Abstract
Tinnitus is the perception of a sound in the absence of a corresponding external sound source. Research has suggested that functional abnormalities in tinnitus patients involve auditory as well as non-auditory brain areas. Transcranial electrical stimulation (tES), such as transcranial direct current stimulation (tDCS) to the dorsolateral prefrontal cortex and transcranial random noise stimulation (tRNS) to the auditory cortex, has demonstrated modulation of brain activity to transiently suppress tinnitus symptoms. Targeting two core regions of the tinnitus network by tES might establish a promising strategy to enhance treatment effects. This proof-of-concept study aims to investigate the effect of a multisite tES treatment protocol on tinnitus intensity and distress. A total of 40 tinnitus patients were enrolled in this study and received either bifrontal tDCS or the multisite treatment of bifrontal tDCS before bilateral auditory cortex tRNS. Both groups were treated on eight sessions (two times a week for 4 weeks). Our results show that a multisite treatment protocol resulted in more pronounced effects when compared with the bifrontal tDCS protocol or the waiting list group, suggesting an added value of auditory cortex tRNS to the bifrontal tDCS protocol for tinnitus patients. These findings support the involvement of the auditory as well as non-auditory brain areas in the pathophysiology of tinnitus and demonstrate the idea of the efficacy of network stimulation in the treatment of neurological disorders. This multisite tES treatment protocol proved to be save and feasible for clinical routine in tinnitus patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tinnitus is considered to be an auditory phantom phenomenon characterized by an ongoing sound perception (e.g., a tone, hissing, or buzzing sound) in the absence of any objective corresponding physical sound source (Jastreboff 1990). About 5–15 % of the population in western societies has chronic tinnitus and many sufferers seek medical care (Axelsson and Ringdahl 1989; Heller 2003). The constant awareness of the phantom sound frequently causes a considerable amount of distress. About 6–25 % of the tinnitus patients report symptoms that are severely debilitating (Baguley 2002; Eggermont and Roberts 2004) with 2–4 % of the total population suffering in the worst degree (Axelsson and Ringdahl 1989). Problems that have been attributed to tinnitus include lifestyle detriment, emotional difficulties, sleep deprivation, difficulties concentrating at work, interference with social interactions, and decreased overall health (Scott and Lindberg 2000).
Based on functional brain imaging studies, it is generally accepted that tinnitus is related to auditory hyperactivity or maladaptive plasticity of the auditory system (Muhlnickel et al. 1998; Lockwood et al. 1999; Langguth et al. 2006; Smits et al. 2007; Weisz et al. 2007; van der Loo et al. 2009b; Vanneste et al. 2010a). However, new insights into the neurobiology of tinnitus suggest that neuronal changes are not limited to the auditory cortex. Co-activation of non-auditory brain areas such as the dorsolateral prefrontal cortex (Schlee et al. 2009; Vanneste et al. 2010a), anterior cingulate cortex (Muhlau et al. 2006; Plewnia et al. 2007; Rauschecker et al. 2010; Vanneste et al. 2010a, 2011), insula (Smits et al. 2007; Vanneste et al. 2010a), and parahippocampus (Carpenter-Thompson et al. 2014; Vanneste and De Ridder 2016) have been described and could explain the potential underlying pathophysiological mechanism for tinnitus (Rauschecker et al. 2010; De Ridder et al. 2011a).
Over the past decade, different non-invasive neuromodulation techniques have been used targeting different tinnitus sites in an attempt to modify local and distant neuroplasticity as to reduce tinnitus symptoms. Transcranial direct current stimulation (tDCS) and transcranial random noise stimulation (tRNS) are two forms of low-intensity transcranial electrical stimulation (tES) applied on the cortical surface using two surface electrodes. TDCS uses continuous electrical current flowing from one electrode serving as the anode to another electrode serving as the cathode to modulate the area of interest. Depending on the polarity of the stimulation, tDCS can increase (anodal stimulation) or decrease (cathodal stimulation) cortical excitability to the targeted brain region (Nitsche and Paulus 2000; Miranda et al. 2006). TRNS is a modification of transcranial alternating current stimulation (tACS) which uses random oscillations with a white structure (i.e., equal amplitude for all frequencies between 0.1 and 640 Hz) in a Gaussian distribution for amplitude, which is no longer sensitive to the direction of the current flow or the polarity (Van Doren et al. 2014).
Joos and her colleagues (2014) have demonstrated that bilateral tDCS over the auditory cortex is able to suppress tinnitus loudness (Joos et al. 2014). Moreover, research demonstrated that tRNS bilaterally over the auditory cortex has a superior effect compared to tDCS in suppressing tinnitus intensity and distress (Vanneste et al. 2013a; Claes et al. 2014), and multiple sessions of auditory cortex tRNS are superior to single sessions (Claes et al. 2014). On the other hand, tDCS was also applied to modulate tinnitus perception targeting the dorsolateral prefrontal cortex (DLPFC) (Vanneste et al. 2010b, 2013b; Vanneste and De Ridder 2011; De Ridder and Vanneste 2012; Faber et al. 2012; Frank et al. 2012). Bifrontal tDCS placing the anodal electrode overlying the right DLPFC and the cathodal electrode overlying the left DLPFC has been demonstrated to suppress tinnitus intensity and distress in multiple studies (Vanneste et al. 2010b; Vanneste and De Ridder 2011; De Ridder and Vanneste 2012; Faber et al. 2012; Frank et al. 2012).
