Abstract
Cognitive impairment is common in patients with the neurodegenerative tauopathy progressive supranuclear palsy (PSP). Although a pattern of ‘subcortical’ cognitive impairment is considered prototypical in PSP, pathological and clinical observations suggest an overlap with frontotemporal dementia (FTD). Our objective was to evaluate behavioural and cognitive symptoms in a retrospective study of patients with PSP syndrome (PSPS) and their relationship to features seen in behavioural variant FTD. We reviewed the records of 62 patients (29 male, 33 female, median age 65.5 years) evaluated at a tertiary cognitive clinic who met NINDS–SPSP criteria for probable or possible PSP, and collected clinical details of their presenting history, cognitive and behavioural features. We also evaluated the proportion of patients fulfilling FTD Consensus criteria. Cognitive and behavioural symptoms were a predominant presenting feature in 58 % of patients evaluated. Cognitive slowing, executive impairments, and inefficient memory recall, consistent with ‘subcortical’ impairment, were identified in the majority of patients. Twenty patients (32 %) fulfilled cognitive and behavioural criteria for possible FTD at initial assessment, whereas behavioural changes not meeting formal diagnostic criteria were present in a greater proportion of the patients. Our findings support the existence of a spectrum of cognitive–behavioural features in PSPS, with significant clinical overlap with behavioural variant FTD. Cognitive and behavioural profiling should be an integral part of the assessment of patients with PSPS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Progressive supranuclear palsy (PSP) is a neurodegenerative disorder characterised by vertical gaze palsy, early postural instability with falls and cognitive decline [17, 28]. The pathological substrate of PSP includes tau-positive neurofibrillary tangles (NFTs), neuropil threads and tufted astrocytes within the basal ganglia, midbrain structures and dentate nucleus [31]. The clinical spectrum of PSP is wider than originally described, with several distinct clinical subtypes now recognised [31]. Heterogeneity in the severity and anatomical distribution of tau pathology appears to underlie these distinctions, with a greater tau burden and more aggressive course in PSP-Richardson’s syndrome compared to PSP-parkinsonism [30].
Cognitive impairment occurs in the majority of patients with pathologically confirmed PSP [6]. Its profile is classically described as ‘subcortical’ in type, characterised by prominent slowing, impaired mental manipulation and inefficient memory recall (as opposed to true amnesia with loss of information over a delay) [2]. Consistent with this concept, PSP patients have been reported to show prolonged initial thinking time on problem-solving tasks and poor performance on timed verbal fluency tasks [24]. However, more recently cortical features, such as apraxia and aphasia, have been documented in a proportion of patients with PSP [13, 27]. Pathological data in several studies suggest the development of dementia in PSP to be more strongly linked to cortical than subcortical tau pathology [5].
There is increasing evidence for a link between PSP and behavioural variant frontotemporal dementia (bvFTD), a neurodegenerative disorder characterised by impaired regulation of conduct, loss of insight and emotional blunting [21, 22]. Tau pathology is the underlying substrate for bvFTD in around 40 % of cases, suggesting a potential clinical overlap with PSP and other tauopathies such as corticobasal degeneration (CBD) [14]. Behavioural symptoms seen in bvFTD, such as apathy and disinhibition, occur more prominently in patients with PSP compared to Alzheimer’s and Parkinson’s disease [1, 19]. A large cross-sectional study reported a predominantly cognitive–behavioural, as opposed to motor, presentation in 20 % of patients with PSP [9]. These findings suggest a spectrum of cognitive–behavioural disturbance in PSP overlapping with that seen in bvFTD. Recent consensus diagnostic criteria for bvFTD are reported to show good sensitivity and specificity [11, 22]. However, to what extent patients with PSP might fulfill behavioural and cognitive criteria for bvFTD is unclear.
In this retrospective study, we aimed to evaluate the link between the clinical PSP syndrome (PSPS) and bvFTD. We predicted a spectrum of cognitive–behavioural impairment in patients with clinically diagnosed PSP, ranging from mild or no cognitive impairment to marked behavioural and executive impairments fulfilling bvFTD consensus (FTDC) criteria. We also aimed to assess differences between patients presenting with predominantly cognitive–behavioural features compared to those with more typical presentations of PSP.
