Abstract
Progressive supranuclear palsy (PSP) is a neurodegenerative disorder characterized by early postural instability and falls, oculomotor dysfunction (vertical supranuclear gaze palsy), parkinsonism with poor response to levodopa, pseudobulbar palsy, and cognitive impairment. This four-repeat tauopathy is morphologically featured by accumulation of tau protein in neurons and glia causing neuronal loss and gliosis in the extrapyramidal system associated with cortical atrophy and white matter lesions. Cognitive impairment being frequent in PSP and more severe than in multiple system atrophy and Parkinson disease, is dominated by executive dysfunction, with milder difficulties in memory, and visuo-spatial and naming dysfunctions. Showing longitudinal decline, it has been related to a variety of pathogenic mechanisms associated with the underlying neurodegenerative process, such as involvement of cholinergic and muscarinergic dysfunctions, and striking tau pathology in frontal and temporal cortical regions associated with reduced synaptic density. Altered striatofrontal, fronto-cerebellar, parahippocampal, and multiple subcortical structures, as well as widespread white matter lesions causing extensive connectivity disruptions in cortico-subcortical and cortico-brainstem connections, support the concept that PSP is a brain network disruption disorder. The pathophysiology and pathogenesis of cognitive impairment in PSP, as in other degenerative movement disorders, are complex and deserve further elucidation as a basis for adequate treatment to improve the quality of life of patients with this fatal disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Progressive supranuclear palsy (PSP), a predominantly sporadic neurodegenerative disorder, is the most common atypical parkinsonian disease with an estimated prevalence of 6.2 to 7.4/ per 100,000 and an annual incidence ranging with increasing age from 1.7 to 14.7/100,000 (Coyle-Gilchrist et al. 2016; Fleury et al. 2018), while it was 0.4 per 100,000 person-years in Olmsted County, MN, USA (Stang et al. 2020). PSP is clinically featured by progressive postural instability and falls, oculomotor dysfunction (supranuclear gaze palsy), parkinsonism with poor response to levodopa, and pseudobulbar palsy and cognitive impairment (CI) with predominant frontal executive dysfunction (Agarwal and Gilbert 2022; Höglinger et al. 2017). This four-repeat tauopathy is morphologically characterized by accumulation of tau protein in neurons and glia, with globose tangles and neuropil threads in cortex and many subcortical regions, associated with neuronal loss and gliosis causing atrophy of cortical and subcortical gray and white matter (Coughlin et al. 2021; Jellinger 2019). PSP shows high clinical and pathological heterogeneity (Campagnolo et al. 2023; Street et al. 2021). The degeneration of specific neuronal pathways is associated with a number of clinical variants that show significant morphological and biochemical differences (Respondek et al. 2017). The new Movement Disorder Society (MDS) PSP criteria classified the core clinical features into four domains: ocular motor dysfunction, postural instability, akinesia, and cognitive dysfunction, thus distinguishing several phenotypes: (1) the most common clinical presentation is Richardson's syndrome (PSP-RS/classic PSP) with early postural instability, falls, vertical gaze palsy, axial rigidity, akinesia, oculomotor dysfunction, cognitive and lingual disorders, and rapid course (Alster et al. 2020a). Other presentations include (2) PSP-parkinsonism (PSP-P), which often mimics Parkinson disease (PD) and accounts for about one-third of autopsy-confirmed cases (Williams and Lees 2009), (3) gait-freezing form (PSP-PGF) with predominant motor blocks, (4) PSP with ocular motor dysfunction (PSP-OM), (5) a frontal behavioral/cognitive subtype (PSP-F) with apathy, bradyphrenia, dysexecutive syndrome, reduced verbal fluency, impulsivity, disinhibition or perseveration and socially inappropriate behavior, showing overlap to behavioral variant frontotemporal dementia/bvFTD, (6) oculomotor dysfunction (PSP-OM), (7) PSP non-fluent agrammatical primary progressive aphasia overlapping with nfvFTD (PSP-SL) (Höglinger et al. 2018), cortico-basal syndrome (PSP-CBS), with orobuccal or limb apraxia, cortical sensory deficits, alien limb phenomena, and movement disorder signs (akinesia, myoclonus, dystonia), associated with severe cortical involvement (Dickson et al. 2010), and other rare variants, such as PSP-primary lateral sclerosis (PSP-PLS), PSP-cerebellar ataxia (PSP-C), and several others (Ali and Josephs 2018; Shoeibi et al. 2019). Furthermore, single cases of PSP showed either a short disease duration or a protracted course with unusual long duration (Couto et al. 2022; Forrest et al. 2022; Zhang et al. 2021). Most of these subtypes show different distribution and density of tau pathology and cellular characteristics as the expression of different progressive patterns of tau pathology (Campagnolo et al. 2023). The most frequent PSP-RS has more severe and extensive tau pathology in basal ganglia, brainstem, and prefrontal cortex, whereas PSP-P and PSP-PGF have a lower tau score with more restricted involvement of substantia nigra (SN), subthalamus, and internal pallidum (Coughlin et al. 2021).
Cognitive and behavioral abnormalities
Cognitive impairment (CI) is a common clinical feature of PSP (Brown et al. 2010; Gerstenecker et al. 2013). It is more frequent and more severe than in multiple system atrophy and PD and shows longitudinal decline (Jia et al. 2022; Sulena et al. 2017). PSP patients have greater difficulty with complex numerical tasks than patients with Lewy body disorders (Howard et al. 2022). They show significant greater impairment in verbal fluency and executive functions than PD patients (Luca et al. 2021). The current MDS criteria for PSP (Höglinger et al. 2017) do not list dementia among supporting features, although its presence may be relevant to consider a possible diagnosis of PSP together with other core features (Campagnolo et al. 2023). Frontal executive dysfunction and verbal fluency deficits are the most distinct and earliest forms of CI in PSP, while memory, construction, naming, and visuo-spatial and social cognition can also be affected (Gerstenecker et al. 2013; Parthimos and Schulpis 2020). PSP in moderate stages of disease shows mild cognitive deficits with predominant executive dysfunction (Caso et al. 2016), about 16% of PSP patients progress to dementia within 1.5 years (Fiorenzato et al. 2019).
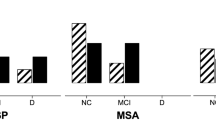
CI is evident in early stages of disease in about 50% of PSP patients, with frontal executive dysfunction in 40.62% (Brown et al. 2010), while in a smaller cohort, at baseline, 15.2% were PSP-NC (not cognitively impaired), 43.4% were classified as PSP-MCI (mild cognitive impairment), and 41.4% as PSP-dementia. Frontal cognitive–behavioral (Han et al. 2010; Hassan et al. 2012) and speech–language disorders (Boeve et al. 2003; Josephs and Duffy 2008) have been consistently reported in PSP. In a small cohort, 52% of PSP patients displayed more than one cognitive–behavioral abnormality, with 81% showing executive dysfunction, 64% cognitive slowing, 55% inefficient memory, 33% impaired word finding, 22% impaired orientation, and 17% visuo-spatial impaired (Kobylecki et al. 2015). After a follow-up of 3 years, 73% of PSP-NC and PSP-MCI converted to dementia, 90% of them developed bvFTD phenotype (Pilotto et al. 2017), while in another study, after 1.5 years, 16% of PSP patients progressed to dementia (Fiorenzato et al. 2019). Among 121 autopsy-confirmed cases of PSP, CI was reported in 74%, among which it was most frequent in PSP-F (100%), followed by PSP-RS (75%) and PSP-P and PSP-CBS (57%), and 80% in unclassified cases, while depression was most frequent in PSP-cognitive impairment (PSP-CI) (59%) (Koga et al. 2017). Recent studies showed that at baseline, MCI was present in 77.8% of PSP patients (48.4% in PD). The 10-year cumulative probability of dementia was 71% in PSP (54% in PD); the cumulative probability of any CI prior to death was 79%; MCI or dementia affected almost all PSP patients during follow-up. The type of cognitive dysfunction in PSP subjects differed from that in PD, with poorer performance in language, episodic memory and psychomotor speed, and attention. Risk factors and predictors of cognitive decline in PSP were impaired olfaction, slow eye movement saccades and peripheral inflammation, and striatal dopamine transporter (DAT) activity (like in PD) (Bäckström et al. 2022).
Cognitive and behavioral profiles varied among clinical PSP phenotypes, although cognitive testing could not clearly differentiate between PSP subtypes. The typical PSP-RS group demonstrated greater impairment in processing speed and executive function compared to the "brainstem predominant" phenotypes (PSP-P and PSP-PGF) (Lee et al. 2018). Early verbal fluency deficit identifies patients with PSP-RS, apathy those with PSP-P (Burrell et al. 2014; Pellicano et al. 2017). In a study of 304 patients with PSP-RS, cognitive scores on the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) were significantly related to other markers of PSP (e.g., motor and functional disabilities, depression, and global cognition). Impairment involved visuo-spatial perception and construction, and attention (Duff et al. 2019). PSP is associated with specific impairment of visuo-spatial cognition which is caused by degeneration of the ocular structures that support exogenous spatial attention and memory, depending on an intact oculomotor system (Smith and Archibald 2020; Smith et al. 2021). Recurrent falls of patients with PSP are associated mainly with executive and visuo-spatial dysfunctions (Kim et al. 2014). Another study revealed more CI in the PSP-RS and PSP-SL groups (with speech and language disorders) than in PSP-PGF and PSP-P groups, mainly due to increased impairment of frontal executive domains in the earlier ones (Horta-Barba et al. 2021). PSP-RS is the subtype with the greatest lifetime prevalence of dementia, while PSP-P has a more benign course (Picillo et al. 2019). Cognitive slowing, executive impairments, and ineffective memory recall were seen in the majority of patients; 32% fulfilled cognitive–behavioral criteria for possible FTD at initial assessment (Kobylecki et al. 2015). In the Mayo Clinic brain bank, almost 50% of pathologically verified PSP cases showed the clinical features of bvFTD (PSP-F), associated with disinhibition (Sakae et al. 2019), whereas in a retrospective multicenter study, FTD-like features were reported in only 10% (Respondek et al. 2014). According to a recent review, cognitive dysfunction was seen in 32% of all clinical PSP phenotypes, with values ranging from 0% in PSP-P to 83.3% in PSP-CBS, with 11.1% in PSP with predominant postural instability, 28.6% in PSP-OM, 47.8% in PSP-RS, and 66.7% in PSP-F (Giagkou et al. 2019). The PSP-F subgroup shows predominant frontal presentation (Donker Kaat et al. 2007), with the typical pattern in the non-fluent variant of primary progressive aphasia and hypometabolism in left frontal–insular and superior–medial frontal cortex involvement (Dodich et al. 2019). Among behavioral abnormalities, apathy (91%), disinhibition (36%), depression (50%), anxiety (37%), and obsessive–compulsive symptoms in 24% were reported (Agarwal and Gilbert 2022). Moreover, there is a broad deficit of social cognition in PSP impairing the recognition of emotion and mind, arising from a variety of related disorders (de Souza et al. 2022; Ghosh et al. 2012). Deficits in emotion recognition correlated with the severity of cognitive deficits but not with disease duration (Ghosh et al. 2009).
