Abstract
Tinnitus is considered as an auditory phantom percept. Preliminary evidence indicates that transcranial direct current stimulation (tDCS) of the temporo-parietal area might reduce tinnitus. tDCS studies of the prefrontal cortex have been successful in reducing depression, impulsiveness and pain. Recently, it was shown that the prefrontal cortex is important for the integration of sensory and emotional aspects of tinnitus. As such, frontal tDCS might suppress tinnitus as well. In an open label study, a total of 478 tinnitus patients received bilateral tDCS on dorsolateral prefrontal cortex (448 patients anode right, cathode left and 30 anode left, cathode right) for 20 min. Treatment effects were assessed with visual analogue scale for tinnitus intensity and distress. No tinnitus-suppressing effect was found for tDCS with left anode and right cathode. Analyses show that tDCS with right anode and left cathode modulates tinnitus perception in 29.9% of the tinnitus patients. For these responders a significant reduction was found for both tinnitus-related distress and tinnitus intensity. In addition, the amount of suppression for tinnitus-related distress is moderated by an interaction between tinnitus type and tinnitus laterality. This was, however, not the case for tinnitus intensity. Our study supports the involvement of the prefrontal cortex in the pathophysiology of tinnitus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tinnitus is a common and disturbing symptom, characterized by the perception of sound or noise in the absence of an auditory stimulus. This auditory phantom percept is often associated with symptoms such as annoyance, concentration problems, anxiety, depression, sleep disturbances, intense worrying and can as a consequence cause a serious amount of distress (Scott and Lindberg 2000). Approximately, 15% of adult population experience tinnitus at some point in their life, and 10–15% of these individuals perceive tinnitus continuously, increasing up to 33% in the elderly population (Axelsson and Ringdahl 1989; Nondahl et al. 2002; Sindhusake et al. 2004). Like auditory hallucinations, tinnitus is also considered an auditory phantom percept related to plastic alterations in the auditory cortex (Muhlnickel et al. 1998). Neuroimaging and electrophysiologic studies indicate an excessive spontaneous activity in the central auditory nervous system and changes in the tonotopic map of the auditory cortex as the neurobiological basis of tinnitus (Weisz et al. 2007; Salvi et al. 2000; Lockwood et al. 1998; Schlee et al. 2009; Smits et al. 2007).
Consistent with the hypothesis that tinnitus is caused by over activation of the auditory cortex, transcranial magnetic stimulation (TMS) can efficiently reduce this cortical hyperactivity temporarily by directly stimulating temporal cortex (De Ridder et al. 2007; Kleinjung et al. 2008). In addition, it was shown that extradural electrical stimulation of the auditory cortex can also suppress tinnitus (De Ridder et al. 2004; Friedland et al. 2007). Interestingly, a recent study demonstrated that transcranial direct current stimulation (tDCS) of the tempororietal area can alter tinnitus perception as well (Fregni et al. 2006). tDCS is an non-invasive and painless tool which modulates cortical excitability in the brain regions of interest through a weak direct current (DC). Depending on the polarity of the stimulation, tDCS can increase or decrease cortical excitability in the brain regions to which it is applied (Miranda et al. 2006). Currently, tDCS is usually applied through two surface electrodes, one serving as the anode and the other as the cathode. Some of the applied current is shunted through scalp tissue and only a part of the applied current passes through the brain. Anodal tDCS typically has an excitatory effect on the local cerebral cortex by depolarizing neurons, while under the cathode hyperpolarization is induced. This effect of tDCS typically outlasts the stimulation by an hour or longer after a single treatment session of sufficiently long stimulation duration (Nitsche and Paulus 2000, 2001; Nitsche et al. 2003; Antal et al. 2004). Compared with extradural electrical stimulation this technique has the advantage of being non-invasive. As an advantage to TMS, tDCS is easier to apply and has not been related to seizures. Taken together, tDCS could potentially be a promising and easily applicable technique to suppress tinnitus.