Methods
Participants
We performed a retrospective review of data acquired in the course of clinical care of patients seen in the Cerebral Function Unit, a tertiary cognitive clinic, at Greater Manchester Neurosciences Centre, UK. Patients were considered for inclusion in the study if they had a clinical diagnosis of PSP recorded at some time during the disease course, and met NINDS–SPSP criteria for possible or probable PSP [17] at some stage during their clinical follow-up in the unit. We did not apply the exclusion criterion of ‘lobar atrophy’ on imaging given the possible overlap with diagnostic features of bvFTD.
In total, 110 patients with a clinical diagnosis of PSP were identified. Of these, clinical records were unavailable in 9, there was insufficient information on clinical features in 20, and a more likely alternative diagnosis in 19 cases. The records of 62 patients (29 male, 33 female) meeting NINDS–SPSP criteria were further analysed. To provide additional information on diagnostic certainty, the NNIPPS criteria for PSP were retrospectively applied to all patients [4]. A pathological diagnosis was available in seven patients: six had confirmed PSP, whilst one had findings of PSP–CBD overlap pathology. Brains were obtained following ethical approval and pre-mortem informed written consent from the patient, or advice from a consultee (usually the next of kin) for patients lacking capacity as per the Mental Capacity Act 2005.
Data collection and definitions
Data were drawn from the clinical notes that included a semi-structured cognitive history, taken both from the patient and an informant, physical and neurological examination, neuropsychological testing and reports from neuroimaging investigations. Demographic characteristics and information on presenting complaint and behavioural features at the time of presentation were recorded. The presenting complaint was defined as ‘cognitive–behavioural’ when no more than one other physical symptom occurred at presentation as described in a previous study [9]. Motor disability was quantified by the Hoehn and Yahr score. Data not explicitly mentioned in the case notes were coded as ‘missing’ as indicated in the tables below.
Neuropsychological assessment was carried out using the Manchester Neuropsychological Profile [29], which has been found to be valuable in distinguishing forms of dementia [25]. Memory was assessed as ‘inefficient’ when recall, though impaired, could be improved with cueing without loss of information over a delay; an ‘amnesic’ picture was recorded when marked loss over a delay was present. Executive dysfunction was defined on the basis of impairment on tests of verbal fluency, set shifting and sequencing. The presence of disorientation, word-finding difficulties, impaired sentence comprehension and perceptuospatial impairment (assessed by line drawing, identification of faces, and a visual object and perception battery), as documented in the neuropsychological assessment report, was recorded [29]. Praxis was assessed as slowed (i.e., predominantly motor impairment) or impaired (i.e., inaccurate or spatially degraded) by performance of limb actions and non-representational hand postures. Cognitive domains were recorded as ‘impaired’ or ‘preserved’ on the basis of the neuropsychologists’ conclusions in their clinical neuropsychological reports, and were, therefore, independent and unbiased by presumptions associated with the present study. Impairment was determined by performance below the 5th percentile, based on published norms where available, or outside the normal range of performance shown by age-matched healthy controls.
To investigate the overlap between PSPS and bvFTD, cognitive and behavioural FTDC criteria were applied based on the clinical information available for each patient at presentation [22]. This analysis was performed by a specialist movement disorder neurologist with training in cognitive neurology (C.K.), who was not involved in the initial clinical or neuropsychological assessment of patients. Patients were classified as ‘possible bvFTD’ if they showed evidence of three or more cognitive–behavioural features. As imaging data were not available for all patients, and were not used in the PSP diagnosis, we did not apply the criteria for ‘probable bvFTD’ which are mainly based on the presence of characteristic imaging findings.
Statistical analysis
Statistical analysis was performed using SPSS 20 (IBM Software). Demographic characteristics of patients with different presenting symptoms were compared using student’s t test or Mann–Whitney U test according to normality of distribution of variables. Categorical data were compared using a Chi-squared test. A significance level of P < 0.05 was used for all analyses with Bonferroni correction for multiple comparisons.
Results
Patient demographics and background clinical information
The median age of patients at initial evaluation was 65.5 years (range 48–78), with a median disease duration of 2.25 years (0.5–10). The median MMSE score was 25 (range 11–29). Median Hoehn and Yahr score at initial assessment was 3.0 (range 2.0–5.0).
