Abstract
Introduction
Movement Disorder Society (MDS) new diagnostic criteria for Progressive Supranuclear palsy (PSP) identifying different disease phenotypes were recently released. The aim of the present study is to report on the cognitive and behavioral features of the different phenotypes diagnosed according to the MDS criteria.
Methods
Forty-nine PSP patients underwent an extensive battery of clinical assessments. Differences between PSP subtypes were computed with χ2 or ANOVA tests. Using the z scores, subjects were classified as having normal cognition, mild cognitive impairment, single or multiple domain, and dementia. A logistic regression model was implemented to investigate the major determinants of PSP non-Richardson’s syndrome phenotype.
Results
Half of the cohort presented Richardson’s syndrome (46.9%), followed by PSP with parkinsonism and corticobasal syndrome (22.4% and 14.2%, respectively). Richardson’s syndrome and PSP with corticobasal syndrome presented a similar burden of disease. The only cognitive testing differentiating the phenotypes were semantic fluency and ideomotor apraxia. The majority of our cohort was either affected by dementia or presented normal cognition. Richardson’s syndrome presented the highest rate of dementia. The only marker of PSP non-Richardson’s syndrome phenotype was better performance in visuo-spatial testing, implying worse visuo-spatial abilities in PSP Richardson’s syndrome.
Conclusion
Available clinical assessments hardly capture differences between PSP phenotypes. The cognitive testing differentiating the PSP phenotypes were semantic fluency and ideomotor apraxia. In PSP, mild cognitive impairment likely represents an intermediate step from normal cognition to dementia. The only marker of PSP non-Richardson’s syndrome phenotype was better performance in visuo-spatial testing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Progressive supranuclear palsy (PSP) is a rare, rapidly progressive, neurodegenerative disease linked to abnormal tau-protein accumulation and characterized by vertical supranuclear gaze palsy as well as postural instability with falls [1]. The latter represents the cornerstones of the National Institute of Neurodegenerative Disorders and Stroke-PSP criteria (NINDS-PSP) recognizing the classic Richardson’s syndrome (PSP-RS) as the only form of disease [2].
New diagnostic criteria were recently released by the Movement Disorder Society (MDS) identifying several PSP phenotypes beyond the PSP-RS with different degrees of diagnostic certainty according to the predominant clinical features [3].
Evidence available so far would suggest that PSP-RS is the most common phenotype (55%), followed by PSP with predominant parkinsonism (PSP-P) (30%) and PSP with predominant frontal presentation (PSP-F) (5%) with the remaining phenotypes accounting for about 1% each [3]. However, large longitudinal natural history studies applying the new MDS criteria are lacking; therefore, prevalence data on phenotypes are expected to change.
In addition to the motor and ocular characteristics, still representing the core of PSP diagnosis, a wide range of cognitive and behavioral disturbances are recognized by the MDS as main disease features characterizing the different phenotypes and determining a major impact on health-related quality of life as well as on survival [3,4,5,6,7].
To date, few studies have attempted to characterize PSP phenotypes focusing on differences between PSP-RS and PSP-P [8, 9, 11].
The MDS criteria theorizing the PSP phenotypes are based on an extensive review of the literature as well as the revision of the largest autopsy-confirmed case series reported so far. However, there are no available studies providing a cognitive–behavioral description of the PSP phenotypes as diagnosed according to the MDS criteria in a prospectively enrolled cohort.
The aim of the present study is to describe motor, cognitive and behavioral differences with available clinical instruments in PSP phenotypes, as diagnosed according to the MDS criteria, in a large sample of prospectively enrolled PSP patients referred to a third-level movement disorders center.
