sexual dimorphism in the control of growth hormone secretion. Endocr Rev 6(2):128–150. doi:10.1210/edrv-6-2-128
Tabb MM, Sun A, Zhou C, Grun F, Errandi J, Romero K, Pham H, Inoue S, Mallick S, Lin M, Forman BM, Blumberg B (2003) Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J Biol Chem 278(45):43919–43927. doi:10.1074/jbc.M303136200
Ichikawa T, Horie-Inoue K, Ikeda K, Blumberg B, Inoue S (2007) Vitamin K2 induces phosphorylation of protein kinase A and expression of novel target genes in osteoblastic cells. J Mol Endocrinol 39(4):239–247. doi:10.1677/JME-07-0048
Hughes JM, Petit MA (2010) Biological underpinnings of Frost’s mechanostat thresholds: the important role of osteocytes. J Musculoskelet Neuronal Interact 10(2):128–135
Acknowledgments
We thank Sarina Benedix and Amon Horngacher (Medizinische Klinik und Poliklinik IV, Munich, Germany) for excellent technical assistance. A. Z was partly funded by a grant from the DAAD (Deutscher Akademischer Austauschdienst). This study was partly supported by a grant from the Else Kröner-Fresenius-Stiftung (Grant to MaBid, No. 2014_A108). A. Z., M. B. and M. B. designed research; A. Z., B. K., Y. C., M. B., R. S., E. S., N. H., F. F., M. M. and S. M. conducted research; A. Z. and M. B. analysed data; A. Z., M. B. and M. B. wrote the paper; M. B. had primary responsibility for final content. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ayse Zengin and Benedikt Kropp have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zengin, A., Kropp, B., Chevalier, Y. et al. Low-carbohydrate, high-fat diets have sex-specific effects on bone health in rats. Eur J Nutr 55, 2307–2320 (2016). https://doi.org/10.1007/s00394-015-1040-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-1040-9