Modes of presentation of patients with PSPS
Cognitive–behavioural symptoms were a predominant feature at presentation in 36 patients (58 %), and were the exclusive presenting complaint in 17 (27 %). Behavioural or personality change was the most common presenting feature in this group (n = 18). Falls or gait disorder (n = 20) and other motor features were the predominant presenting feature in patients with a non-cognitive–behavioural presentation. There was no significant difference in median age in patients with a cognitive–behavioural vs. motor presentation (64.0 vs. 69.5 years, P > 0.05). However, those with a cognitive–behavioural presentation were assessed at a shorter median disease duration of 2.0 years (range 0.5–8) vs. 4.0 (1–10) (P < 0.05) at first evaluation compared to those presenting with mainly motor features. There were no significant differences between these two groups in MMSE score, digit span, verbal fluency or performance on Weigl’s block tests (P > 0.05). There was no significant difference between the two groups in proportion of patients with probable or possible PSP.
Cognitive features at initial assessment
The cognitive features at initial assessment are summarised in Table 1. Findings compatible with ‘subcortical’ impairment, such as cognitive slowing and executive impairment, were present in the majority of patients. Inefficient memory recall was a common finding, but few patients showed features of true amnesia. Whilst word finding was impaired in around 30 % of patients, comprehension was relatively preserved. Whereas slowing of movements was common, true impairment of praxis was uncommonly seen. There was no significant difference in the proportion of patients with motor vs. cognitive–behavioural presentations of PSPS showing impairment in the domains listed above, including praxis (P > 0.05 in all cases).
Behavioural overlap with FTD
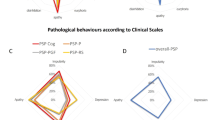
Thirty-two (52 %) patients displayed more than one early cognitive–behavioural abnormality as defined in the FTDC criteria [22]. The variation in number of early behavioural symptoms is illustrated in Fig. 1. Apathy was present in 50 % of patients, whilst other behavioural features such as disinhibition, perseverative behaviours and hyperorality occurred less commonly (Table 2). Twenty patients (32 %) fulfilled diagnostic criteria for possible bvFTD, with three or more clinical criteria, including executive dysfunction in 13 cases. All but five of these met NINDS–SPSP criteria for probable or possible PSP at presentation, whilst all met PSP criteria during their disease course. Of the seven patients with pathologically confirmed PSP, two met clinical FTDC criteria; three others with a cognitive–behavioural presentation did not fulfill the criteria. The proportion of patients meeting the varying criteria for PSP and bvFTD during their disease course is summarised in Fig. 2.
Behavioural impairment not meeting the formal FTDC definition of ‘early’ (within 3 years of presentation) was noted at initial assessment in a proportion of patients; for example, apathy was present in an additional eight cases at initial assessment. Lack of insight, whilst not a formal FTDC criterion, was common, recorded in 35 of 57 patients (61 %). Follow-up data on behavioural features were available for 28 patients, for a median duration of 1.8 years (range 0.3–6.4). During this time, additional behavioural features were reported in 12 patients, six of whom had initially met criteria for bvFTD. The development of apathy (5 cases) and perseverative symptoms (5) were most common.
Discussion
Our findings in a clinically diagnosed series of patients with PSPS suggest that cognitive or behavioural features are common and may occur as the predominant feature at presentation. We observed a spectrum of impairment ranging from mild cognitive symptoms to fulfillment of clinical diagnostic criteria for bvFTD.
Cognitive and behavioural presentation in PSPS
We identified cognitive slowing, inefficient memory recall and executive dysfunction in the majority of our cohort, consistent with a degree of ‘subcortical’ impairment. Prominent behavioural features were also seen in a significant number; moreover, cognitive–behavioural symptoms were the presenting feature in almost two-thirds of patients. This is similar to findings from a large prospective study of over 300 patients with PSP, of whom 80 % were cognitively impaired, with two or more domains affected in 40 % [6]. The most frequent and marked impairments were in the domains of initiation and perseveration, including verbal fluency, and memory [6]; however, that study did not address behavioural change. The demographic characteristics of patients in that study were comparable to those reported here. In a further large cross-sectional study of patients with PSP, those presenting with cognitive–behavioural symptoms were younger, with a longer latency to diagnosis, but their overall disease course did not differ from those presenting with mainly motor features [9]. The mean disease duration (5.4 years) and motor severity of those patients were higher than reported here. In this previous study, the diagnosis of bvFTD had initially been considered in around 30 % of patients with cognitive–behavioural presentations of PSP [9], although the precise symptomatology of the patients was also not addressed in detail. We did not observe significant differences in demographics or cognitive profile between patients with motor vs. cognitive–behavioural onset, although those with cognitive–behavioural presentations were assessed at a shorter disease duration.