Neuroimaging findings in PSP with cognitive impairment
Conventional brain imaging in PSP usually reveals atrophy of midbrain and superior cerebellar peduncle (Luca et al. 2021; Quattrone et al. 2008; Whitwell et al. 2013). MRI in PSP patients with CI showed reduced gray matter (GM) volumes in several cortical and subcortical areas, including frontal, temporal and parahippocampal structures, basal ganglia, midbrain, and cerebellum, reduced cortical thickness in left entorhinal and fusiform gyrus (Nicastro et al. 2020). A data-driven model of brain volume changes estimated that the earliest atrophy occurs in the brainstem and subcortical regions followed by progression caudally into the superior cerebellar peduncle and deep cerebellar nuclei, and rostral to the cortex. The sequence of cortical atrophy progresses in an anterior to posterior direction, beginning in the insula and then the frontal lobe before spreading to the temporal, parietal and finally the occipital lobe (Scotton et al. 2022). This in vivo model accords with the post-mortem neuropathological staging of tau pathology in PSP (Kovacs et al. 2020). PSP-CI has been related to decreased GM volume in frontotemporal regions, thalamus and globus pallidus (GP), with predominant involvement of midbrain, subcortical structures, basal ganglia, and cerebellum (Stezin et al. 2017). Significant correlation has been found between fronto-cerebellar GM atrophy and executive CI (Giordano et al. 2013); prominent executive dysfunction is related to bilateral cortical thinning in prefrontal/precentral cortex and temporal poles as well as in dorsolateral anterior cortical regions (Caso et al. 2016). Episodic memory impairment correlated with hippocampal/ parahippocampal volume (Macedo et al. 2022a) or with lesions to striatofrontal structures (Macedo et al. 2022b). The involvement of the limbic and paralimbic systems contributes to memory impairment and personality changes (Braak et al. 1992; Higuchi et al. 1995). Voxel-based morphometry shows GM atrophy in orbitofrontal and medial frontal cortices (Brodman areas 44, 45, 47), in cingulate and paralimbic association cortex (Brodman areas 23, 24, 32) (Cordato et al. 2005). Involved are also frontal operculum, insula, precentral, superior frontal, postcentral gyrus, and the superior parietal lobule (Ghosh et al. 2012). Corpus callosum atrophy may reflect the changes in the cerebral cortex, accentuated in the frontal region, which contributes to frontal lobe dysfunction in PSP (Yamauchi et al. 1997). A systemic review of GM abnormalities in PSP showed significant reduction in both cortical and subcortical regions including frontal motor, medial and lateral frontal cortices, superior temporal gyrus, insula, striatum, thalamus, midbrain, and anterior cerebellum, causing involvement of cortico-subcortical circuits (Pan et al. 2017). PSP clinical variants have different patterns of involvement of subcortical areas and circuitries, suggesting different patterns of disease spreading through the brain. Volume loss in frontal lobes is seen in PSP-SL, PSP-CBS, and PSP-F (Whitwell et al. 2020), which can be considered PSP-cortical phenotypes (Guasp et al. 2021; Jabbari et al. 2020; Kovacs et al. 2020), while PSP-P and PSP-OM show more restricted patterns of neurodegeneration involving striatum, thalamus, GP, and subthalamic nucleus. PSP-SL shows greater volume loss and tau burden in supplementary motor area and motor cortex, but less involvement of midbrain and subthalamic nucleus, while PSP-P has larger midbrain volume and greater tau load in the putamen. Both PSP-F and bvFTD show similar patterns of brain changes with atrophy in frontal medial cortex, cingulum, insular, and limbic structures, with significant cluster in anterior temporal lobe (de Souza et al. 2022).
White matter (WM) atrophy involves dorsomedial midbrain, corpus callosum and periventricular WM (Cordato et al. 2005). It was greatest in midbrain, cerebellar tracts, and cerebral peduncles, but was also present in orbitofrontal and superior frontal regions (Ghosh et al. 2012). Diffusion tensor MRI shows abnormalities of corpus callosum, superior cerebellar peduncle, cingulum, uncinate fascicles bilaterally, in right inferior longitudinal fasciculus as best predictor of executive dysfunction (Agosta et al. 2014). WM tract degeneration further affects corona radiata, corticospinal tract, superior longitudinal fasciculus, anterior thalamic radiation, medial lemniscus, anterior limb of internal capsule, and right posterior thalamic radiation (Worker et al. 2014). Moreover, widespread WM changes affect cerebellar peduncles, thalamic radiations, corticospinal tracts, corpus callosum, and superior longitudinal fasciculi (Nicastro et al. 2020; Piattella et al. 2015b). Lesions involving the main tracts including superior cerebellar peduncle, corpus callosum, corticospinal tracts, inferior fronto-occipital, and superior longitudinal and uncinate fascicles bilaterally are associated with cognitive deficits in PSP (Caso et al. 2016). Increased mean diffusivity was observed on superior cerebellar peduncle, sagittal striatum, posterior corona radiata, body of corpus callosum, anterior and superior corona radiata (Sintini et al. 2019), and hypometabolism in medial prefrontal and frontal cortices, caudate nucleus, and mesencephalon (Tomše et al. 2022).
Functional imaging
PSP has long been proposed as a network-based disorder, showing altered functional connectivity (FC). fMRI in PSP patients shows significant disruptions within the connections between brainstem–cerebellar, diencephalic–basal ganglia, and cortical connections, particularly within cortico-subcortical and cortico-brainstem interactions. Patients with more severe CI showed lower mean midbrain–cortical network connectivity scores and widespread disruption of cortico-subcortical connectivity (Gardner et al. 2013). Decreased pallidum functional connectivity correlated with CI. Lower FC in resting state networks mainly involves basal ganglia, thalamus anterior cingulate, dorsolateral prefrontal and temporo-occipital cortices, supramarginal gyrus, supplementary motor cortex, and cerebellum (Piattella et al. 2015a). Increasing tau burden in midbrain and deep nuclei is associated with disrupted cortico-subcortical and cortico-brainstem connections (Cope et al. 2018). Earlier stage subcortical–anterior cortical lesions with declining FC are followed by later-stage subcortical–posterior cortical changes with prefrontal–paralimbic FC decline (Brown et al. 2017). Disrupted structural connectivity of frontal-deep GM pathways (Abos et al. 2019) and decreased FC in prefrontal cortex is significantly correlated with cognitive and behavioral performance (and dementia severity) (Rosskopf et al. 2017). Local frontal language networks may be impaired in PSP-RS (Ransmayr et al. 2022). In PSP-RS, alterations in the structural connectivity of the whole brain connectome are seen, in particular affecting frontal connections, deep GM connections between basal ganglia structures, and between frontal lobe and basal ganglia, supporting the concept that PSP is a network-based disorder.
Apathy in PSP is rather frequent. In the apathetic subtype, marked atrophy was observed in GM volumes in the inferior frontal gyrus, anterior cingulate cortex, and in subcortical areas that were positively correlated with apathy scale and tau deposition. This phenotype may arise from tau aggregation and oxidative stress compromising the neuronal circuit resilience in the posterior cortex along with neuronal loss in the anterior cortex (Matsuoka et al. 2023).
Tau imaging
Tau PET imaging detected the distribution and severity of specific forms of tau pathology (Saint-Aubert et al. 2017; Whitwell et al. 2017). 18F-AV 1451 PET binds to the PSP-specific subcortical areas with tau pathology, showing increased retention in basal ganglia, midbrain and cerebellar dentate nucleus (Cho et al. 2017; Passamonti et al. 2017; Whitwell et al. 2017). In PSP-CI patients, higher 18F-AV PET binding correlated with cortical thinning in parietooccipital areas (Nicastro et al. 2020). 18FFlortaucipir uptake increased in striatum, GP, and thalamus. Volume loss in frontal lobes in PSP-SL, PSP-F, and PSP-CBS was associated with highest cortical tau burden (Whitwell et al. 2020). Tau uptake appears related to GM volume reduction and WM degeneration (Sintini et al. 2019). Executive dysfunction relates to tau deposition in superior frontal gyrus and supramarginal cortices, language deficits to neuronal loss and tau deposition in perirhinal gyrus and Broca's area, while visuo-spatial dysfunctions and global CI relate to tau deposition in supramarginal gyrus (Schofield et al. 2012).
Recent tau-PET studies in PSP patients showed tracer uptake in the midbrain, followed by GP, frontal cortex, and medulla oblongata, which correlated well with clinical severity (Brendel et al. 2018), and improved the imaging diagnosis of PSP (Messerschmidt et al. 2022). The distribution patterns of 18F-Florzolotau PET imaging in living brains showed a remarkable similarity to those reported in post-mortem studies, with binding intensity being markedly higher in PSP-RS (Liu et al. 2023a). Patients with PSP showed significant hypoperfusion predominantly in thalamus, caudate nucleus, and anterior cingulate cortex, fitting the topology of the 4-repeat tauopathies disease spectrum (PSP and CBS) (Katzdobler et al. 2023).