Methods
Between November 2015 and April 2018, consecutive cases of suspected PSP referred to the Center for Neurodegenerative Diseases of the University of Salerno were proposed a dedicated set of assessments including a clinical interview, a motor evaluation, extensive cognitive and behavioral testing. The purpose of this effort was to create a PSP registry for the Salerno County. The project was approved by the local Ethics Committee and each subject was included upon signature of the informed consent form. Each enrolled patient underwent the MDS-proposed diagnostic flowchart with two movement disorders specialists who retrospectively defined the PSP phenotypes according to the predominant clinical features and expressed the degree of diagnostic certainty [3]. Details on the methods are available elsewhere [12]. Briefly, two movement disorders specialists (M.P. and R.E.) independently reviewed all the data collected for each subject (including the videotaped motor assessment) and applied the new criteria proposed by the task force (detailed in Tables 1–5 of [3]). Any case arising disagreement between the two evaluators was submitted to a third blinded expert (P.B.) and finally discussed to reach a unanimous agreement on diagnosis and predominance type.
Severity of the disease was evaluated with the PSP rating scale (PSP-rs) as well as the Natural history and Neuroprotection in Parkinson Plus Syndrome (NNIPPS) scale [13, 14].
Due to the ease of use, health-related quality of life was assessed with the EQ-5D Visual Analog Scale (EQ-5D-VAS) [15].
Cognitive abilities were screened with the Montreal Cognitive Assessment (MOCA). Memory domain was investigated with the delayed recall scores of the Rey auditory verbal learning test (15-RAWLT) and the prose memory test. Attention domain was explored through the trail making test (TMT) and the short version of the Stroop interference test. Executive functions were assessed with the Clock design test (CDT) and semantic verbal fluency test (SVF). Visuo-spatial functions were tested with the constructional apraxia test and Benton orientation line test (BJLO). Language domain was explored with two sub-tests from the Neuropsychological Examination of Aphasia battery (ENPA): the non-word repetition test and the hearing comprehension test of sentences. We explored praxic abilities through the bucco-facial and ideomotor apraxia test [4].
Functional autonomy was evaluated with the Instrumental Activities of Daily Life (IADL), while depression and apathy with the Beck Depression Inventory II (BDI-II) and Apathy Evaluation Scale (AES), respectively. Patients with BDI-II score greater than 12 were defined depressed, while patients with AES score greater than 37 were defined apathetic [16, 17].
Using the z scores of the individual tests and a control group consisting of twenty-six healthy participants with age and education matched to the patients, enrolled subjects were classified as having PSP with normal cognition (PSP-NC), PSP with MCI-single domain (PSP-MCIsd), PSP with MCI-multiple domain (PSP-MCImd) and PSP with dementia (PSP-D).
Due to the lack of specific MCI criteria for PSP, MDS MCI criteria for Parkinson’s disease were applied [18]. As such, MCI was defined as impairment in neuropsychological tests (score below 1.5 standard deviation) with no impairment in IADL. In detail, patients having compromised two tests within one single cognitive domain with other domains unimpaired were classified as PSP-MCIsd, while patients having impaired at least one test in two or more cognitive domains were codified as PSP-MCImd [18]. Patients presenting any type of cognitive/behavioral decline associated with impairment of IADL were considered as affected by dementia (PSP-D), according to Statistical Diagnostic Manual of Psychiatry-5th Edition (DSM-5).
Statistical analysis
After checking for normality distribution with the Kolmogorov–Smirnov test, differences in variables between PSP subtypes were computed with χ2 or ANOVA tests as appropriate. Post hoc comparisons were run with Bonferroni test. Pearson’s correlation has been performed to explore the relationship between motor symptoms, as assessed with PSP-rs and NNIPPS, and cognitive tests. Significance level was set at p ≤ 0.004 to correct for multiple comparisons.
Multivariate logistic regression was implemented to explore the major determinants of PSP non-RS phenotype (dependent variables PSP-RS = 0 versus PSP non-RS = 1 encompassing all the other phenotypes). All models were adjusted for age and education. All the clinical, cognitive and behavioral variables were assessed individually and if any were found to be significant at the p < 0.2 level, they were assessed together in a single backwards regression model with a p < 0.1 inclusion level. All β values and p values are from the backwards selection models. Significance level was set at ≤ 0.05. Data analysis was conducted with SPSS (version 23.0).