The cognitive profile of patients with PSP has been encapsulated by the concept of the ‘subcortical syndrome’, in which prominent slowing and inefficient recall are emphasised, although behavioural features other than apathy were not noted [2]. Our work suggests significant behavioural impairment in a substantial proportion of patients with clinically diagnosed PSPS at a relatively early stage in the disease course, at a frequency consistent with previous reports. Litvan and colleagues reported a study using the neuropsychiatric inventory (NPI) in 22 patients with PSP with disease duration of 4.5 years; moderate to severe apathy, more severe than in Alzheimer’s disease, was seen in 80 % of patients whilst disinhibition was evident in 36 % [19]. Similarly, PSP patients exhibit greater apathy and disinhibition scores on the NPI compared to those with Parkinson’s disease [1]. Our work adds to these studies by providing useful insight into early symptomatology not afforded from studies in which the disease duration at assessment was typically longer and reflective of more advanced patients [9, 19].
Methodological considerations
One of the principal limitations of our study is the absence of pathological confirmation in all cases studied. We, therefore, cannot exclude diagnostic uncertainty. The clinical syndrome of PSP has good predictive value for underlying tau pathology as opposed to other, non-tau FTLD pathological entities, although the possibility of CBD pathology cannot be excluded [15]. Other coexistent pathology may also have influenced the cognitive–behavioural presentation. The NINDS–SPSP criteria have been shown to have 93–99 and 100 % specificity for ‘possible’ and ‘probable’ PSP, respectively, in two clinicopathological studies [18, 20]. We acknowledge, however, that these findings have yet to be validated in larger studies, and that more recent work has suggested a lower specificity of these criteria [23]. Whereas twelve patients (one subsequently pathologically confirmed) in our series did not meet the NNIPPS criteria, in eight cases this was due to the disease duration longer than 8 years rather than atypical clinical features. We, therefore, feel that the overall validity of our study is not compromised by the use of the NINDS–SPSP criteria despite the lack of pathological confirmation in all cases. Given the documented patterns of cortical atrophy and hypometabolism seen in PSP, and the potential overlap with bvFTD, we did not apply the criterion of atrophy seen on imaging to our patient cohort, and to ensure consistency did not apply the imaging criteria to the bvFTD criteria either.
There is also a possibility of selection bias, in that patients with cognitive complaints early in their disease course are likely to have been disproportionately referred given our specialist interest. This may also account for the shorter disease duration observed in the ‘cognitive–behavioural’ group and lead to overestimation of the proportion of patients presenting with cognitive and behavioural problems. However, this also represents a relative strength of the study, in that cognitive and behavioural problems were explored in detail, as compared to other studies in PSP in which the movement disorder may have been the main focus of investigation. The fact that this was not a prospective study means that the data collected were not identical for all patients; however, the use of a standardised template for collection of historical and neuropsychological data should help to mitigate against this limitation. More prospective data on the evolution of cognitive and behavioural changes from unselected populations of patients with PSPS would help to clarify the true prevalence of these symptoms.
Clinical overlap between PSPS and bvFTD
Recent work analysing the FTDC criteria excluded patients with PSPS for methodological reasons, but noted the importance of examining to what extent patients with PSPS might fulfill criteria for bvFTD [11]. Our findings suggest a spectrum of early behavioural disturbance in PSP significantly overlapping with bvFTD, as evidenced by the fact that over 30 % of our cohort met clinical criteria for possible bvFTD and a further significant proportion exhibited bvFTD-like behavioural characteristics, albeit insufficient to formally satisfy these criteria. Our findings contrast with a comparative study, which suggested that behavioural features other than apathy are rare in PSP compared to bvFTD; however, the behavioural features assessed were not identical to those in the current study [3]. Significant overlap between PSP and bvFTD in other neuropsychological domains, such as emotion recognition, has also been identified [10]. Our findings suggest that the behavioural changes seen in PSP are not simply an inevitable consequence of disease progression, but support the existence of a ‘PSP-FTD’ subtype, as recently proposed [8]. The percentage of patients exhibiting cognitive impairment did not differ in those with cognitive–behavioural vs. motor presentations. The study did not compare detailed quantitative and qualitative characteristics of performance in the two subgroups, so inferences are necessarily limited. Nevertheless, the finding highlights the fact that a degree of cognitive impairment may reasonably be considered an integral part of PSP whereas frank ‘frontal’ behavioural change may be limited to a subgroup of patients. Further prospective longitudinal research in patients with PSPS would help to determine the relative weighting of cognitive and behavioural features and their underlying substrates. The available longitudinal data suggest that behavioural disturbance may evolve over time in patients with PSPS, indicating the importance of prospective longitudinal studies better to characterise the evolution of these changes. Limited longitudinal data on cognitive dysfunction in PSP suggest a more rapid rate of deterioration compared to other parkinsonian syndromes, possibly predicated on greater rates of atrophy in frontal and subcortical structures [26]. A better knowledge of the behavioural progression of PSP would aid in identifying strategies to manage such disturbances.