The topography of tau deposition in PSP patients differed from that of other neurodegenerative disorders (Jin et al. 2023). It confirmed that the patient-level tau patterns are associated with the tau connectivity of subcortical tau epicenters (Franzmeier et al. 2022). Network incorporating GM structures demonstrated hyperconnectivity and cerebellar hypoconnectivity, while cortico-subcortical connections showed variable changes. Tau load in right GP and left dentate nucleus is associated with functional networks which strengthen intra-opercular and intra-cerebellar connections and weaken operculo-cerebellar connections. These findings support the concept of tau-dependent functional network changes in PSP, providing evidence for downstream effects of tau pathology on brain functionality in this disorder (Aghakhanyan et al. 2022).
Neuropathology in PSP with cognitive impairment
The pathological hallmark of PSP consists of 4-R tau deposition in both neurons and glial cells as neurofibrillary tangles (NFTs), neuropil threads, tufted astroglia, and coiled bodies, accompanied by neuronal loss and gliosis predominantly affecting GP, subthalamic nucleus, and SN and cerebellar dentate nucleus (Hauw et al. 1994; Litvan et al. 1996). In general, subcortical and brainstem tau may deposit earlier before cortical tau, the earliest lesions being NFTs in the SN before tufted astrocytes develop. It is well documented that cognitive decline in PSP is associated with more severe tau burden in the neocortex and hippocampus (Bigio et al. 1999; Jellinger 2008) and that the occurrence of CI/dementia in PSP is influenced by the distribution and severity of tau pathology (Koga et al. 2017; Williams et al. 2007; Williams and Lees 2009). Histopathological findings are qualitatively similar in the various subtypes of PSP but differ in the relative distribution and severity of neuronal loss and gliosis as well as in the regional intensity of the tau pathology (Williams and Lees 2009). Tau burden in PSP-P was significantly lower than in PSP-RS (Robinson et al. 2020), whereas frontal and temporal neuronal tau pathology as well as that in WM was greater in PSP-F than in PSP-RS (Sakae et al. 2019). In general, the PSP-related tau burden is significantly correlated with executive, language and visuo-spatial impairments, less severe with memory and behavioral impairments. A comparison between PSP-CI and PSP-NC cases showed that although Braak tau stage, Thal amyloid phase, and the frequency of other lesions were not different between both subtypes, the total and regional tau burden in pontine base and cerebellar WM was significantly higher in PSP-CI (Koga et al. 2017), supporting previous studies showing that multiple cognitive domains, in particular executive functions, are affected in PSP (Bak et al. 2005; Brown et al. 2010). These data were confirmed in a recent study showing that PSP-F has more severe tau pathology in frontal cortex, extending to precentral gyrus, associated with cortical atrophy and decreased frontal blood flow with right-sided predominance, while tau pathology was mild in SN, locus ceruleus, and subthalamic nucleus (Ono et al. 2022). PSP-RS cases show marked changes in cortical neurophysiology, associated with a decline in cognitive functions due to a reduction of synaptic density in inferior frontal cortex, affecting superficial and granular layer glutamatergic excitation (Adams et al. 2022). In PSP, there is general synaptic loss of both excitatory executive and inhibitory bipartite synapses in the frontal cortex (Briel et al. 2021). Higher 18F-AV-1451 PET binding correlates with higher synaptic density in PSP, but this association diminishes with disease severity; moreover, higher cortical 18F-AV-1451 binding correlates with lower subcortical synaptic density. Given the importance of synaptic function for cognition and action, the complex relationship between molecular pathology including tau and synaptic density may explain changes in cognitive and motor physiology, elucidating the pathophysiology of PSP and other primary tauopathies (Holland et al. 2022).
Co-pathologies
CI in PSP patients, primarily involving executive functions, is related to specific PSP-related tau burden but not to Alzheimer-type tau pathology, although PSP may co-exist with AD (Koga et al. 2017; Sakamoto et al. 2009). A comparison of pathological changes between PSP and PSP with coexisting AD (PSD/AD) showed that Braak NFT stage and Thal amyloid phase were higher in patients with PSD/AD. However, the latter had lower total tau burden, although the frequency of CI did not differ between both groups, indicating that coexisting AD did not significantly impact the frequency or severity of CI in PSP (Koga et al. 2017). Other comparative studies of a cohort of PSP cases revealed that APOE ε4 carriers had more plaques, while APOE ε2 carriers had fewer plaques. TDP-43 co-pathology was present in 14% of PSP cases and was significantly associated with age at onset and death. The distribution of TDP-43 pathology in PSP was primarily observed in limbic-predominant age-related TDP-43 encephalopathy (LATE) pattern (Nelson et al. 2019). Lewy body co-pathology was seen in 15% of PSP cases and was not age-associated. Taken together, the primary tauopathy burden is the strongest correlate of CI in PSP, while co-pathologies are principally determined by age and genetic risk factors, and do not correlate with clinical symptoms (Koga et al. 2017; Robinson et al. 2020). In another study, TDP-43-positive inclusions were observed in 26% of PSP cases, in which regional tau burden tended to be higher, and a significant correlation between tau and TDP-43 burden was noted in the occipitotemporal gyrus. Hippocampal sclerosis (HS) was found in 3/5 TDP-43-positive PSP cases, but HS was more frequent in TDP-43-positive than -negative cases. Dementia was present in 58% of PSP cases (Pillon et al. 1991) but in 4/5 of TDP-43-positive ones. TDP-43 and tau were frequently co-localized in the amygdala but not in hippocampus. These data suggest that PSP is a primary tauopathy in which TDP-43 accumulation can occur in the limbic system, and may be associated with the occurrence of HS (Yokota et al. 2010). Although in this particular cohort, the frequency of CI/dementia in PSP cases with both TDP-43 and HS (100%) and that with TDP-43 pathology (80%) was higher than in those lacking both lesions (50%), the potential comorbid effect of concurrent TDP-43 pathology and/or HS on CI in PSP needs to be further elucidated.
Genetic influence on cognition in PSP
PSP is genetically and phenotypically a complex disorder. The major genetic risk factor for sporadic PSP, which represents around 85% of all cases, is a variant in the MAPT (microtubule-associated tau) gene with over-representation of the H1 haplotype and the H1/H1 genotype (Chen et al. 2019; Heckman et al. 2019; Houlden et al. 2001), and within H1, a sub-haplotype (H1c) is associated with PSP (Im et al. 2015). MAPT mutations/variants and its haplotypes are the major genetic risk factors of PSP, both in the sporadic and familial forms (Debnath et al. 2022). The H1 haplotype present in 95% of the PSP patients compared to 75% in the general population, indicates it as a risk for PSP similar to that of APOE ε4 risk allele for AD (Farrer et al. 1997; Stefansson et al. 2005). While MAPT mutations are the most common causes of familial PSP, the leucine-rich repeat kinase 2 (LRRK2) is a rare monogenic cause of PSP. More than 15 MAPT mutations have been identified in cases with PSP, and the mean age at onset is earlier in carriers of LRRK2 or dynactin subunit 1 (DCTN1) mutations (Wen et al. 2021). LRRK2 has been associated with sporadic and familial forms of PD, which may suggest a genetic overlap with PSP (Jabbari et al. 2021). Genome-wide association studies (GWAS) also identified several novel risk variants, and it has become apparent that interactions between genetic and environmental factors, the latter mediated through epigenetic modifications, significantly contribute to PSP development (Debnath et al. 2022). Although CI is common in PSP, the influence of genetics on cognition in this atypical parkinsonism is poorly understood. In a study of 305 patients with possible or probable PSP, genetic information determined by genotyping assays revealed no significant differences between individuals homo- or heterozygous for the MAPT H1 haplotype, although it varied considerably at the sub-haplotype level: According to the MAPT status, H1c-specific rs242557/A allele carriers showed better cognitive abilities than noncarriers (Gerstenecker et al. 2017). While the H1 haplotype has been associated with progression of MCI to dementia (Samaranch et al. 2010), there were no clinical or pathological differences between H1/H1 and H1/H2 carriers and no significant differences in any regional tau burden between APOE genotype or MAPT haplotype carriers. Moreover, cognition in this cohort was not significantly affected by APOE genotype, and no associations were found for other genes. In a series of 121 autopsy-confirmed PSP cases, genetic analysis also revealed no significant differences for either APOE ε4 frequencies or the MAPT H1/H1 haplotype carriers between PSP with and without CI (Koga et al. 2017). Although APOE ε4 carriers have higher Thal amyloid plaques, the frequency of CI was lower in these carriers, suggesting that ε4 is not associated with CI in PSP. In conclusion, both studies clearly indicated that, whereas AD-related pathology is driven by APOE ε4, this genotype does not increase the frequency or severity of CI in PSP. Analysis of modular gene co-expression revealed changes in the molecular pathology underlying AD and PSP: Immune-inflammatory responses preponderate in younger, those associated with synaptic transmission in older AD patients, whereas in PSP, both of these changes overlap. Twelve AD and 4 PSP genetic risk factors were detected (Iohan et al. 2022). On the other hand, AD and PSP have been shown to share similar transcriptomic changes in distinct brain regions (temporal and cerebellar cortices). Many genes have concordant protein changes and evidence of epigenetic control, indicating that complex molecular alterations are similar in both disorders (Wang et al. 2022). A recent genetic survival study of the mitochondrial genome suggested that mitochondrial haplogroups may be associated with the progression of cognitive decline in PD over time (Liu et al. 2023b). Similar studies in PSP, to the best of our knowledge, are currently not available, but could bring further insight into the cognitive progression in this and other movement disorders.