Several studies using different functional imaging modalities such as EEG (van der Loo et al. 2009a), MEG (Weisz et al. 2007), PET scan (Eichhammer et al. 2007) and fMRI (Smits et al. 2007) have shown that tinnitus is associated with hyperactivity of the auditory cortex. However, tinnitus distress and tinnitus loudness are also associated with changes in the anterior cingulate cortex and insula (Vanneste et al. 2010a; De Ridder et al. 2011b, 2015), suggesting that loudness and distress perception are a network phenomenon, rather than hyperactivity of a single area. Based on the principles of network science, it has, therefore, been proposed that targeting multiple hubs in a network may be superior in modulating network activity than manipulating a single region (Albert et al. 2000) and the same principle might be applicable for tinnitus (Mohan et al. 2016a, b). Recent studies using repetitive transcranial magnetic stimulation (rTMS) have investigated the effect of a multisite approach obtained by sequential excitatory stimulation (high-frequency rTMS) of the prefrontal cortex and inhibitory stimulation (low-frequency rTMS) of the auditory cortex, with mixed results. However, it was suggested that a consecutive treatment that consists of excitatory left dorsal lateral prefrontal cortex stimulation before inhibitory left (and right) auditory cortex stimulation leads to more pronounced long-term effects compared to auditory cortex stimulation (Kleinjung et al. 2008; Lehner et al. 2013). A different study by Langguth and his colleagues in 2014 did not find a superior effect of this multisite approach (Langguth et al. 2014).
It has been shown that dorsolateral prefrontal cortex stimulation with tDCS can modulate tinnitus-related anterior cingulate activity (Vanneste and De Ridder 2011) improving both tinnitus loudness and tinnitus-related distress (Vanneste and De Ridder 2011), and that tRNS is superior to tDCS and tACS of the auditory cortex (Vanneste et al. 2013a). Therefore, a combined stimulation paradigm that inhibits auditory cortex activity by means of tRNS and facilitates prefrontal cortex output by tDCS may provide stronger relief (Pal et al. 2015).
In this proof-of-concept study, we aim to explore the effectiveness of a multisite consecutive treatment approach using transcranial electrical stimulation, more specifically tDCS and tRNS. We hypothesize that a multisite consecutive treatment protocol consisting of bilateral DLPFC tDCS followed by bilateral auditory cortex tRNS will result in an immediate and superior effect compared to bilateral DLPFC tDCS.
Materials and methods
Participants
Forty subjects (22 males and 18 females) with chronic tinnitus (>1 year) participated in this study, with a mean age of 48.33 years (SD 10.74). The mean tinnitus duration was 10.82 years (SD 14.35). See descriptions of the tinnitus characteristics in Table 1. To obtain a homogeneous sample and exclude potential variables that would interfere with response to tES, we excluded subjects based on the following criteria: individuals with pulsatile tinnitus, a history of epileptic insults, severe organic co-morbidity, a pacemaker or defibrillator, current pregnancy, neurological disorders such as brain tumors, and individuals being treated for mental disorders. All prospective subjects underwent a complete ENT and neurological examination to rule out possible treatable causes for their tinnitus.
Transcranial direct current stimulation (tDCS)
Direct current was transmitted by a saline-soaked pair of surface sponges (35 cm2) and delivered by a battery-driven, constant current stimulator with a maximum output of 10 mA (NeuroConn; http://www.neuroconn.de/). For each subject, we used a bilateral montage over the left and right DLPFC. For all subjects, an anodal electrode was placed over the right dorsolateral prefrontal cortex and the cathodal electrode or return electrode over the left dorsolateral prefrontal cortex. The site for stimulation was determined by the International 10/20 Electroencephalogram System corresponding to F3 and F4, respectively. The direct current was initially increased in a ramp-like fashion over several seconds (10 s) until reaching 1.5 mA. TDCS stimulation was maintained for a total of 20 min.
Transcranial random noise stimulation (tRNS)
The tRNS consisted of an alternating current of 2.0 mA intensity with a 0 mA offset applied at random frequencies. The frequencies ranged from 0.1 to 100 Hz, i.e., low-frequency tRNS. Similar to tDCS, the current was transmitted by a saline-soaked pair of surface sponges (35 cm2) and delivered by specially developed, battery-driven, constant current stimulator with a maximum output of 10 mA (NeuroConn; http://www.neuroconn.de/). For each patient receiving tRNS, one electrode was placed on the T3 and one was placed on T4 as determined by the International 10/20 Electroencephalogram System. The alternating current was initially increased in a ramp-like fashion over several seconds (10 s) until reaching 2.0 mA. In tRNS, stimulation was maintained for a total of 20 min.