Clinicopathological studies indicate that 4-repeat (4R) tauopathies such as PSP and CBD are the underlying pathological substrate in a proportion of patients with bvFTD [15]. Patients with bvFTD and confirmed 4R tauopathies exhibit significantly greater apathy compared to 3R tauopathies such as Pick’s disease, as well as impaired planning and judgment, a finding that is thought to reflect a greater tau burden in the basal ganglia in 4R vs. 3R tauopathies [12]. It has been demonstrated that cortical pathological tau burden is higher in PSP patients with cognitive and behavioural changes [5], whereas patients with the PSP-parkinsonism phenotype exhibit considerably lower tau burden compared to ‘classical’ PSP [30]. Whilst we did not have sufficient pathological data to confirm a greater frontal tau burden in our patients fulfilling criteria for FTD, it is likely that differential distribution of pathology underlies to some extent the varied presentations seen here. Behavioural features, particularly apathy, have been linked to frontal and putaminal atrophy in imaging studies of PSP [16]. A common area of midcingulate hypoperfusion is reported in an [99Tc]-HMPAO SPECT study of patients with PSP and tau-positive FTD [7], and taken together these results suggest a common structural and functional substrate for the overlaps in cognitive and behavioural symptoms of PSP and bvFTD.
In conclusion, our findings support the concept of a spectrum of cognitive–behavioural disturbance in PSPS significantly overlapping with that seen in bvFTD. Cognitive and behavioural profiling should be an integral component of clinical assessment of patients with suspected PSP. Prospective longitudinal studies with pathological confirmation would improve our understanding of the evolution of these disabling symptoms in the time course of the disease.
References
Aarsland D, Litvan I, Larsen JP (2001) Neuropsychiatric symptoms of patients with progressive supranuclear palsy and Parkinson’s disease. J Neuropsychiatry Clin Neurosci 13:42–49
Albert ML, Feldman RG, Willis AL (1974) The ‘subcortical dementia’ of progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 37:121–130
Bak TH, Crawford LM, Berrios G, Hodges JR (2010) Behavioural symptoms in progressive supranuclear palsy and frontotemporal dementia. J Neurol Neurosurg Psychiatry 81:1057–1059
Bensimon G, Ludolph A, Agid Y, Vidailhet M, Payan C, Leigh PN (2009) Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study. Brain 132:156–171
Bigio EH, Brown DF, White CL 3rd (1999) Progressive supranuclear palsy with dementia: cortical pathology. J Neuropathol Exp Neurol 58:359–364
Brown RG, Lacomblez L, Landwehrmeyer BG, Bak T, Uttner I, Dubois B, Agid Y, Ludolph A, Bensimon G, Payan C, Leigh NP (2010) Cognitive impairment in patients with multiple system atrophy and progressive supranuclear palsy. Brain 133:2382–2393
Chiu WZ, Papma JM, de Koning I, Donker Kaat L, Seelaar H, Reijs AE, Valkema R, Hasan D, Boon AJ, van Swieten JC (2012) Midcingulate involvement in progressive supranuclear palsy and tau positive frontotemporal dementia. J Neurol Neurosurg Psychiatry 83:910–915
Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA (2010) Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol 23:394–400
Donker Kaat L, Boon AJ, Kamphorst W, Ravid R, Duivenvoorden HJ, van Swieten JC (2007) Frontal presentation in progressive supranuclear palsy. Neurology 69:723–729
Ghosh BC, Rowe JB, Calder AJ, Hodges JR, Bak TH (2009) Emotion recognition in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 80:1143–1145
Harris JM, Gall C, Thompson JC, Richardson AM, Neary D, du Plessis D, Pal P, Mann DM, Snowden JS, Jones M (2013) Sensitivity and specificity of FTDC criteria for behavioral variant frontotemporal dementia. Neurology 80:1881–1887