Pathogenic factors of cognitive impairment in PSP
Whereas the contribution of α-synuclein, Aβ and tau pathologies as well as cerebrovascular lesions and TDP-43 pathology appear well documented in the pathogenesis of CI in PD (Jellinger 2022), the pathogenic factors implicated in cognitive decline in PSP are poorly understood. There is evidence from both in vivo and post-mortem studies suggesting that mitochondrial energy metabolism is impaired in PSP. Failure in mitochondrial energy production may act as an upstream event in the chain of pathological events leading to the aggregation of tau protein and neuronal cell death (Ries et al. 2011). In the SN of PSP patients, strong mitochondrial ferritin, a mitochondrial iron storage protein, was observed, which is related to mitophagy. Mitochondrial ferritin was localized in dopaminergic neurons, microglia and astroglia in SN and ventral tegmental area in PSP, and was co-localized with tau immunoreactivity. This may explain the function of mitochondrial ferritin in PSP progression (Abu Bakar et al. 2021, 2022). Furthermore, pathological tau was noticed in PSP astrocytes and microglia (Montalbano et al. 2023), leading to general synaptic loss, suggesting a hyperphosphorylated tau protein pathology-associated role of astroglia in maintaining connections between neuronal circuits, considered as the microscopic substrate of cognitive dysfunction in PSP (Briel et al. 2021).
Earlier studies provided insight into widespread degenerative changes in PSP involving subcortical cholinergic and muscarinergic systems. In PSP, there is a significant loss of neurons immunoreactive for choline acetyltransferase (ChAT) in the nucleus of Edinger–Westphal, rostral medial longitudinal fasciculus, interstitial nucleus of Cajal, and the deep layers of the superior colliculus (69–93%), whereas the other ChAT-immunoreactive cells in cranial nerves III and IV and other mesencephalic cholinergic nuclei were not involved (Juncos et al. 1991). In the nucleus basalis of Meynert (nbM), the number of neurons and the ChAT-positivity rate of remaining neurons are reduced, as they are in the pedunculopontine tegmental nuclei (tgN), where many NFTs are present. In PSP, cholinergic neurons in the laterodorsal tgN are more vulnerable than those in the posterior tgN and in the nbM (Kasashima and Oda 2003). Other studies revealed a selective and marked degeneration of cholinergic and dopaminergic terminals in the striatum with significant reduction of vesicular acetylcholine transporter (VAChT) density in caudate, putamen and SN compacta, consistent with degeneration of dopaminergic nigrostriatal projection neurons in PSP (Suzuki et al. 2002). Furthermore, in PSP, muscarinic M2 receptors were reduced in the thalamic nucleus, M4 receptors in the mediodorsal thalamus as well as in subthalamic nucleus, with normal binding in other thalamic nuclei, indicating loss of cholinergic afferents that may contribute to behavioral and cognitive disturbances in PSP (Warren et al. 2007a, b), while cortical muscarinic receptors M1, M2, and M4 densities and M1 coupling were preserved (Warren et al. 2008). On the other hand, PSP showed reduced acetylcholinesterase activity in precentral cortex (9.4%) and thalamus (24%) (Hirano et al. 2010). Recent studies revealed that the volume of nbM is reduced both in PD and PSP. The lack of significant correlation between nbM volume and cognitive measures suggests that other factors, such as frontal atrophy, may play a more important role than subcortical cholinergic atrophy in PSP (Rogozinski et al. 2022). Furthermore, tau pathology and neuroinflammation have been shown to colocalize in PSP, and correlate with clinical severity (Malpetti et al. 2020). Neuroinflammation in PSP has been reviewed recently (Alster et al. 2020b). Other pathogenic factors of CI in PSP—and related movement disorders—include autosomal/proteasomal dysfunction, oxidative stress (Aoyama et al. 2006; Matsuoka et al. 2023), loss of neurotrophic factors, and others (Schapira 2011), but their complex interplay in the development of cognitive deterioration in PSP deserves further elucidation.
Conclusion and outlook
PSP, a primary 4-repeat tauopathy, is a pathological entity with a wide range of presenting clinical features, among which CI is an important and frequent lesion associated with progressive dysfunction. Its neuropathology is characterized by the deposition of phosphorylated tau protein in neurons and glia, associated with neuronal and synapse loss and gliosis in widespread disease-specific cortical and subcortical regions. Both tau pathology and the related neurodegenerative lesions are more extensive and more severe in PSP patients with CI that is dominated by executive dysfunction but also difficulties in memory, naming, language, visuo-spatial, and other domains. Modern neuroimaging methods have demonstrated the distribution and intensity of tau depositions in widespread brain regions that mirror quite well the tau pathology detected post-mortem. MRI and related methods revealed both extensive GM lesions in cortical and other subcortical regions with hypometabolism in frontal and other cortical regions, as well as widespread WM lesions involving the corona radiata and many cortico-basal ganglia–cerebellar pathways. These lesions are accompanied by widespread disturbances of cortico-cortical, cortico-subcortical, fronto-striatal, and other related connections causing widespread disruptions of cognition–/behavior-related and other important functional brain networks. However, the available data about functional disconnections associated with CI in PSP are still limited. Further lesions associated with PSP-CI are disorders of cholinergic, dopaminergic, and muscarinic dysfunctions caused by degeneration of subcortical nuclei. The pathophysiology and pathogenesis of cognitive dysfunctions in PSP are complex as in related movement disorders. There are both similarities and differences in the pathogenic and genetic mechanisms in PSP and AD, although combinations of PSP with AD-like pathology occur. Despite available standard diagnostic criteria for both PSP and cognitive deficits, the basic pathogenic mechanisms are still poorly understood, and adequate treatments of CI in PSP are still limited since despite severe involvement of cholinergic mechanisms, treatment with cholinesterase inhibitors had no reliable effects. Although improved specific biomarkers (Coughlin and Litvan 2020) and quite exact methods for the assessment of cognitive dysfunctions in PSP are available, better insight into its complex molecular pathogenesis may expand access to hopefully beneficial clinical trials.
Data availability
Not applicable.
Abbreviations
- AD:
-
Alzheimer disease
- bvFTD:
-
Behavioral variant frontotemporal dementia
- ChAT:
-
Choline acetyltransferase
- CI:
-
Cognitive impairment
- FC:
-
Functional connectivity
- FTD:
-
Frontotemporal dementia
- GM:
-
Gray matter
- GP:
-
Globus pallidus
- HS:
-
Hippocampal sclerosis
- MCI:
-
Mild cognitive impairment
- nbM:
-
Nucleus basalis of Meynert
- NFTs:
-
Neurofibrillary tangles
- PD:
-
Parkinson disease
- SN:
-
Substantia nigra
- tgN:
-
Tegmental nuclei
- WM:
-
White matter
- PSP:
-
Progressive supranuclear palsy
- PSP-CBS:
-
PSP cortico-basal syndrome
- PSP-CI:
-
PSP-cognitive impairment
- PSP-D:
-
PSP-dementia
- PSP-F:
-
PSP frontal behavioral/cognitive subtype
- PSP-MCI:
-
PSP with mild cognitive impairment
- PSP-NC:
-
PSP not cognitively impaired
- PSP-OM:
-
PSP with ocular motor dysfunction
- PSP-P:
-
PSP-parkinsonism
- PSP-PGF:
-
Gait-freezing form
- PSP-RS:
-
PSP-Richardson's Syndrome
- PSP-SL:
-
PSP non-fluent agrammatical primary progressive aphasia overlapping with nfvFTD
References
Abos A, Segura B, Baggio HC, Campabadal A, Uribe C, Garrido A, Camara A, Muñoz E, Valldeoriola F, Marti MJ, Junque C, Compta Y (2019) Disrupted structural connectivity of fronto-deep gray matter pathways in progressive supranuclear palsy. Neuroimage Clin 23:101899
Abu Bakar ZH, Kato T, Yanagisawa D, Bellier JP, Mukaisho KI, Tooyama I (2021) Immunohistochemical study of mitochondrial ferritin in the midbrain of patients with progressive supranuclear palsy. Acta Histochem Cytochem 54:97–104
Abu Bakar ZH, Bellier JP, Yanagisawa D, Kato T, Mukaisho KI, Tooyama I (2022) LC3/FtMt colocalization patterns reveal the progression of FtMt accumulation in nigral neurons of patients with progressive supranuclear palsy. Int J Mol Sci 23:537. https://doi.org/10.3390/ijms23010537