Experimental design
The study was in accordance with the ethical standards of the Helsinki declaration (1964) and was approved by the institutional ethics committee. Informed consent was obtained from all individuals included in the study. Patients were randomly assigned to one of the three groups, namely waiting list, tDCS or multisite (tDCS–tRNS). Both the tDCS and multisite group received eight sessions (two times a week for 4 weeks) of treatment, while the waiting list group did not receive treatment for one month. The multisite group first received tDCS for 20 min followed by 20 min of tRNS. The three groups did not differ significantly in age, tinnitus duration, tinnitus type, laterality, and other questionnaires that measure the emotional and loudness component of tinnitus. See descriptions of the tinnitus characteristics per group in Table 1.
Evaluation
Before and after the experimental procedures, the subjects completed a set of validated self-report inventories used before in our studies. Primary outcome of treatment was evaluated for the changes of tinnitus loudness using a Numeric Rating Scale for Loudness (NRS), the Tinnitus Questionnaire (TQ), and the Tinnitus Handicap Inventory (THI).
NRS
A visual analog scale for tinnitus loudness (‘How loud is your tinnitus? 0 = no tinnitus and 100 = as loud as imaginable’) was used.
TQ
Patients were also given the Tinnitus Questionnaire (Meeus et al. 2007). The TQ is a global index of tinnitus distress based on the total score on the TQ, participants were assigned to a distress category: slight (0–30 points; grade 1), moderate (31–46; grade 2), severe (47–59; grade 3), and very severe (60–84; grade 4) distress. Furthermore, Goebel and Hiller (1994) stated that grade 4 tinnitus patients are psychologically decompensated, indicating that patients categorized into this group cannot cope with their tinnitus. In contrast, patients that have a score lower than 60 on the TQ can cope with their tinnitus.
THI
The Tinnitus Handicap Inventory was included because it is a brief and easy-to-administer questionnaire that is suitable for use in busy clinical settings (Newman et al. 1996). The THI is a 25-item self-administered questionnaire that aims to quantify the impact of tinnitus on daily life. Respondents are asked to answer the questions with ‘Yes’ (4 points), ‘Sometimes’ (2 points) or ‘No’ (0 points). A higher THI score (maximum 100) is indicative of a greater tinnitus handicap.
Secondary outcome of treatment was measured using the DS14 (i.e., standard assessment of negative affectivity, social inhibition), the Beck Depression Inventory (BDI) and the Hospital Anxiety and Depression Scale (HADS).
DS14
The DS14 is a 14-item questionnaire that assesses the presence of a Type-D personality. Half the items refer to negative affectivity and the other half refer to social inhibition. A score 10 or above (range 0–28) on both scales classifies a person as a Type-D personality (Denollet 2005).
BDI
The Beck Depression Inventory is a questionnaire to evaluate the severity of depressive mood states. It scores components like hopelessness and feelings of guilt, as well as fatigue and other physical symptoms. It consists of 21 questions rated between 0 (no symptom impact) and 3 (maximum symptom impact) with a maximum score of 63 (Richter et al. 1998).
HADS
The Hospital Anxiety and Depression Scale is designed as a simple yet reliable tool for use in medical practice (Zigmond and Snaith 1983) and considered to be a measure of general distress (Grulke et al. 2005; McCue et al. 2006; Robjant et al. 2009). This scale consists of 14 questions, seven measuring anxiety (score from 0 to 21) and seven measuring depression (score from 0 to 21). Each question is rated on a four-point scale.
Statistical analyses
A repeated measures MANOVA with pre- and post-measure as within-subjects variable and group (waiting list, tDCS, multisite) as between-subjects variable for the primary outcome measures (NRS loudness, TQ, THI) was used. Based on these findings, a univariate repeated measures ANOVA was conducted pre- and post-measure as within-subjects variable and group (waiting list, tDCS, multisite) for the specific primary outcome measure. To further explore the data, the individual percentage of improvement for NRS loudness, TQ, and THI was calculated. These scores were used as the dependent measures using an ANOVA with the group variable (waiting list, tDCS, multisite) as independent measurement. For the secondary outcome measures, we used a similar method and applied this method for the DS14 (negative affectivity and social inhibition) and mood questionnaires (BDI, HADS depression, HADS anxiety), respectively.
Results
Primary outcome measures
A repeated measures MANOVA including the pre- and post-measure as within-subjects variable and group (waiting list, tDCS, multisite) as between-subjects variable for the NRS loudness, TQ and THI showed a significant effect for the pre–post-measurement (F = 6.11, p = .002) as well as an interaction effect for the pre–post-measurement and group (F = 3.92, p = .002). No significant main effect was obtained for group (F = 1.57, p = .17).