Results
Forty-nine out of 60 patients referred to our center for suspected PSP underwent the complete set of assessments. Eleven patients (18.3%) fulfilled one of the MDS mandatory exclusion criteria preventing further evaluation according to the PSP diagnostic flowchart (3 for severe leukoencephalopathy and 3 for the presence of normal pressure hydrocephalus disclosed by the MRI, 2 for predominant autonomic dysfunction, 2 for positive familial history, 1 for predominant multisegmental upper and lower motor signs suggestive of motor neuron disease).
Out of the 49 patients enrolled, 55.1% (27) were men, 23 patients qualified for PSP-RS, 11 with PSP-P, and 7 with PSP with predominant corticobasal syndrome (PSP-CBS), 4 with PSP-F and 4 with PSP with predominant progressive gait freezing (PSP-PGF). All patients reached the degree of certainty of Probable PSP, but those—by definition—with PSP-CBS.
PSP phenotypes were similar for age, education and disease duration (Table 1). Although PSP-rs total score was similar among subtypes, PSP-CBS tended to score worse in the PSP-rs ocular and limb exam sections (p = 0.022 and p = 0.041, respectively). According to the NNIPPS total score, PSP-CBS and PSP-RS presented the highest burden of disease (p = 0.010). As for the subcores, NNIPPS rigidity, axial bradykinesia and ocular motor function subscores were higher in PSP-RS and PSP-CBS compared with PSP-P (p = 0.002, p < 0.001 and p = 0.008, respectively). As expected, NNIPPS myoclonus subscore was higher in PSP-CBS compared with PSP-RS and PSP-P (p = 0.020). Finally, NNIPPS limb bradykinesia subscore was higher in PSP-RS compared with PSP-P (p = 0.012) (Table 2).
Health-related quality of life, as evaluated with the EQ-5D-VAS, did not show significant differences among PSP phenotypes (Table 2).
Regarding the cognitive–behavioral evaluation, significant differences among groups were detected for the SVF with PSP-RS and PSP-CBS scoring lower than PSP-PGF (p = 0.020). Differences between phenotypes emerged also for left-side ideomotor apraxia with a tendency for PSP-CBS to score lower than PSP-RS and PSP-F (p = 0.028) (Table 3). The phenotypes also presented differences in the BDI-II with trends towards significance for higher scores in the cognitive factor in PSP-CBS and PSP-F compared to PSP-P (p = 0.036) (Table 3).
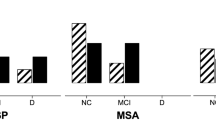
According to the BDI-II, 77.1% of the total sample presented depression: 78.9% of PSP-RS, 66.7% of PSP-P, 100% of PSP-CBS, 100% of PSP-F and 50% of PSP-PGF. Differences among subtypes were not significant (p = 0.426) (Fig. 1a).
a Patients with clinically significant depression according to phenotype. Patients with BDI-II score greater than 12. b Patients with clinically significant apathy according to phenotype. Patients with AES score greater than 37. c Cognitive status according to phenotype. D dementia, NC normal cognition, MCImd mild cognitive impairment multiple domain, MCIsd mild cognitive impairment single domain, PSP-CBS progressive supranuclear palsy-corticobasal syndrome, PSP-F progressive supranuclear palsy with predominant frontal presentation, PSP-P progressive supranuclear palsy with predominant parkinsonism, PSP-PGF progressive supranuclear palsy with predominant gait freezing
According to the AES, 82.6% of the whole sample was apathetic: 81.8% of PSP-RS, 77.8% of PSP-P, 100% of PSP-CBS, 75% of PSP-F and 75% of PSP-PGF. Differences among subtypes were not significant (p = 0.745) (Fig. 1b).
Regarding the cognitive status of the whole sample, 24.5% had a normal cognitive status, 18.4% had MCI-sd, 14.3% had MCI-md and 42.9% had dementia. Details of cognitive status according to PSP subtypes are shown in Fig. 1c.
Pearson’s correlation showed a significant inverse correlation between NNIPPS and SVF (r = − 0.525, p = 0.001). No other significant correlations were detected.