Adams NE, Jafarian A, Perry A, Rouse MA, Shaw AD, Murley AG, Cope TE, Bevan-Jones WR, Passamonti L, Street D, Holland N, Nesbitt D, Hughes LE, Friston KJ, Rowe JB (2022) Neurophysiological consequences of synapse loss in progressive supranuclear palsy. Brain Online. https://doi.org/10.1093/brain/awac1471
Agarwal S, Gilbert R (2022) Progressive Supranuclear Palsy, 2022/04/03 Edition. StatPearls Publishing, Treasure Island, FL
Aghakhanyan G, Rullmann M, Rumpf J, Schroeter ML, Scherlach C, Patt M, Brendel M, Koglin N, Stephens AW, Classen J, Hoffmann KT, Sabri O, Barthel H (2022) Interplay of tau and functional network connectivity in progressive supranuclear palsy: a [(18)F]PI-2620 PET/MRI study. Eur J Nucl Med Mol Imaging 50:103–114
Agosta F, Galantucci S, Svetel M, Lukic MJ, Copetti M, Davidovic K, Tomic A, Spinelli EG, Kostic VS, Filippi M (2014) Clinical, cognitive, and behavioural correlates of white matter damage in progressive supranuclear palsy. J Neurol 261:913–924
Ali F, Josephs K (2018) The diagnosis of progressive supranuclear palsy: current opinions and challenges. Expert Rev Neurother 18:603–616
Alster P, Madetko N, Koziorowski D, Friedman A (2020a) Progressive supranuclear palsy-parkinsonism predominant (PSP-P) - A clinical challenge at the boundaries of PSP and Parkinson’s disease (PD). Front Neurol 11:180
Alster P, Madetko N, Koziorowski D, Friedman A (2020b) Microglial activation and inflammation as a factor in the pathogenesis of progressive supranuclear palsy (PSP). Front Neurosci 14:893
Aoyama K, Matsubara K, Kobayashi S (2006) Aging and oxidative stress in progressive supranuclear palsy. Eur J Neurol 13:89–92
Bäckström D, Granåsen G, Mo SJ, Riklund K, Trupp M, Zetterberg H, Blennow K, Forsgren L, Domellöf ME (2022) Prediction and early biomarkers of cognitive decline in Parkinson disease and atypical parkinsonism: a population-based study. Brain Commun 4:fcac040
Bak TH, Crawford LM, Hearn VC, Mathuranath PS, Hodges JR (2005) Subcortical dementia revisited: similarities and differences in cognitive function between progressive supranuclear palsy (PSP), corticobasal degeneration (CBD) and multiple system atrophy (MSA). Neurocase 11:268–273
Bigio EH, Brown DF, White CL 3rd (1999) Progressive supranuclear palsy with dementia: cortical pathology. J Neuropathol Exp Neurol 58:359–364
Boeve B, Dickson D, Duffy J, Bartleson J, Trenerry M, Petersen R (2003) Progressive nonfluent aphasia and subsequent aphasic dementia associated with atypical progressive supranuclear palsy pathology. Eur Neurol 49:72–78
Braak H, Jellinger K, Braak E, Bohl J (1992) Allocortical neurofibrillary changes in progressive supranuclear palsy. Acta Neuropathol 84:478–483
Brendel M, Schönecker S, Höglinger G, Lindner S, Havla J, Blautzik J, Sauerbeck J, Rohrer G, Zach C, Vettermann F, Lang AE, Golbe L, Nübling G, Bartenstein P, Furukawa K, Ishiki A, Bötzel K, Danek A, Okamura N, Levin J, Rominger A (2018) [(18)F]-THK5351 PET correlates with topology and symptom severity in progressive supranuclear palsy. Front Aging Neurosci 9:440
Briel N, Pratsch K, Roeber S, Arzberger T, Herms J (2021) Contribution of the astrocytic tau pathology to synapse loss in progressive supranuclear palsy and corticobasal degeneration. Brain Pathol 31:e12914
Brown RG, Lacomblez L, Landwehrmeyer BG, Bak T, Uttner I, Dubois B, Agid Y, Ludolph A, Bensimon G, Payan C, Leigh NP (2010) Cognitive impairment in patients with multiple system atrophy and progressive supranuclear palsy. Brain 133:2382–2393
Brown JA, Hua AY, Trujllo A, Attygalle S, Binney RJ, Spina S, Lee SE, Kramer JH, Miller BL, Rosen HJ, Boxer AL, Seeley WW (2017) Advancing functional dysconnectivity and atrophy in progressive supranuclear palsy. Neuroimage Clin 16:564–574
Burrell JR, Hodges JR, Rowe JB (2014) Cognition in corticobasal syndrome and progressive supranuclear palsy: a review. Mov Disord 29:684–693
Campagnolo M, Weis L, Fogliano C, Cianci V, Garon M, Fiorenzato E, Carecchio M, Ferreri F, Bisiacchi P, Antonini A, Biundo R (2023) Clinical, cognitive, and morphometric profiles of progressive supranuclear palsy phenotypes. J Neural Transm (vienna) 130:97–109
Caso F, Agosta F, Volonté MA, Ferraro PM, Tiraboschi P, Copetti M, Valsasina P, Falautano M, Comi G, Falini A, Filippi M, (2016) Cognitive impairment in progressive supranuclear palsy-Richardson’s syndrome is related to white matter damage. Parkinsonism Relat Disord 31:65–71
Chen Z, Chen JA, Shatunov A, Jones AR, Kravitz SN, Huang AY, Lawrence L, Lowe JK, Lewis CM, Payan CAM, Lieb W, Franke A, Deloukas P, Amouyel P, Tzourio C, Dartigues JF, Ludolph A, Bensimon G, Leigh PN, Bronstein JM, Coppola G, Geschwind DH, Al-Chalabi A (2019) Genome-wide survey of copy number variants finds MAPT duplications in progressive supranuclear palsy. Mov Disord 34:1049–1059
Cho H, Choi JY, Hwang MS, Lee SH, Ryu YH, Lee MS, Lyoo CH (2017) Subcortical (18) F-AV-1451 binding patterns in progressive supranuclear palsy. Mov Disord 32:134–140
Cope TE, Rittman T, Borchert RJ, Jones PS, Vatansever D, Allinson K, Passamonti L, Vazquez Rodriguez P, Bevan-Jones WR, O’Brien JT, Rowe JB (2018) Tau burden and the functional connectome in Alzheimer’s disease and progressive supranuclear palsy. Brain 141:550–567
Cordato NJ, Duggins AJ, Halliday GM, Morris JG, Pantelis C (2005) Clinical deficits correlate with regional cerebral atrophy in progressive supranuclear palsy. Brain 128:1259–1266
Coughlin DG, Litvan I (2020) Progressive supranuclear palsy: Advances in diagnosis and management. Parkinsonism Relat Disord 73:105–116
Coughlin DG, Dickson DW, Josephs KA, Litvan I (2021) Progressive supranuclear palsy and corticobasal degeneration. Adv Exp Med Biol 1281:151–176
Couto B, Martinez-Valbuena I, Lee S, Alfradique-Dunham I, Perrin RJ, Perlmutter JS, Cruchaga C, Kim A, Visanji N, Sato C, Rogaeva E, Lang AE, Kovacs GG (2022) Protracted course progressive supranuclear palsy. Eur J Neurol 29:2220–2231
Coyle-Gilchrist IT, Dick KM, Patterson K, Vázquez Rodríquez P, Wehmann E, Wilcox A, Lansdall CJ, Dawson KE, Wiggins J, Mead S, Brayne C, Rowe JB (2016) Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology 86:1736–1743
de Souza LC, Bertoux M, Radakovic R, Hornberger M, Mariano LI, Resende EPF, Quesque F, Guimarães HC, Gambogi LB, Tumas V, Camargos ST, Cardoso FEC, Teixeira AL, Caramelli P (2022) I’m looking through you: Mentalizing in frontotemporal dementia and progressive supranuclear palsy. Cortex 155:373–389
Debnath M, Dey S, Sreenivas N, Pal PK, Yadav R (2022) Genetic and epigenetic constructs of progressive supranuclear palsy. Ann Neurosci 29:177–188
Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA (2010) Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol 23:394–400
Dodich A, Cerami C, Inguscio E, Iannaccone S, Magnani G, Marcone A, Guglielmo P, Vanoli G, Cappa SF, Perani D (2019) The clinico-metabolic correlates of language impairment in corticobasal syndrome and progressive supranuclear palsy. Neuroimage Clin 24:102009
Donker Kaat L, Boon AJ, Kamphorst W, Ravid R, Duivenvoorden HJ, van Swieten JC (2007) Frontal presentation in progressive supranuclear palsy. Neurology 69:723–729
Duff K, McDermott D, Luong D, Randolph C, Boxer AL (2019) Cognitive deficits in progressive supranuclear palsy on the Repeatable Battery for the Assessment of Neuropsychological Status. J Clin Exp Neuropsychol 41:469–475
Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM (1997) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. a meta-analysis. APOE and alzheimer disease meta analysis consortium. JAMA 278:1349–1356
Fiorenzato E, Antonini A, Camparini V, Weis L, Semenza C, Biundo R (2019) Characteristics and progression of cognitive deficits in progressive supranuclear palsy vs. multiple system atrophy and Parkinson’s disease. J Neural Transm (vienna) 126:1437–1445
Fleury V, Brindel P, Nicastro N, Burkhard PR (2018) Descriptive epidemiology of parkinsonism in the Canton of Geneva, Switzerland. Parkinsonism Relat Disord 54:30–39
Forrest SL, Tartaglia MC, Kim A, Alcaide-Leon P, Rogaeva E, Lang A, Kovacs GG (2022) Progressive supranuclear palsy syndrome associated with a novel tauopathy: case study. Neurology 99(24):1094–1098
Franzmeier N, Brendel M, Beyer L, Slemann L, Kovacs GG, Arzberger T, Kurz C, Respondek G, Lukic MJ, Biel D, Rubinski A, Frontzkowski L, Hummel S, Müller A, Finze A, Palleis C, Joseph E, Weidinger E, Katzdobler S, Song M, Biechele G, Kern M, Scheifele M, Rauchmann BS, Perneczky R, Rullman M, Patt M, Schildan A, Barthel H, Sabri O, Rumpf JJ, Schroeter ML, Classen J, Villemagne V, Seibyl J, Stephens AW, Lee EB, Coughlin DG, Giese A, Grossman M, McMillan CT, Gelpi E, Molina-Porcel L, Compta Y, van Swieten JC, Laat LD, Troakes C, Al-Sarraj S, Robinson JL, Xie SX, Irwin DJ, Roeber S, Herms J, Simons M, Bartenstein P, Lee VM, Trojanowski JQ, Levin J, Höglinger G, Ewers M, (2022) Tau deposition patterns are associated with functional connectivity in primary tauopathies. Nat Commun 13:1362