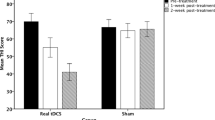
A univariate repeated measures ANOVA indicated that for NRS loudness there was a significant effect for the pre–post-measurement (F = 6.93, p = .001) as well as a significant interaction effect (F = 3.98, p = .002). The main effect for the pre–post-measurement (F = 7.63, p = .002) showed a reduction in the post-measurement (M = 68.10, SD = 10.85) in comparison to the pre-measurement (M = 76.58, SD = 16.81). This effect was moderated by the group the patient was assigned to; that is, the interaction effect revealed for the tDCS group a significant reduction of 14.20 % when comparing pre- versus post-measurement for NRS loudness (F = 5.69, p = .022). For the sequential stimulation of bifrontal tDCS followed by auditory cortex tRNS, results showed a significant suppression of 21.26 % when comparing pre- versus post-treatment (F = 10.14, p = .003). No significant effect was obtained when comparing pre versus post for the waiting list group (F = .71, p = .40). A comparison between the groups showed that the suppression effect obtained by the multisite protocol (tDCS–tRNS) was larger than those obtained for tDCS and the waiting list group (F = 5.09, p = .011). In addition, the group that received only tDCS also had a larger effect than the waiting list group (F = 1.52, p = .030) (see Fig. 1).
A univariate repeated measures ANOVA showed that for the TQ there was an effect for the pre–post-measurement (F = 17.01, p < .001) and an interaction effect (F = 10.19, p < .001). The main effect showed that post-measurement (M = 43.88, SD = 13.45) had a reduced score on the TQ in comparison to the pre-measurement (M = 49.52, SD = 11.46). A closer look at the data revealed that this effect was moderated by the group the patient was assigned to. No significant effect was obtained when comparing pre versus post for the waiting list group (F = .85, p = .36). However, for the tDCS group, a significant reduction of 13.03 % was obtained when comparing pre- versus post-measurements for the TQ (F = 6.86, p = .013). In addition, the sequential stimulation of bifrontal tDCS followed by auditory cortex tRNS showed a significant suppression of 25.90 % when comparing pre- versus post-treatment (F = 29.85, p < .001). A comparison between the groups specified that the suppression effect obtained by the multisite protocol was larger than for the tDCS and waiting list group (F = 12.12, p < .001) (see Fig. 1).
A univariate repeated measures ANOVA showed for the THI a main effect for the pre–post-measurement (F = 11.72, p = .002) indicating a reduction in the post-measurement (M = 46.50, SD = 17.52) in comparison to the pre-measurement (M = 53.45, SD = 16.76). This effect was moderated by group showing an interaction effect (F = 6.02, p = .005) for the THI. For the waiting list group as well as for the tDCS group, no effect was obtained when comparing the pre- and post-measurement (waiting list: F = .002, p = .97; tDCS: F = 1.37, p = .002). However, for the sequential stimulation of bifrontal tDCS followed by auditory cortex tRNS, a significant effect was obtained (F = 22.90, p < .001) demonstrating a suppression of 27.79 %. A comparison between the groups indicated that the effect obtained for the multisite treatment was larger than for the TDCS and the waiting list group (F = 5.61, p = .007) (see Fig. 1).
Secondary outcome measures
For the DS14, a repeated measures MANOVA including the pre- and post-measure, as within-subjects variable, and group (waiting list, tDCS, multisite), as between-subjects variable, for both the negative affect and social inhibition showed only an effect for the pre–post-measurement (F = 5.83, p = .006). No effect was obtained for group (F = .47, p = .76) or for the interaction effect (F = 1.06, p = .39). A univariate repeated measures ANOVA revealed a significant main effect for the pre–post-measurement of negative affect (F = 6.98, p = .012), but not for the pre–post-measurement of social inhibition (F = .13, p = .72). For negative affect, we found a decrease in negative affect for the post-measurement (M = 11.63, SD = 5.96) in comparison to the pre-measurement (M = 14.05, SD = 6.37) (see Fig. 2).
For the BDI and both subscales of the HADS, a repeated measures MANOVA including the pre- and post-measure as within-subjects variable and group (waiting list, tDCS, multisite) as between-subjects variable indicated only an effect for the pre–post-measurement (F = 3.63, p = .023). No effect was obtained for group (F = .11, p = .99) or for the interaction effect (F = 1.50, p = .19). A univariate repeated measures ANOVA showed a significant main effect for the pre–post-measurement of BDI (F = 4.88, p = .03), HADS depression (F = 7.80, p = .008) and HADS anxiety (F = 6.83, p = .013). For BDI, we found a decrease in depressive feelings for the post-measurement (M = 13.50, SD = 7.79) in comparison to the pre-measurement (M = 15.63, SD = 8.04). For HADS depression, we saw a similar effect with a decrease in depressive feelings for the post-measurement (M = 8.00, SD = 3.42) in comparison to the pre-measurement (M = 9.55, SD = 3.83). For HADS anxiety, we demonstrated a decrease in anxiety levels for the post-measurement (M = 7.18, SD = 3.17) in comparison to the pre-measurement (M = 8.50, SD = 2.94) (see Fig. 2).
Discussion
This proof-of-concept study shows that a multisite treatment protocol that consists of bifrontal tDCS followed by auditory cortex tRNS results in more pronounced effects when compared with the bifrontal tDCS protocol or a waiting list group, suggesting an added value of auditory cortex tRNS to the bifrontal tDCS protocol for tinnitus patients. There were no adverse effects associated with this new treatment protocol of eight sessions and using the same transcranial electrical stimulator for performing the tDCS and the tRNS consequently is feasible for clinical routine.