Multivariate logistic regression showed that the only significant marker of PSP non-RS was better scores in the BJLO [OR = 1.788 (95% CI 0.908–3.520), p = 0.093] (Table 4).
Discussion
Here, we provide a comprehensive clinical, cognitive and behavioral characterization of PSP phenotypes diagnosed according to the MDS criteria in a prospectively enrolled single-center cohort [3, 12].
As expected, the most prevalent phenotype was PSP-RS (46.9%), followed by PSP-P (22.4%) and PSP-CBS (14.2%). The high prevalence of PSP-CBS is justified by the application of the joint classification of overlapping PSP and corticobasal disease (CBD) syndromes as clinically probable 4R-tauopathy operationalized for the first time by the MDS [3]. This approach avoids separating PSP and CBD on a clinical ground, as these entities are undistinguishable with current available methods [19].
Similarly, our analysis shows that available clinical assessments hardly capture differences between PSP phenotypes. As such, scant differences were detected despite the use of an extensive battery of validated motor, cognitive and behavioral instruments used.
The comparison of disease severity between phenotypes disclosed that both PSP-RS and PSP-CBS have the highest motor burden of disease in terms of limb impairment and rigidity as well as ocular involvement [20]. In line with the presence of myoclonus among the PSP-CBS diagnostic criteria, such patients presented higher scores in the myoclonus section of the NNIPPS [3]. Our data also point out that PSP-rs and NNIPPS are not completely equivalent. The NNIPPS provides a more detailed and comprehensive evaluation of the wide spectrum of symptoms featuring the PSP phenotypes, including myoclonus. As such, although it has been used in a number of multicenter clinical trials and natural history studies, the PSP-rs lacks of specific items dedicated to myoclonus or freezing of gait still core features of PSP-CBS and PSP-PGF [13, 20].
Regarding the cognitive evaluation, the PSP phenotype showed significant differences in the semantic verbal fluency with PSP-CBS having the lowest scores followed by PSP-RS. This is in line with the prominent deficits in attention and executive functions described in PSP, with verbal fluency being specifically affected [4, 9]. Semantic fluency is a widely used measure of frontal–temporal cortical functioning and its alteration provides additional frontal–executive cognitive demand as it requires both alternating between two semantic categories and inhibiting category unrelated responses [21, 22]. Semantic verbal fluency task needs the retrieval of hierarchically organized contents with search strategies adapted to a categorical structure, including semantically associated sub-domains. Thus, our results would suggest that PSP-CBS as well as PSP-RS has the most extensive dysfunction in cortical and subcortical networks, and especially in the left inferior frontal cortex as well as in temporal areas compared to the other phenotypes with comparable disease duration and severity [23,24,25]. Furthermore, the significant correlation between motor impairment and SVF detected in the whole PSP sample further supports a role for semantic fluency as a marker of disease.
As expected, PSP-CBS had the lowest scores with the ideomotor apraxia test, especially on the left side, in line with both the criteria identifying such phenotype and the left-side predominance in our cohort (Table 1) [3].
Regarding the behavioral evaluation, PSP-CBS and PSP-F showed a trend towards significance for higher scores in the cognitive factor of the BDI-II. Although there is a scant of studies on the factor structure of the BDI-II in movement disorders, this would be in line with the hypothesis that the cognitive factor of the BDI-II better mirrors depressive symptoms irrespective of body problems [26].
In line with other studies [4, 27], in our sample, depressive symptoms are less prevalent than the apathetic symptoms (77.1% versus 82.6%). Although there is scant of studies considering behavioral disorders in PSP phenotypes, our data would suggest that depressive symptoms are more prevalent in patients with PSP-CBS and PSP-F (100%) compared to PSP-PGF (50%). Indeed, we acknowledge that symptoms of depression can be subtle and often overlooked as reactions to the severity of motor symptoms [28]. However, neuroimaging and postmortem studies suggest that depression in movement disorders is underlined by mesolimbic dopaminergic pathways and basal ganglia dysfunction and, thus, is an intrinsic feature of the disease [29, 30].