Gardner RC, Boxer AL, Trujillo A, Mirsky JB, Guo CC, Gennatas ED, Heuer HW, Fine E, Zhou J, Kramer JH, Miller BL, Seeley WW (2013) Intrinsic connectivity network disruption in progressive supranuclear palsy. Ann Neurol 73:603–616
Gerstenecker A, Mast B, Duff K, Ferman TJ, Litvan I (2013) Executive dysfunction is the primary cognitive impairment in progressive supranuclear palsy. Arch Clin Neuropsychol 28:104–113
Gerstenecker A, Roberson ED, Schellenberg GD, Standaert DG, Shprecher DR, Kluger BM, Litvan I (2017) Genetic influences on cognition in progressive supranuclear palsy. Mov Disord 32:1764–1771
Ghosh BC, Rowe JB, Calder AJ, Hodges JR, Bak TH (2009) Emotion recognition in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 80:1143–1145
Ghosh BC, Calder AJ, Peers PV, Lawrence AD, Acosta-Cabronero J, Pereira JM, Hodges JR, Rowe JB (2012) Social cognitive deficits and their neural correlates in progressive supranuclear palsy. Brain 135:2089–2102
Giagkou N, Höglinger GU, Stamelou M (2019) Progressive supranuclear palsy. Int Rev Neurobiol 149:49–86
Giordano A, Tessitore A, Corbo D, Cirillo G, de Micco R, Russo A, Liguori S, Cirillo M, Esposito F, Tedeschi G (2013) Clinical and cognitive correlations of regional gray matter atrophy in progressive supranuclear palsy. Parkinsonism Relat Disord 19:590–594
Guasp M, Molina-Porcel L, Painous C, Caballol N, Camara A, Perez-Soriano A, Sánchez-Gómez A, Garrido A, Muñoz E, Marti MJ, Valldeoriola F, Grau O, Gelpí E, Respondek G, Höglinger GH, Compta Y (2021) Association of PSP phenotypes with survival: A brain-bank study. Parkinsonism Relat Disord 84:77–81
Han HJ, Kim H, Park JH, Shin HW, Kim GU, Kim DS, Lee EJ, Oh HE, Park SH, Kim YJ (2010) Behavioral changes as the earliest clinical manifestation of progressive supranuclear palsy. J Clin Neurol 6:148–151
Hassan A, Parisi JE, Josephs KA (2012) Autopsy-proven progressive supranuclear palsy presenting as behavioral variant frontotemporal dementia. Neurocase 18:478–488
Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I (1994) Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 44:2015–2019
Heckman MG, Brennan RR, Labbé C, Soto AI, Koga S, DeTure MA, Murray ME, Petersen RC, Boeve BF, van Gerpen JA, Uitti RJ, Wszolek ZK, Rademakers R, Dickson DW, Ross OA (2019) Association of MAPT subhaplotypes with risk of progressive supranuclear palsy and severity of tau pathology. JAMA Neurol 76:710–717
Higuchi Y, Iwaki T, Tateishi J (1995) Neurodegeneration in the limbic and paralimbic system in progressive supranuclear palsy. Neuropathol Appl Neurobiol 21:246–254
Hirano S, Shinotoh H, Shimada H, Aotsuka A, Tanaka N, Ota T, Sato K, Ito H, Kuwabara S, Fukushi K, Irie T, Suhara T (2010) Cholinergic imaging in corticobasal syndrome, progressive supranuclear palsy and frontotemporal dementia. Brain 133:2058–2068
Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Müller U, Nilsson C, Whitwell JL, Arzberger T, Englund E, Gelpi E, Giese A, Irwin DJ, Meissner WG, Pantelyat A, Rajput A, van Swieten JC, Troakes C, Antonini A, Bhatia KP, Bordelon Y, Compta Y, Corvol JC, Colosimo C, Dickson DW, Dodel R, Ferguson L, Grossman M, Kassubek J, Krismer F, Levin J, Lorenzl S, Morris HR, Nestor P, Oertel WH, Poewe W, Rabinovici G, Rowe JB, Schellenberg GD, Seppi K, van Eimeren T, Wenning GK, Boxer AL, Golbe LI, Litvan I (2017) Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 32:853–864
Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Muller U, Nilsson C, Whitwell JL, Arzberger T, Englund E, Gelpi E, Giese A, Irwin DJ, Meissner WG, Pantelyat A, Rajput A, van Swieten JC, Troakes C, Antonini A, Bhatia KP, Bordelon Y, Compta Y, Corvol JC, Colosimo C, Dickson DW, Dodel R, Ferguson L, Grossman M, Kassubek J, Krismer F, Levin J, Lorenzl S, Morris HR, Nestor P, Oertel WH, Poewe W, Rabinovici G, Rowe JB, Schellenberg GD, Seppi K, van Eimeren T, Wenning GK, Boxer AL, Golbe LI, Litvan I (2018) Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord 32:853–864
Holland N, Malpetti M, Rittman T, Mak EE, Passamonti L, Kaalund SS, Hezemans FH, Jones PS, Savulich G, Hong YT, Fryer TD, Aigbirhio FI, O’Brien JT, Rowe JB (2022) Molecular pathology and synaptic loss in primary tauopathies: an 18F-AV-1451 and 11C-UCB-J PET study. Brain 145:340–348
Horta-Barba A, Pagonabarraga J, MartÃnez-Horta S, Busteed L, Pascual-Sedano B, Illán-Gala I, Marin-Lahoz J, Aracil-Bolaños I, Pérez-Pérez J, Sampedro F, Bejr-Kasem H, Kulisevsky J, (2021) Cognitive and behavioral profile of progressive supranuclear palsy and its phenotypes. J Neurol 268:3400–3408
Houlden H, Baker M, Morris HR, MacDonald N, Pickering-Brown S, Adamson J, Lees AJ, Rossor MN, Quinn NP, Kertesz A, Khan MN, Hardy J, Lantos PL, St George-Hyslop P, Munoz DG, Mann D, Lang AE, Bergeron C, Bigio EH, Litvan I, Bhatia KP, Dickson D, Wood NW, Hutton M (2001) Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology 56:1702–1706
Howard E, Ballinger S, Kinney NG, Balgenorth Y, Ehrhardt A, Phillips JS, Irwin DJ, Grossman M, Cousins KAQ (2022) Frontal atrophy and executive dysfunction relate to complex numbers impairment in progressive supranuclear palsy. J Alzheimers Dis 88:1553–1566
Im SY, Kim YE, Kim YJ (2015) Genetics of progressive supranuclear palsy. J Mov Disord 8:122–129
Iohan L, Lambert JC, Costa MR (2022) Analysis of modular gene co-expression networks reveals molecular pathways underlying Alzheimer’s disease and progressive supranuclear palsy. PLoS ONE 17:e0266405
Jabbari E, Holland N, Chelban V, Jones PS, Lamb R, Rawlinson C, Guo T, Costantini AA, Tan MMX, Heslegrave AJ, Roncaroli F, Klein JC, Ansorge O, Allinson KSJ, Jaunmuktane Z, Holton JL, Revesz T, Warner TT, Lees AJ, Zetterberg H, Russell LL, Bocchetta M, Rohrer JD, Williams NM, Grosset DG, Burn DJ, Pavese N, Gerhard A, Kobylecki C, Leigh PN, Church A, Hu MTM, Woodside J, Houlden H, Rowe JB, Morris HR (2020) Diagnosis across the spectrum of progressive supranuclear palsy and corticobasal syndrome. JAMA Neurol 77:377–387
Jabbari E, Koga S, Valentino RR, Reynolds RH, Ferrari R, Tan MMX, Rowe JB, Dalgard CL, Scholz SW, Dickson DW, Warner TT, Revesz T, Höglinger GU, Ross OA, Ryten M, Hardy J, Shoai M, Morris HR, (2021) Genetic determinants of survival in progressive supranuclear palsy: a genome-wide association study. Lancet Neurol 20:107–116
Jellinger KA (2008) Different tau pathology pattern in two clinical phenotypes of progressive supranuclear palsy. Neurodegener Dis 5:339–346
Jellinger KA (2019) Neuropathology and pathogenesis of extrapyramidal movement disorders: a critical update-I Hypokinetic-Rigid Movement Disorders. J Neural Transm (vienna) 126:933–995
Jellinger KA (2022) Morphological basis of Parkinson disease-associated cognitive impairment: an update. J Neural Transm (vienna) 129:977–999
Jia P, Zhang J, Han J, Ji Y (2022) Clinical outcomes and cognitive impairments between progressive supranuclear palsy and multiple system atrophy. Brain Behav 12:e2827
Jin J, Su D, Zhang J, Li X, Feng T (2023) Tau PET imaging in progressive supranuclear palsy: a systematic review and meta-analysis. J Neurol. https://doi.org/10.1007/s00415-022-11556-3
Josephs KA, Duffy JR (2008) Apraxia of speech and nonfluent aphasia: a new clinical marker for corticobasal degeneration and progressive supranuclear palsy. Curr Opin Neurol 21:688–692
Juncos JL, Hirsch EC, Malessa S, Duyckaerts C, Hersh LB, Agid Y (1991) Mesencephalic cholinergic nuclei in progressive supranuclear palsy. Neurology 41:25–30
Kasashima S, Oda Y (2003) Cholinergic neuronal loss in the basal forebrain and mesopontine tegmentum of progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol 105:117–124
Katzdobler S, Nitschmann A, Barthel H, Bischof G, Beyer L, Marek K, Song M, Wagemann O, Palleis C, Weidinger E, Nack A, Fietzek U, Kurz C, Häckert J, Stapf T, Ferschmann C, Scheifele M, Eckenweber F, Biechele G, Franzmeier N, Dewenter A, Schönecker S, Saur D, Schroeter ML, Rumpf JJ, Rullmann M, Schildan A, Patt M, Stephens AW, van Eimeren T, Neumaier B, Drzezga A, Danek A, Classen J, Bürger K, Janowitz D, Rauchmann BS, Stöcklein S, Perneczky R, Schöberl F, Zwergal A, Höglinger GU, Bartenstein P, Villemagne V, Seibyl J, Sabri O, Levin J, Brendel M (2023) Additive value of [(18)F]PI-2620 perfusion imaging in progressive supranuclear palsy and corticobasal syndrome. Eur J Nucl Med Mol Imaging 50:423–434