These results are, to our knowledge, the first to demonstrate an immediate and superior improvement of a combination of frontal and auditory transcranial electrical stimulation. The study of Pal and his colleagues (2015) did not show a beneficial effect on tinnitus with their tDCS protocol. They simultaneously tried to stimulate the frontal cortex and inhibit left and right auditory cortex by placing the anode over F3–Fz–F4 for prefrontal cortex stimulation and two cathodes at T3 and T4 corresponding to the left and right auditory cortex. The stimulation protocol used by Pal and his colleagues (2015) is different from our multisite stimulation protocol as (1) our stimulations were conducted sequentially instead of simultaneously, (2) anodal right DLPFC was targeted in our protocol instead of a more central prefrontal area and (3) tRNS was applied over the auditory cortex instead of two cathodes of tDCS over the auditory cortex, as tRNS has been found to be more effective than tDCS when targeting the auditory cortex. Comparing our results using transcranial electrical stimulation (i.e., tDCS and tRNS) with the multisite rTMS studies, the multisite rTMS studies only reported long-term superior effects (after 3 months), but no immediate effects of combining DLPFC rTMS followed by auditory cortex rTMS.
Different explanations may account for the more pronounced effects of the multisite treatment protocol compared to the bifrontal DLPFC tDCS. A possible explanation of the improved results of the multisite treatment protocol is the additive effect of combining two effective treatments for tinnitus targeting two core regions of the tinnitus network. If a network consisting of auditory and non-auditory brain areas and altered connectivity between these areas forms the neural basis for tinnitus, targeting the whole network (Schlee et al. 2009) by stimulating multiple core regions in the network might enhances the effect (Lehner et al. 2013). Bifrontal tDCS to the DLPFC (Vanneste et al. 2010b, 2013b; Vanneste and De Ridder 2011; De Ridder and Vanneste 2012; Faber et al. 2012; Frank et al. 2012) as well as tRNS to the auditory cortex (Vanneste et al. 2013a), separately, has been found beneficial for suppressing tinnitus symptoms. Therefore, combining two effective techniques sequentially would explain the enhanced or added treatment effects.
Another hypothesis for the added effect can be explained by the preconditioning phenomenon. This is the potential of the stimulation to interact with the prior state of the cortex. We can postulate that by preconditioning the brain state with one stimulation protocol targeting a core region of the tinnitus network, the effect of the second stimulation protocol targeting another region of the tinnitus network can be enhanced. Studies have mostly investigated the preconditioning or priming effects of tDCS on the aftereffects of rTMS targeting the same brain region (Lang et al. 2004; Siebner et al. 2004). However, this has not been explored for priming effects of tDCS on the aftereffects of tRNS on different brain regions. It is of interest, however, that frontal tDCS has been shown to change auditory cortex activity (Vanneste and De Ridder 2011), in keeping with this hypothesis.
Interestingly, the multisite treatment protocol was only found superior for the primary outcome measures for tinnitus, which are the more general tinnitus assessments measuring tinnitus intensity and tinnitus distress as a transient aversive state (Joos et al. 2012), such as on the Visual Analog Scale (“how loud is your tinnitus?”—loudness), the Tinnitus Questionnaire (global index of distress—distress), and the Tinnitus Handicap Inventory (impact of tinnitus on daily life—handicap). This points to an added value of auditory cortex tRNS to the bifrontal DLFPC tDCS on more general tinnitus aspects. The superior effect was not found for the secondary outcome measures that are more related to emotional components of tinnitus as a constant emotional state, namely the DS14, BDI, and the HADS showing that auditory cortex tRNS does not add value to the bifrontal DLFPC tDCS regarding these measures. Bifrontal DLPFC tDCS has repeatedly been found to modulate affective processing and to be effective for depression (Fregni et al. 2006). For tinnitus, bilateral tDCS of the DLPFC has been found to interfere with the emotional processing of tinnitus (i.e., tinnitus-related distress) by modulating an alpha oscillatory network consisting of the parahippocampus, subgenual anterior cingulate cortex, dorsal lateral prefrontal cortex, amygdala, and insula and associated with beta activity in the dorsal anterior cingulate cortex (Vanneste et al. 2010a; Vanneste and De Ridder 2011). Furthermore, DLPFC tDCS had been shown to reduce tinnitus intensity by modulating gamma band activity in the auditory cortex (van der Loo et al. 2009b; Vanneste and De Ridder 2011). Thus, it appears that bifrontal tDCS, but not auditory cortex tRNS, is targeting the tinnitus distress network (Schlee et al. 2009; Vanneste et al. 2010a; Langguth et al. 2012). Therefore, it is not surprising that for the secondary outcome measures, the auditory cortex tRNS does not provide an added effect on the emotional components of tinnitus.