As for apathy, significant symptoms were more prevalent in PSP-CBS (100%) and PSP-RS (81.8%) compared with PSP-PGF and PSP-F (75%). As opposite to our data, Pellicano and colleagues identified apathy as a predictor of the PSP-P compared with PSP-RS [11]. The use of different tools to quantify apathy can account for such discrepancy [17]. Indeed, the high prevalence of apathy in PSP is explained by the profound degeneration of the prefrontal areas and the dysfunction of the frontal–subcortical connections, also shown by MRI studies [10].
Ours is one of the few studies considering the concept of MCI in atypical parkinsonism [6]. The majority of our cohort either was affected by dementia (42.9%) or presented normal cognition (24.5%), with a minority presenting MCI (18.4% MCI-md and 14.3% MCI-sd). Confirming previous findings, these data would suggest that MCI in PSP represent an intermediate step from normal cognition to dementia [6].
When investigating the prevalence of cognitive status across PSP phenotypes, the highest rates of dementia were disclosed in PSP-RS (47.8%), followed by PSP-P (45.5%) and PSP-CBS (42.9%), with PSP-PGF presenting the highest prevalence of normal cognition (75%). These results are in line with available literature describing PSP-RS as the most severe and PSP-PGF as the most benign phenotype [3, 31].
Finally, we attempted at exploring the major determinants of PSP-RS versus PSP non-RS phenotypes. Among all the clinical, cognitive and behavioral variables considered, the only predictor of PSP non-RS was better scores in visuo-spatial testing (i.e., BJLO). Our results would imply worse visuo-spatial functions in PSP-RS compared to the other phenotypes, excluding PSP-SL. Further studies should focus on a wider examination of the other cognitive domains beyond attention and executive functions in PSP to confirm such data. Indeed, we acknowledge the possible impact of supranuclear gaze palsy on BJLO performances. As such, PSP non-RS may have higher BJLO scores in light of the milder ocular impairment reflected by lower scores in the PSP-rs ocular section for all the PSP non-RS phenotypes but PSP-CBS.
Limits of the present study include exploratory nature of the study, the number of included cases from a single center (49), the lack of longitudinal follow-up as well as pathological confirmation, still the gold standard for PSP diagnosis. Furthermore, we acknowledge that the prevalence of the PSP-F subtype may be underrepresented due to the enrollment performed in a movement disorders center.
In conclusion, we report on the clinical, cognitive and behavioral features of MDS PSP phenotypes. Current clinical instruments hardly detect differences between phenotypes. The lack of specific clinical assessments differentiating PSP phenotypes in vivo will preclude future advances in understanding the underlying mechanisms of these distinct pathological processes as well as in their treatment.
Abbreviations
- 15-RAWLT:
-
Rey’s auditory 15-word learning test
- AES:
-
Apathy evaluation scale
- ANOVA:
-
Analysis of variance
- BDI-II:
-
Beck depression inventory II
- BJLO:
-
Benton’s judgment of line orientation
- CDT:
-
Clock drawing test
- DSM-5th:
-
Statistical Diagnostic Manual of Psychiatry-5th edition
- ENPA:
-
Neuropsychological examination of aphasia battery
- IADL:
-
Instrumental activities of daily life
- MCI:
-
Mild cognitive impairment
- MoCA:
-
Montreal cognitive assessment battery
- MRI:
-
Magnetic resonance imaging
- MDS:
-
Movement disorders society
- NINDS-PSP criteria:
-
National Institute of Neurodegenerative Disorders and Stroke-progressive supranuclear palsy criteria
- NNIPPS:
-
Natural history and neuroprotection in Parkinson plus syndrome
- PSP:
-
Progressive supranuclear palsy
- PSP-CBS:
-