Kim SL, Lee MJ, Lee MS (2014) Cognitive dysfunction associated with falls in progressive supranuclear palsy. Gait Posture 40:605–609
Kobylecki C, Jones M, Thompson JC, Richardson AM, Neary D, Mann DM, Snowden JS, Gerhard A (2015) Cognitive-behavioural features of progressive supranuclear palsy syndrome overlap with frontotemporal dementia. J Neurol 262:916–922
Koga S, Parks A, Kasanuki K, Sanchez-Contreras M, Baker MC, Josephs KA, Ahlskog JE, Uitti RJ, Graff-Radford N, van Gerpen JA, Wszolek ZK, Rademakers R, Dickson DW (2017) Cognitive impairment in progressive supranuclear palsy is associated with tau burden. Mov Disord 32:1772–1779
Kovacs GG, Lukic MJ, Irwin DJ, Arzberger T, Respondek G, Lee EB, Coughlin D, Giese A, Grossman M, Kurz C, McMillan CT, Gelpi E, Compta Y, van Swieten JC, Laat LD, Troakes C, Al-Sarraj S, Robinson JL, Roeber S, Xie SX, Lee VM, Trojanowski JQ, Höglinger GU (2020) Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol 140:99–119
Lee YC, Williams DR, Anderson JFI (2018) Prospective characterization of cognitive function in typical and “brainstem predominant” progressive supranuclear palsy phenotypes. J Mov Disord 11:72–77
Litvan I, Hauw JJ, Bartko JJ, Lantos PL, Daniel SE, Horoupian DS, McKee A, Dickson D, Bancher C, Tabaton M, Jellinger K, Anderson DW (1996) Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol 55:97–105
Liu FT, Lu JY, Li XY, Liang XN, Jiao FY, Ge JJ, Wu P, Li G, Shen B, Wu B, Sun YM, Zhu YH, Luo JF, Yen TC, Wu JJ, Zuo CT, Wang J (2023a) (18)F-Florzolotau PET imaging captures the distribution patterns and regional vulnerability of tau pathology in progressive supranuclear palsy. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-00022-06104-00250
Liu G, Ni C, Zhan J, Li W, Luo J, Liao Z, Locascio JJ, Xian W, Chen L, Pei Z, Corvol JC, Maple-Grødem J, Campbell MC, Elbaz A, Lesage S, Brice A, Hung AY, Schwarzschild MA, Hayes MT, Wills AM, Ravina B, Shoulson I, Taba P, Kõks S, Beach TG, Cormier-Dequaire F, Alves G, Tysnes OB, Perlmutter JS, Heutink P, van Hilten JJ, Barker RA, Williams-Gray CH, Scherzer CR (2023b) Mitochondrial haplogroups and cognitive progression in Parkinson’s disease. Brain 146:42–49
Luca A, Nicoletti A, Donzuso G, Terravecchia C, Cicero CE, D’Agate C, Rascuná C, Manna R, Mostile G, Zappia M (2021) Phonemic verbal fluency and midbrain atrophy in progressive supranuclear palsy. J Alzheimers Dis 80:1669–1674
Macedo AC, Caramelli P, de Paula França Resende E, Mariano LI, Tumas V, Teixeira AL, Camargos ST, Cardoso FEC, de Souza LC, (2022a) Episodic memory in progressive supranuclear palsy: a neuropsychological and neuroimaging study. Neurol Sci 43:5363–5368
Macedo AC, Mariano LI, Martins MI, Friedlaender CV, Ventura JM, de Faria Rocha JV, Camargos ST, Cardoso FEC, Caramelli P, de Souza LC (2022b) Do patients with progressive supranuclear palsy have episodic memory impairment? A systematic review. Mov Disord Clin Pract 9:436–445
Malpetti M, Passamonti L, Rittman T, Jones PS, Vázquez Rodríguez P, Bevan-Jones WR, Hong YT, Fryer TD, Aigbirhio FI, O’Brien JT, Rowe JB (2020) Neuroinflammation and tau colocalize in vivo in progressive supranuclear palsy. Ann Neurol 88:1194–1204
Matsuoka K, Takado Y, Tagai K, Kubota M, Sano Y, Takahata K, Ono M, Seki C, Matsumoto H, Endo H, Shinotoh H, Sahara Y, Obata T, Near J, Kawamura K, Zhang MR, Suhara T, Shimada H, Higuchi M (2023) Two pathways differentially linking tau depositions, oxidative stress, and neuronal loss to apathetic phenotypes in progressive supranuclear palsy. J Neurol Sci 444:120514
Messerschmidt K, Barthel H, Brendel M, Scherlach C, Hoffmann KT, Rauchmann BS, Rullmann M, Marek K, Villemagne VL, Rumpf JJ, Saur D, Schroeter ML, Schildan A, Patt M, Beyer L, Song M, Palleis C, Katzdobler S, Fietzek UM, Respondek G, Scheifele M, Nitschmann A, Zach C, Barret O, Madonia J, Russell D, Stephens AW, Koglin N, Roeber S, Herms J, Bötzel K, Bartenstein P, Levin J, Seibyl JP, Höglinger G, Classen J, Sabri O (2022) (18)F-PI-2620 Tau PET Improves the Imaging Diagnosis of Progressive Supranuclear Palsy. J Nucl Med 63:1754–1760
Montalbano M, Majmundar L, Sengupta U, Fung L, Kayed R (2023) Pathological tau signatures and nuclear alterations in neurons, astrocytes and microglia in Alzheimer’s disease, progressive supranuclear palsy, and dementia with Lewy bodies. Brain Pathol 33:e13112
Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, Rademakers R, Alafuzoff I, Attems J, Brayne C, Coyle-Gilchrist ITS, Chui HC, Fardo DW, Flanagan ME, Halliday G, Hokkanen SRK, Hunter S, Jicha GA, Katsumata Y, Kawas CH, Keene CD, Kovacs GG, Kukull WA, Levey AI, Makkinejad N, Montine TJ, Murayama S, Murray ME, Nag S, Rissman RA, Seeley WW, Sperling RA, White CL 3rd, Yu L, Schneider JA (2019) Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain 142:1503–1527
Nicastro N, Rodriguez PV, Malpetti M, Bevan-Jones WR, Simon Jones P, Passamonti L, Aigbirhio FI, O’Brien JT, Rowe JB (2020) (18)F-AV1451 PET imaging and multimodal MRI changes in progressive supranuclear palsy. J Neurol 267:341–349
Ono Y, Higashida K, Yoshikura N, Hayashi Y, Kimura A, Iwasaki Y, Yoshida M, Shimohata T (2022) Progressive supranuclear palsy with predominant frontal presentation exhibiting progressive nonfluent aphasia due to crossed aphasia. Neuropathology 42:232–238
Pan P, Liu Y, Zhang Y, Zhao H, Ye X, Xu Y (2017) Brain gray matter abnormalities in progressive supranuclear palsy revisited. Oncotarget 8:80941–80955
Parthimos TP, Schulpis KH (2020) The progressive supranuclear palsy: past and present aspects. Clin Gerontol 43:155–180
Passamonti L, Vázquez Rodríguez P, Hong YT, Allinson KS, Williamson D, Borchert RJ, Sami S, Cope TE, Bevan-Jones WR, Jones PS, Arnold R, Surendranathan A, Mak E, Su L, Fryer TD, Aigbirhio FI, O’Brien JT, Rowe JB (2017) 18F-AV-1451 positron emission tomography in Alzheimer’s disease and progressive supranuclear palsy. Brain 140:781–791
Pellicano C, Assogna F, Cellupica N, Piras F, Pierantozzi M, Stefani A, Cerroni R, Mercuri B, Caltagirone C, Pontieri FE, Spalletta G (2017) Neuropsychiatric and cognitive profile of early Richardson’s syndrome, Progressive Supranuclear Palsy-parkinsonism and Parkinson’s disease. Parkinsonism Relat Disord 45:50–56
Piattella MC, Tona F, Bologna M, Sbardella E, Formica A, Petsas N, Filippini N, Berardelli A, Pantano P (2015a) Disrupted resting-state functional connectivity in progressive supranuclear palsy. AJNR Am J Neuroradiol 36:915–921
Piattella MC, Upadhyay N, Bologna M, Sbardella E, Tona F, Formica A, Petsas N, Berardelli A, Pantano P (2015b) Neuroimaging evidence of gray and white matter damage and clinical correlates in progressive supranuclear palsy. J Neurol 262:1850–1858
Picillo M, Cuoco S, Tepedino MF, Cappiello A, Volpe G, Erro R, Santangelo G, Pellecchia MT, Barone P (2019) Motor, cognitive and behavioral differences in MDS PSP phenotypes. J Neurol 266:1727–1735
Pillon B, Dubois B, Ploska A, Agid Y (1991) Severity and specificity of cognitive impairment in Alzheimer’s, Huntington’s, and Parkinson’s diseases and progressive supranuclear palsy. Neurology 41:634–643
Pilotto A, Gazzina S, Benussi A, Manes M, Dell’Era V, Cristillo V, Cosseddu M, Turrone R, Alberici A, Padovani A, Borroni B (2017) Mild cognitive impairment and progression to dementia in progressive supranuclear palsy. Neurodegener Dis 17:286–291
Quattrone A, Nicoletti G, Messina D, Fera F, Condino F, Pugliese P, Lanza P, Barone P, Morgante L, Zappia M, Aguglia U, Gallo O (2008) MR imaging index for differentiation of progressive supranuclear palsy from Parkinson disease and the Parkinson variant of multiple system atrophy. Radiology 246:214–221
Ransmayr L, Fuchs A, Ransmayr-Tepser S, Kommenda R, Kögl M, Schwingenschuh P, Fellner F, Guger M, Eggers C, Darkow R, Mangesius S, Ransmayr G (2022) Differences in aphasia syndromes between progressive supranuclear palsy-Richardson’s syndrome, behavioral variant frontotemporal dementia and Alzheimer’s dementia. J Neural Transm (vienna) 129:1039–1048
Respondek G, Stamelou M, Kurz C, Ferguson LW, Rajput A, Chiu WZ, van Swieten JC, Troakes C, Al Sarraj S, Gelpi E, Gaig C, Tolosa E, Oertel WH, Giese A, Roeber S, Arzberger T, Wagenpfeil S, Höglinger GU (2014) The phenotypic spectrum of progressive supranuclear palsy: a retrospective multicenter study of 100 definite cases. Mov Disord 29:1758–1766