This study has some limitations. First, the stimulation duration was not equal over the compared treatment protocols. The patients receiving only tDCS were treated with 20 min of tDCS per day, whereas patients receiving the multisite stimulation were treated with 40 min of stimulation (20-min tDCS and 20-min tRNS) per day. It remains unclear whether the superior effect of the multisite protocol is due to the longer duration of stimulation (2 × 20 min) or due to the fact that more stimulation sites were targeted. However, there is no evidence, to our knowledge, for tinnitus, that the effect of transcranial electrical stimulation is dose dependent with longer stimulation resulting in more tinnitus reduction. Moreover, studies have found that increasing the stimulation duration on one stimulation site does not seem to be a successful approach to increase the efficacy of tDCS (Batsikadze et al. 2013; Nitsche et al. 2015; To et al. 2016). Therefore, the superior effects of the multisite stimulation protocol seem to be caused more by the combination effect of tDCS on the DLPFC and tRNS on the auditory cortex. Second, our study design did not allow us to elucidate the mechanisms of effects in the multisite protocol on the different components. Because we only measured the effect of the multisite protocol after both bifrontal tDCS and auditory cortex tRNS and not after each separate intervention (i.e., measurement after tDCS and measurement after tDCS and tRNS), we cannot disentangle whether bifrontal tDCS acted on the emotional component of tinnitus first and then the tinnitus loudness component or vice versa or whether the emotional component mediated the improvement in the tinnitus loudness component. More research is needed to investigate the mechanisms of effect in multisite treatment protocols and the mechanisms of effect in bifrontal tDCS protocols as the emotional component of diseases may have an important influence on the disorder in general. This will help us to further understand the mechanisms of tinnitus. Third, this study used a waiting list group as a control condition and not a sham stimulation not being able to fully control for a possible placebo response in any active conditions. Fourth, the study did not include a long-term follow-up of the tinnitus patients, not being able to measure possible long-term effects of the multisite stimulation protocol. Lastly, the sample used in this study is relatively small. Therefore, the results need to be interpreted with caution and further research is needed before implementing this multisite treatment protocol as a routine administration.
In conclusion, this multisite transcranial electrical stimulation protocol showed superior and promising effects for the suppression of tinnitus loudness and distress, therefore, supporting the involvement of the prefrontal and auditory cortex in the pathophysiology of tinnitus and demonstrating the idea of a network stimulation. The stimulation protocol is feasible for clinical routine and was well tolerated by all participants. Further studies should take the limitations of this study into account and analyze the neurobiological effects of this new stimulation paradigm.
References
Albert R, Jeong H, Barabasi AL (2000) Error and attack tolerance of complex networks. Nature 406:378–382. doi:10.1038/35019019
Axelsson A, Ringdahl A (1989) Tinnitus–a study of its prevalence and characteristics. Br J Audiol 23:53–62. doi:10.3109/03005368909077819
Baguley DM (2002) Mechanisms of tinnitus. Br Med Bull 63:195–212
Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA (2013) Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol 591:1987–2000. doi:10.1113/jphysiol.2012.249730
Carpenter-Thompson JR, Akrofi K, Schmidt SA, Dolcos F, Husain FT (2014) Alterations of the emotional processing system may underlie preserved rapid reaction time in tinnitus. Brain Res 1567:28–41. doi:10.1016/j.brainres.2014.04.024
Claes L, Stamberger H, Van de Heyning P, De Ridder D, Vanneste S (2014) Auditory cortex tACS and tRNS for tinnitus: single versus multiple sessions. Neural Plast 2014:436713. doi:10.1155/2014/436713
De Ridder D, Vanneste S (2012) EEG driven tDCS versus bifrontal tDCS for tinnitus. Front Psychiatry 3:84. doi:10.3389/fpsyt.2012.00084
De Ridder D, Elgoyhen AB, Romo R, Langguth B (2011a) Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci USA 108:8075–8080. doi:10.1073/pnas.1018466108
De Ridder D, Vanneste S, Congedo M (2011b) The distressed brain: a group blind source separation analysis on tinnitus. PLoS One 6:e24273. doi:10.1371/journal.pone.0024273
De Ridder D, Congedo M, Vanneste S (2015) The neural correlates of subjectively perceived and passively matched loudness perception in auditory phantom perception. Brain Behav. doi:10.1002/brb3.331
Denollet J (2005) DS14: standard assessment of negative affectivity, social inhibition, and type D personality. Psychosom Med 67:89–97. doi:10.1097/01.psy.0000149256.81953.49