Progressive supranuclear palsy with predominant corticobasal syndrome
- PSP-D:
-
Progressive supranuclear palsy with dementia
- PSP-F:
-
Progressive supranuclear palsy with predominant frontal presentation
- PSP-MCImd:
-
Progressive supranuclear palsy with mild cognitive impairment-multiple domain
- PSP-MCI-sd:
-
Progressive supranuclear palsy with mild cognitive impairment-single domain
- PSP-NC:
-
Progressive supranuclear palsy with normal cognition
- PSP-P:
-
Progressive supranuclear palsy with predominant parkinsonism
- PSP-PGF:
-
Progressive supranuclear palsy with predominant progressive gait freezing
- PSP-rs:
-
Progressive supranuclear palsy-rating scale
- PSP-RS:
-
Progressive supranuclear palsy with predominant Richardson’s syndrome subtype
- PSP-SL:
-
Progressive supranuclear palsy with predominant speech/language disorder
- SPSS:
-
Statistical package for social science
- SVP:
-
Semantic verbal fluency
- TMT:
-
Trial making test
- VAS:
-
Visual analog scale
References
Boxer AL, Yu JT, Golbe LI, Litvan I, Lang AE, Höglinger GU (2017) Advances in progressive supranuclear palsy: new diagnostic criteria, biomarkers, and therapeutic approaches. Lancet Neurol 16(7):552–563
Litvan I, Agid Y, Calne D, Campbell B et al (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-PSP international workshop. Neurology 47(1):1–9
Höglinger GU, Respondek G, Stamelou M et al (2017) Clinical diagnosis of progressive supranuclear palsy: the Movement Disorder Society criteria. Mov Disord 32(6):853–864
Santangelo G, Cuoco S, Pellecchia MT, Erro R, Barone P, Picillo M (2018) Comparative cognitive and neuropsychiatric profiles between Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy. J Neurol 265(11):2602–2613
Gerstnecker A (2017) The neuropsychology (broadly conceived) of multiple system atrophy, progressive supranuclear palsy, and corticobasal degeneration. Arch Clin Neuropsychol 32(7):861–875
Pilotto A, Gazzina S, Benussi A et al (2017) Mild cognitive impairment and progression to dementia in progressive supranuclear palsy. Neurodegener Dis 17(6):286–291
Gerstenecker A, Mast B, Duff K, Ferman TJ (2013) Executive dysfunction is the primary cognitive impairment in progressive supranuclear palsy. Arch Clin Neuropsychol 28(2):104–113
Millar D, Griffiths P, Zermansky AJ, Burn DJ (2016) Characterizing behavioural and cognitive dysexecutive changes in progressive supranuclear palsy. Mov Disord 21(2):199–207
Brown RG, Lacomblez L, Landwehrmeyer BG et al (2010) Cognitive impairment in patients with multiple system atrophy and progressive supranuclear palsy. Brain 133(8):2382–2393
Cordato N, Pantelis C, Halliday GM et al (2002) Frontal atrophy correlates with behavioural changes in progressive supranuclear palsy. Brain 125(4):789–800
Pellicano C, Assogna F, Cellupica N et al (2017) Neuropsychiatric and cognitive profile of early Richardson’s syndrome, progressive supranuclear palsy-parkinsonism and Parkinson’s disease. Parkinsonism Relat Disord 45:50–56
Picillo M, Erro R, Cuoco S, Tepedino MF, Manara R, Pellecchia MT, Barone P (2017) MDS PSP criteria in real-life clinical setting: motor and cognitive characterization of subtypes. Mov Disord 33(8):1361–1365
Golbe LI, Ohman-Strickland PA (2007) A clinical rating scale for progressive supranuclear palsy. Brain 130(Pt 6):1552–1565
Payan CA, Viallet F, Landwehrmeyer BG et al (2011) NNIPPS Study Group. Disease severity and progression in progressive supranuclear palsy and multiple system atrophy: validation of the NNIPPS–Parkinson Plus Scale. PLoS One 6(8):e22293
The EuroQol Group (1990) EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 16:199–208
Kjaergaard M, Arfwedson W, Waterloo K, Jorde R (2014) A study of the psychometric properties of the Beck Depression Inventory-II, the Montgomery and Åsberg Depression Rating Scale, and the Hospital Anxiety and Depression Scale in a sample from a healthy population. Scand J Psychol 55(1):83–89