Hu WT, Parisi JE, Knopman DS, Boeve BF, Dickson DW, Ahlskog JE, Petersen RC, Josephs KA (2007) Clinical features and survival of 3R and 4R tauopathies presenting as behavioral variant frontotemporal dementia. Alzheimer Dis Assoc Disord 21:S39–S43
Josephs KA, Boeve BF, Duffy JR, Smith GE, Knopman DS, Parisi JE, Petersen RC, Dickson DW (2005) Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase 11:283–296
Josephs KA, Hodges JR, Snowden JS, Mackenzie IR, Neumann M, Mann DM, Dickson DW (2011) Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol 122:137–153
Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, Parisi JE, Dickson DW (2006) Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology 66:41–48
Josephs KA, Whitwell JL, Eggers SD, Senjem ML, Jack CR Jr (2011) Gray matter correlates of behavioral severity in progressive supranuclear palsy. Mov Disord 26:493–498
Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47:1–9
Litvan I, Campbell G, Mangone CA, Verny M, McKee A, Chaudhuri KR, Jellinger K, Pearce RK, D’Olhaberriague L (1997) Which clinical features differentiate progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) from related disorders? A clinicopathological study. Brain 120(Pt 1):65–74
Litvan I, Mega MS, Cummings JL, Fairbanks L (1996) Neuropsychiatric aspects of progressive supranuclear palsy. Neurology 47:1184–1189
Lopez OL, Litvan I, Catt KE, Stowe R, Klunk W, Kaufer DI, Becker JT, DeKosky ST (1999) Accuracy of four clinical diagnostic criteria for the diagnosis of neurodegenerative dementias. Neurology 53:1292–1299
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134:2456–2477
Respondek G, Roeber S, Kretzschmar H, Troakes C, Al-Sarraj S, Gelpi E, Gaig C, Chiu WZ, van Swieten JC, Oertel WH, Hoglinger GU (2013) Accuracy of the National Institute for Neurological Disorders and Stroke/Society for Progressive Supranuclear Palsy and neuroprotection and natural history in Parkinson plus syndromes criteria for the diagnosis of progressive supranuclear palsy. Mov Disord 28:504–509
Robbins TW, James M, Owen AM, Lange KW, Lees AJ, Leigh PN, Marsden CD, Quinn NP, Summers BA (1994) Cognitive deficits in progressive supranuclear palsy, Parkinson’s disease, and multiple system atrophy in tests sensitive to frontal lobe dysfunction. J Neurol Neurosurg Psychiatry 57:79–88
Snowden JS, Thompson JC, Stopford CL, Richardson AM, Gerhard A, Neary D, Mann DM (2011) The clinical diagnosis of early-onset dementias: diagnostic accuracy and clinicopathological relationships. Brain 134:2478–2492
Soliveri P, Monza D, Paridi D, Carella F, Genitrini S, Testa D, Girotti F (2000) Neuropsychological follow up in patients with Parkinson’s disease, striatonigral degeneration-type multisystem atrophy, and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 69:313–318
Soliveri P, Piacentini S, Girotti F (2005) Limb apraxia in corticobasal degeneration and progressive supranuclear palsy. Neurology 64:448–453
Steele JC, Richardson JC, Olszewski J (1964) Progressive Supranuclear Palsy. a heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy. Nuchal dystonia and dementia. Arch Neurol 10:333–359
Thompson JC, Stopford CL, Snowden JS, Neary D (2005) Qualitative neuropsychological performance characteristics in frontotemporal dementia and Alzheimer’s disease. J Neurol Neurosurg Psychiatry 76:920–927
Williams DR, Holton JL, Strand C, Pittman A, de Silva R, Lees AJ, Revesz T (2007) Pathological tau burden and distribution distinguishes progressive supranuclear palsy-parkinsonism from Richardson’s syndrome. Brain 130:1566–1576
Williams DR, Lees AJ (2009) Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol 8:270–279
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobylecki, C., Jones, M., Thompson, J.C. et al. Cognitive–behavioural features of progressive supranuclear palsy syndrome overlap with frontotemporal dementia. J Neurol 262, 916–922 (2015). https://doi.org/10.1007/s00415-015-7657-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7657-z