Eggermont JJ, Roberts LE (2004) The neuroscience of tinnitus. Trends Neurosci. doi:10.1016/j.tins.2004.08.010
Eichhammer P, Hajak G, Kleinjung T, Landgrebe M, Langguth B (2007) Functional imaging of chronic tinnitus: the use of positron emission tomography. Prog Brain Res 166:83–88. doi:10.1016/S0079-6123(07)66008-7
Faber M, Vanneste S, Fregni F, De Ridder D (2012) Top down prefrontal affective modulation of tinnitus with multiple sessions of tDCS of dorsolateral prefrontal cortex. Brain Stimul 5:492–498. doi:10.1016/j.brs.2011.09.003
Frank E, Schecklmann M, Landgrebe M et al (2012) Treatment of chronic tinnitus with repeated sessions of prefrontal transcranial direct current stimulation: outcomes from an open-label pilot study. J Neurol 259:327–333. doi:10.1007/s00415-011-6189-4
Fregni F, Boggio PS, Nitsche MA, Marcolin MA, Rigonatti SP, Pascual-Leone A (2006) Treatment of major depression with transcranial direct current stimulation. Bipolar Disord 8:203–204. doi:10.1111/j.1399-5618.2006.00291.x
Goebel G, Hiller W (1994) The tinnitus questionnaire. A standard instrument for grading the degree of tinnitus. Results of a multicenter study with the tinnitus questionnaire. HNO 42:166–172
Grulke N, Bailer H, Kachele H, Bunjes D (2005) Psychological distress of patients undergoing intensified conditioning with radioimmunotherapy prior to allogeneic stem cell transplantation. Bone Marrow Transplant 35:1107–1111. doi:10.1038/sj.bmt.1704971
Heller AJ (2003) Classification and epidemiology of tinnitus. Otolaryngol Clin North Am 36:239–248
Jastreboff PJ (1990) Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res 8:221–254
Joos K, Vanneste S, De Ridder D (2012) Disentangling depression and distress networks in the tinnitus brain. PLoS One 7:e40544. doi:10.1371/journal.pone.0040544
Joos K, De Ridder D, Van de Heyning P, Vanneste S (2014) Polarity specific suppression effects of transcranial direct current stimulation for tinnitus. Neural Plast 2014:930860. doi:10.1155/2014/930860
Kleinjung T, Eichhammer P, Landgrebe M et al (2008) Combined temporal and prefrontal transcranial magnetic stimulation for tinnitus treatment: a pilot study. Otolaryngol Head Neck Surg 138:497–501. doi:10.1016/j.otohns.2007.12.022
Lang N, Siebner HR, Ernst D, Nitsche MA, Paulus W, Lemon RN, Rothwell JC (2004) Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects. Biol Psychiatry 56:634–639. doi:10.1016/j.biopsych.2004.07.017
Langguth B, Eichhammer P, Kreutzer A et al (2006) The impact of auditory cortex activity on characterizing and treating patients with chronic tinnitus–first results from a PET study. Acta Otolaryngol. doi:10.1080/03655230600895317
Langguth B, Schecklmann M, Lehner A et al (2012) Neuroimaging and neuromodulation: complementary approaches for identifying the neuronal correlates of tinnitus. Front Syst Neurosci 6:15. doi:10.3389/fnsys.2012.00015
Langguth B, Landgrebe M, Frank E et al (2014) Efficacy of different protocols of transcranial magnetic stimulation for the treatment of tinnitus: pooled analysis of two randomized controlled studies. World J Biol Psychiatry 15:276–285. doi:10.3109/15622975.2012.708438
Lehner A, Schecklmann M, Poeppl TB et al (2013) Multisite rTMS for the treatment of chronic tinnitus: stimulation of the cortical tinnitus network—a pilot study. Brain Topogr 26:501–510. doi:10.1007/s10548-012-0268-4
Lockwood AH, Salvi RJ, Burkard RF, Galantowicz PJ, Coad ML, Wack DS (1999) Neuroanatomy of tinnitus. Scand Audiol Suppl 51:47–52
McCue P, Buchanan T, Martin CR (2006) Screening for psychological distress using internet administration of the Hospital Anxiety and Depression Scale (HADS) in individuals with chronic fatigue syndrome. Br J Clin Psychol 45:483–498. doi:10.1348/014466505X82379
Meeus O, Blaivie C, Van de Heyning P (2007) Validation of the Dutch and the French version of the Tinnitus Questionnaire. B-Ent 3(Suppl 7):11–17
Miranda PC, Lomarev M, Hallett M (2006) Modeling the current distribution during transcranial direct current stimulation. Clin Neurophysiol 117:1623–1629. doi:10.1016/j.clinph.2006.04.009
Mohan A, De Ridder D, Vanneste S (2016a) Emerging hubs in phantom perception connectomics. Neuroimage Clin 11:181–194. doi:10.1016/j.nicl.2016.01.022
Mohan A, De Ridder D, Vanneste S (2016b) Graph theoretical analysis of brain connectivity in phantom sound perception. Sci Rep 6:19683. doi:10.1038/srep19683
Muhlau M, Rauschecker JP, Oestreicher E et al (2006) Structural brain changes in tinnitus. Cereb Cortex 16:1283–1288. doi:10.1093/cercor/bhj070
Muhlnickel W, Elbert T, Taub E, Flor H (1998) Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci USA 95:10340–10343
Newman CW, Jacobson GP, Spitzer JB (1996) Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg 122:143–148
Nitsche MA, Paulus W (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527(Pt 3):633–639