Respondek G, Kurz C, Arzberger T, Compta Y, Englund E, Ferguson LW, Gelpi E, Giese A, Irwin DJ, Meissner WG, Nilsson C, Pantelyat A, Rajput A, van Swieten JC, Troakes C, Josephs KA, Lang AE, Mollenhauer B, Müller U, Whitwell JL, Antonini A, Bhatia KP, Bordelon Y, Corvol JC, Colosimo C, Dodel R, Grossman M, Kassubek J, Krismer F, Levin J, Lorenzl S, Morris H, Nestor P, Oertel WH, Rabinovici GD, Rowe JB, van Eimeren T, Wenning GK, Boxer A, Golbe LI, Litvan I, Stamelou M, HÃöglinger GU, (2017) Which ante mortem clinical features predict progressive supranuclear palsy pathology? Mov Disord 32:995–1005
Ries V, Oertel WH, Höglinger GU (2011) Mitochondrial dysfunction as a therapeutic target in progressive supranuclear palsy. J Mol Neurosci 45:684–689
Robinson JL, Yan N, Caswell C, Xie SX, Suh E, Van Deerlin VM, Gibbons G, Irwin DJ, Grossman M, Lee EB, Lee VM, Miller B, Trojanowski JQ (2020) Primary tau pathology, not copathology, correlates with clinical symptoms in PSP and CBD. J Neuropathol Exp Neurol 79:296–304
Rogozinski S, Klietz M, Respondek G, Oertel WH, Grothe MJ, Pereira JB, Höglinger GU (2022) Reduction in volume of nucleus basalis of Meynert is specific to Parkinson’s disease and progressive supranuclear palsy but not to multiple system atrophy. Front Aging Neurosci 14:851788
Rosskopf J, Gorges M, Müller HP, Lulé D, Uttner I, Ludolph AC, Pinkhardt E, Juengling FD, Kassubek J, (2017) Intrinsic functional connectivity alterations in progressive supranuclear palsy: Differential effects in frontal cortex, motor, and midbrain networks. Mov Disord 32:1006–1015
Saint-Aubert L, Lemoine L, Chiotis K, Leuzy A, Rodriguez-Vieitez E, Nordberg A (2017) Tau PET imaging: present and future directions. Mol Neurodegener 12:19
Sakae N, Josephs KA, Litvan I, Murray ME, Duara R, Uitti RJ, Wszolek ZK, Graff-Radford NR, Dickson DW (2019) Neuropathologic basis of frontotemporal dementia in progressive supranuclear palsy. Mov Disord 34:1655–1662
Sakamoto R, Tsuchiya K, Yoshida R, Itoh Y, Furuta N, Kosuga A, Sugai Y, Mimura M (2009) Progressive supranuclear palsy combined with Alzheimer’s disease: a clinicopathological study of two autopsy cases. Neuropathology 29:219–229
Samaranch L, Cervantes S, Barabash A, Alonso A, Cabranes JA, Lamet I, Ancín I, Lorenzo E, Martínez-Lage P, Marcos A, Clarimón J, Alcolea D, Lleó A, Blesa R, Gómez-Isla T, Pastor P (2010) The effect of MAPT H1 and APOE epsilon 4 on transition from mild cognitive impairment to dementia. J Alzheimers Dis 22:1065–1071
Schapira AH (2011) Aetiopathogenesis of Parkinson’s disease. J Neurol 258:S307-310
Schofield EC, Hodges JR, Bak TH, Xuereb JH, Halliday GM (2012) The relationship between clinical and pathological variables in Richardson’s syndrome. J Neurol 259:482–490
Scotton WJ, Bocchetta M, Todd E, Cash DM, Oxtoby N, VandeVrede L, Heuer H, Alexander DC, Rowe JB, Morris HR, Boxer A, Rohrer JD, Wijeratne PA (2022) A data-driven model of brain volume changes in progressive supranuclear palsy. Brain Commun 4:fcac098
Shoeibi A, Olfati N, Litvan I (2019) Frontrunner in translation: progressive supranuclear palsy. Front Neurol 10:1125
Sintini I, Schwarz CG, Senjem ML, Reid RI, Botha H, Ali F, Ahlskog JE, Jack CR Jr, Lowe VJ, Josephs KA, Whitwell JL (2019) Multimodal neuroimaging relationships in progressive supranuclear palsy. Parkinsonism Relat Disord 66:56–61
Smith DT, Archibald N (2020) Spatial working memory in Progressive Supranuclear Palsy. Cortex 122:115–122
Smith DT, Casteau S, Archibald N (2021) Spatial attention and spatial short term memory in PSP and Parkinson’s disease. Cortex 137:49–60
Stang CD, Turcano P, Mielke MM, Josephs KA, Bower JH, Ahlskog JE, Boeve BF, Martin PR, Upadhyaya SG, Savica R (2020) Incidence and trends of progressive supranuclear palsy and corticobasal syndrome: a population-based study. J Parkinsons Dis 10:179–184
Stefansson H, Helgason A, Thorleifsson G, Steinthorsdottir V, Masson G, Barnard J, Baker A, Jonasdottir A, Ingason A, Gudnadottir VG, Desnica N, Hicks A, Gylfason A, Gudbjartsson DF, Jonsdottir GM, Sainz J, Agnarsson K, Birgisdottir B, Ghosh S, Olafsdottir A, Cazier JB, Kristjansson K, Frigge ML, Thorgeirsson TE, Gulcher JR, Kong A, Stefansson K (2005) A common inversion under selection in Europeans. Nat Genet 37:129–137
Stezin A, Lenka A, Jhunjhunwala K, Saini J, Pal PK (2017) Advanced structural neuroimaging in progressive supranuclear palsy: Where do we stand? Parkinsonism Relat Disord 36:19–32
Street D, Malpetti M, Rittman T, Ghosh BCP, Murley AG, Coyle-Gilchrist I, Passamonti L, Rowe JB (2021) Clinical progression of progressive supranuclear palsy: impact of trials bias and phenotype variants. Brain Commun 3:fcab206
Sulena GD, Sharma AK, Kumar N (2017) Clinical profile of cognitive decline in patients with Parkinson’s disease, progressive supranuclear palsy, and multiple system atrophy. J Neurosci Rural Pract 8:562–568
Suzuki M, Desmond TJ, Albin RL, Frey KA (2002) Cholinergic vesicular transporters in progressive supranuclear palsy. Neurology 58:1013–1018
Tomše P, Rebec E, Studen A, Perovnik M, Rus T, Ležaic L, Tang CC, Eidelberg D, Trošt M (2022) Abnormal metabolic covariance patterns associated with multiple system atrophy and progressive supranuclear palsy. Phys Med 98:131–138
Wang X, Allen M, Is Ö, Reddy JS, Tutor-New FQ, Castanedes Casey M, Carrasquillo MM, Oatman SR, Min Y, Asmann YW, Funk C, Nguyen T, Ho CC, Malphrus KG, Seyfried NT, Levey AI, Younkin SG, Murray ME, Dickson DW, Price ND, Golde TE, Ertekin-Taner N (2022) Alzheimer’s disease and progressive supranuclear palsy share similar transcriptomic changes in distinct brain regions. J Clin Invest 132:e149904
Warren NM, Piggott MA, Lees AJ, Burn DJ (2007a) The basal ganglia cholinergic neurochemistry of progressive supranuclear palsy and other neurodegenerative diseases. J Neurol Neurosurg Psychiatry 78:571–575
Warren NM, Piggott MA, Lees AJ, Burn DJ (2007b) Muscarinic receptors in the thalamus in progressive supranuclear palsy and other neurodegenerative disorders. J Neuropathol Exp Neurol 66:399–404
Warren NM, Piggott MA, Lees AJ, Perry EK, Burn DJ (2008) Intact coupling of M1 receptors and preserved M2 and M4 receptors in the cortex in progressive supranuclear palsy: contrast with other dementias. J Chem Neuroanat 35:268–274
Wen Y, Zhou Y, Jiao B, Shen L (2021) Genetics of progressive supranuclear palsy: a review. J Parkinsons Dis 11:93–105
Whitwell JL, Jack CR Jr, Parisi JE, Gunter JL, Weigand SD, Boeve BF, Ahlskog JE, Petersen RC, Dickson DW, Josephs KA (2013) Midbrain atrophy is not a biomarker of progressive supranuclear palsy pathology. Eur J Neurol 20:1417–1422
Whitwell JL, Lowe VJ, Tosakulwong N, Weigand SD, Senjem ML, Schwarz CG, Spychalla AJ, Petersen RC, Jack CR Jr, Josephs KA (2017) [(18) F]AV-1451 tau positron emission tomography in progressive supranuclear palsy. Mov Disord 32:124–133
Whitwell JL, Tosakulwong N, Botha H, Ali F, Clark HM, Duffy JR, Utianski RL, Stevens CA, Weigand SD, Schwarz CG, Senjem ML, Jack CR, Lowe VJ, Ahlskog JE, Dickson DW, Josephs KA (2020) Brain volume and flortaucipir analysis of progressive supranuclear palsy clinical variants. Neuroimage Clin 25:102152
Williams DR, Lees AJ (2009) Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol 8:270–279
Williams DR, Holton JL, Strand C, Pittman A, de Silva R, Lees AJ, Revesz T (2007) Pathological tau burden and distribution distinguishes progressive supranuclear palsy-parkinsonism from Richardson’s syndrome. Brain 130:1566–1576
Worker A, Blain C, Jarosz J, Chaudhuri KR, Barker GJ, Williams SC, Brown RG, Leigh PN, Dell’Acqua F, Simmons A (2014) Diffusion tensor imaging of Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy: a tract-based spatial statistics study. PLoS ONE 9:e112638
Yamauchi H, Fukuyama H, Nagahama Y, Katsumi Y, Dong Y, Konishi J, Kimura J (1997) Atrophy of the corpus callosum, cognitive impairment, and cortical hypometabolism in progressive supranuclear palsy. Ann Neurol 41:606–614
Yokota O, Davidson Y, Bigio EH, Ishizu H, Terada S, Arai T, Hasegawa M, Akiyama H, Sikkink S, Pickering-Brown S, Mann DM (2010) Phosphorylated TDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol 120:55–66
Zhang L, Toyoshima Y, Takeshima A, Shimizu H, Tomita I, Onodera O, Takahashi H, Kakita A (2021) Progressive supranuclear palsy: neuropathology of patients with a short disease duration due to unexpected death. Neuropathology 41:174–182
Acknowledgements
The author thanks Mr. E. Mitter-Ferstl for secretarial and editorial work.
Funding
The study was funded by the Society for the Promotion of Research in Experimental Neurology, Vienna, Austria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jellinger, K.A. Pathomechanisms of cognitive impairment in progressive supranuclear palsy. J Neural Transm 130, 481–493 (2023). https://doi.org/10.1007/s00702-023-02613-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-023-02613-w