Santangelo G, Barone P, Cuoco S, Raimo S, Pezzella D, Picillo M, Vitale C (2014) Apathy in untreated, de novo patients with Parkinson‘s disease: validation study of apathy evaluation scale. J Neurol 261:2319–2328
Litvan I, Goldman JG, Tröster AI et al (2012) Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: movement Disorder Society Task Force guidelines. Mov Disord 27(3):349–356
Höglinger GU (2018) Is it useful to classify progressive supranuclear palsy and corticobasal degeneration as different disorders? No. Mov Disord Clin Pract 5(2):141–144
Brittain C, McCarthy A, Irizarry MC et al (2018) Severity dependent distribution of impairments in PSP and CBS: interactive visualizations. Parkinsonism Relat Disord. https://doi.org/10.1016/j.parkreldis.2018.08.025
Vita MG, Marra C, Spinelli P et al (2014) Typicality of words produced on a semantic fluency task in amnesic mild cognitive impairment: linguistic analysis and risk of conversion to dementia. J Alzheimers Dis 42(4):1171–1178
Owen AM, James M, Leigh PN et al (1992) Fronto-striatal cognitive deficits at different stages of Parkinson’s disease. Brain 16:1727–1751
Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS (2006) A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp 27(10):799–810
Henry JD, Crawford JR (2004) A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology 18(2):284–295
Rittman T, Ghosh BC, McColgan P et al (2013) The Addenbrooke’s Cognitive Examination for the differential diagnosis and longitudinal assessment of patients with parkinsonian disorders. J Neurol Neurosurg Psychiatry 84(5):544–551
Wang YP, Gorenstein C (2013) Psychometric properties of the Beck Depression Inventory-II: a comprehensive review. Rev Bras Psiquiatr 35:416–431
Ječmenica-Lukić M, Pekmezović T, Petrović IN, Tomić A, Svetel M, Kostić VS (2018) Use of the neuropsychiatric inventory to characterize the course of neuropsychiatric symptoms in progressive supranuclear palsy. J Neuropsychiatry Clin Neurosci 30(1):38–44
Almeida L, Ahmed B, Walz R et al (2017) Depressive Symptoms are frequent in atypical parkinsonian disorders. Mov Disord Clin Pract 4(2):191–197
Wilson RS, Nag S, Boyle PA et al (2013) Brainstem aminergic nuclei and late-life depressive symptoms. JAMA Psychiatry 70:1320–1328
Benoit M, Robert PH (2011) Imaging correlates of apathy and depression in Parkinson’s disease. J Neurol Sci 310:58–60
Jellinger KA (2008) Different tau pathology pattern in two clinical phenotypes of progressive supranuclear palsy. Neurodegener Dis 5(6):339–346
Acknowledgements
The PSP Salerno Study Group: Marina Picillo, Roberto Erro, Sofia Cuoco, Maria Francesca Tepedino, Renzo Manara, Maria Teresa Pellecchia, Paolo Barone, Marianna Amboni, Arianna Cappiello, Immacolata Carotenuto, Giovanna Dati, Gabriella Santangelo, Pietro Siano, Annamaria Vallelunga, Giampiero Volpe.
Funding
There is no specific source of funding for this manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
MP is supported by the Michael J Fox Foundation for Parkinson’s research; PB received consultancies as a member of the advisory board for Zambon, Lundbeck, UCB, Chiesi, Abbvie and Acorda; RE received consultancies from Zambon and honoraria from TEVA; the other authors report no financial disclosures.
Additional information
The PSP Salerno study group is listed in Acknowledgements section.
Rights and permissions
About this article
Cite this article
Picillo, M., Cuoco, S., Tepedino, M.F. et al. Motor, cognitive and behavioral differences in MDS PSP phenotypes. J Neurol 266, 1727–1735 (2019). https://doi.org/10.1007/s00415-019-09324-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09324-x