Nitsche MA, Polania R, Kuo MF (2015) Transcranial direct current stimulation: modulation of brain pathways and potential clinical applications. In: Reti IM (ed) Brain stimulation: methodologies & interventions. Wiley-Blackwell, Hoboken
Pal N, Maire R, Stephan MA, Herrmann FR, Benninger DH (2015) Transcranial direct current stimulation for the treatment of chronic tinnitus: a randomized controlled study. Brain Stimul 8:1101–1107. doi:10.1016/j.brs.2015.06.014
Plewnia C, Reimold M, Najib A, Brehm B, Reischl G, Plontke SK, Gerloff C (2007) Dose-dependent attenuation of auditory phantom perception (tinnitus) by PET-guided repetitive transcranial magnetic stimulation. Hum Brain Mapp 28:238–246. doi:10.1002/hbm.20270
Rauschecker JP, Leaver AM, Muhlau M (2010) Tuning out the noise: limbic–auditory interactions in tinnitus. Neuron 66:819–826. doi:10.1016/j.neuron.2010.04.032
Richter P, Werner J, Heerlein A, Kraus A, Sauer H (1998) On the validity of the Beck Depression Inventory. A review. Psychopathology 31:160–168
Robjant K, Robbins I, Senior V (2009) Psychological distress amongst immigration detainees: a cross-sectional questionnaire study. Br J Clin Psychol. doi:10.1348/014466508X397007
Schlee W, Mueller N, Hartmann T, Keil J, Lorenz I, Weisz N (2009) Mapping cortical hubs in tinnitus. BMC Biol 7:80. doi:10.1186/1741-7007-7-80
Scott B, Lindberg P (2000) Psychological profile and somatic complaints between help-seeking and non-help-seeking tinnitus subjects. Psychosomatics 41:347–352. doi:10.1176/appi.psy.41.4.347
Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC (2004) Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci 24:3379–3385. doi:10.1523/JNEUROSCI.5316-03.2004
Smits M, Kovacs S, de Ridder D, Peeters RR, van Hecke P, Sunaert S (2007) Lateralization of functional magnetic resonance imaging (fMRI) activation in the auditory pathway of patients with lateralized tinnitus. Neuroradiology 49:669–679. doi:10.1007/s00234-007-0231-3
To WT, Hart J, De Ridder D, Vanneste S (2016) Considering the influence of stimulation parameters on the effect of conventional and high-definition transcranial direct current stimulation. Expert Rev Med Devices 13:391–404. doi:10.1586/17434440.2016.1153968
van der Loo E, Gais S, Congedo M et al (2009a) Tinnitus intensity dependent gamma oscillations of the contralateral auditory cortex. PLoS One 4(e7396):7391–7395. doi:10.1371/journal.pone.0007396
van der Loo E, Gais S, Congedo M et al (2009b) Tinnitus intensity dependent gamma oscillations of the contralateral auditory cortex. PLoS One 4:e7396. doi:10.1371/journal.pone.0007396
Van Doren J, Langguth B, Schecklmann M (2014) Electroencephalographic effects of transcranial random noise stimulation in the auditory cortex. Brain Stimul 7:807–812. doi:10.1016/j.brs.2014.08.007
Vanneste S, De Ridder D (2011) Bifrontal transcranial direct current stimulation modulates tinnitus intensity and tinnitus-distress-related brain activity. Eur J Neurosci 34:605–614. doi:10.1111/j.1460-9568.2011.07778.x
Vanneste S, De Ridder D (2016) Deafferentation-based pathophysiological differences in phantom sound: tinnitus with and without hearing loss. Neuroimage 129:80–94. doi:10.1016/j.neuroimage.2015.12.002
Vanneste S, Plazier M, der Loo E, de Heyning PV, Congedo M, De Ridder D (2010a) The neural correlates of tinnitus-related distress. Neuroimage 52:470–480. doi:10.1016/j.neuroimage.2010.04.029
Vanneste S, Plazier M, Ost J, van der Loo E, Van de Heyning P, De Ridder D (2010b) Bilateral dorsolateral prefrontal cortex modulation for tinnitus by transcranial direct current stimulation: a preliminary clinical study. Exp Brain Res 202:779–785. doi:10.1007/s00221-010-2183-9
Vanneste S, van de Heyning P, De Ridder D (2011) The neural network of phantom sound changes over time: a comparison between recent-onset and chronic tinnitus patients. Eur J Neurosci 34:718–731. doi:10.1111/j.1460-9568.2011.07793.x
Vanneste S, Fregni F, De Ridder D (2013a) Head-to-head comparison of transcranial random noise stimulation, transcranial AC stimulation, and transcranial DC stimulation for tinnitus. Front Psychiatry 4:158. doi:10.3389/fpsyt.2013.00158
Vanneste S, Walsh V, Van De Heyning P, De Ridder D (2013b) Comparing immediate transient tinnitus suppression using tACS and tDCS: a placebo-controlled study. Exp Brain Res 226:25–31. doi:10.1007/s00221-013-3406-7
Weisz N, Muller S, Schlee W, Dohrmann K, Hartmann T, Elbert T (2007) The neural code of auditory phantom perception. J Neurosci 27:1479–1484. doi:10.1523/JNEUROSCI.3711-06.2007
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67:361–370
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
To, W.T., Ost, J., Hart, J. et al. The added value of auditory cortex transcranial random noise stimulation (tRNS) after bifrontal transcranial direct current stimulation (tDCS) for tinnitus. J Neural Transm 124, 79–88 (2017). https://doi.org/10.1007/s00702-016-1634-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-016-1634-2