New insights into the neurobiology of tinnitus suggest that neuronal changes are not limited to the classical auditory pathways. In particular, the dorsolateral prefrontal cortex (DLPFC) seems to play a specific role in auditory processing. That is, the DLPC has a bilateral facilitatory effect on auditory memory storage and contains auditory memory cells (Bodner et al. 1996). The DLPFC also exerts early inhibitory modulation of input to primary auditory cortex in humans (Knight et al. 1989) and has been found to be associated with auditory attention (Voisin et al. 2006; Lewis et al. 2000; Alain et al. 1998) resulting in top-down modulation of auditory processing (Mitchell et al. 2005). This was further confirmed by electrophysiological data indicating that tinnitus might occur as the result of a dysfunction in the top-down inhibitory processes (Norena et al. 1999). Interestingly, several tDCS studies focused on DLPFC and found successful results for treating major depression (Fregni et al. 2006) and mood changes in depression (Fregni et al. 2006), as well as reducing impulsiveness (Beeli et al. 2008) and the pain threshold (Boggio et al. 2008, 2009). As the DLPFC is involved in attention-mediated top-down control of auditory processing and tinnitus, and tDCS seems like a promising technique for modulating the DLPFC, it is possible that using frontal tDCS might be a useful technique for the suppressing tinnitus.
The aim of the present study is to (1) investigate whether tDCS of the DLPFC is a valuable technique for treating tinnitus and (2) verify whether tDCS is a good alternative for TMS. Furthermore, a comparison will be made between different types of tinnitus (pure tone vs. narrow band), and tinnitus laterality (unilateral and bilateral), as previous studies already revealed that these variables have an important impact on TMS results.
Method
Subjects
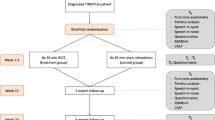
We studied 543 patients (male 195, female 348) with chronic tinnitus (>1 year). The mean age was 56.83 years (SD 11.49). See Table 1 for an overview of demographic and tinnitus-related data.
tDCS is performed as a neuromodulation technique in the treatment for tinnitus in the multidisciplinary TRI tinnitus clinic of the University of Antwerp, Belgium, which was approved by the Ethical committee of the Antwerp University Hospital. All prospective participants underwent a complete audiological, ENT and neurological investigation to rule out possible treatable causes for their tinnitus. Tinnitus matching is performed by presenting sounds to the ear in which the tinnitus is not perceived. Technical investigations include MRI of the brain and posterior fossa, pure tone and speech audiometry and tympanometry.
Transcranial direct current stimulation
Direct current was transmitted by a saline-soaked pair of surface sponge (35 cm2) and delivered by specially developed, battery-driven, constant current stimulator with a maximum output of 10 mA (Eldith©; http://www.eldith.de). For each patient receiving tDCS, one electrode was placed over the left DLPFC and one was placed on the right DLPFC as determined by the International 10/20 Electroencephalogram System corresponding to F3 and F4, respectively. A constant current of 1.5 mA intensity was applied for 20 min.
Four-hundred and forty-eight patients received tDCS with cathodal electrode placed over the left DLPFC and the anode placed overlying the right DLPFC; 30 patients received tDCS with anode electrode placed over the left DLPFC and the cathode placed on the right DLPFC; and 65 perceived no tDCS (i.e. waiting list control group).
Evaluation
A visual analogue scale for tinnitus intensity (‘How loud is your tinnitus?’, 0 = no tinnitus and 10 = as loud as imaginable) and tinnitus distress (‘How stressful is your tinnitus?’, 0 = no distress and 10 = suicidal distress) was administered before (pre) and directly after (post) tDCS stimulation.
Statistical analyses
Calculations were performed using SPSS software package. A paired two-sample t test was conducted for VAS intensity pre and post and VAS distress pre and post, respectively.
A linear regression analysis was conducted with gender, age, tinnitus type, tinnitus laterality and tinnitus duration as independent variables with, respectively, tinnitus perception and tinnitus-related distress as dependent variables. The variables gender, tinnitus type and tinnitus laterality were recoded in contrast variables, respectively, gender (women 1 and man −1), tinnitus type (narrow band noise 1 and pure tone −1) and tinnitus laterality (bilateral 1 and unilateral −1).
Responders are defined as patients that respond to tDCS treatment (VAS pre–VAS post >0), while non-responders are defined as patients that do not respond to tDCS treatment (VAS pre–VAS post ≤0). A logistic regression analysis was conducted to verify whether the independent variables gender, age, tinnitus type, tinnitus laterality and tinnitus duration could predict if a patient would respond to tDCS treatment.
A paired two-sample t test is conducted for VAS intensity pre and post and VAS distress pre and post, respectively. A univariate ANOVA on the amount of reduction between pre and post VAS intensity with type (pure tone vs. narrow band noise) and laterality (unilateral vs. bilateral) as fixed factors and tinnitus durations as a covariate was conducted. A similar analysis was conducted for tinnitus-related distress. Type I error risk was set up at 0.05. If otherwise indicated data are given as mean (M) and standard deviation (SD).
Results
For patients assigned to the waiting list control group no significant effects were obtained for, respectively, VAS intensity and VAS distress pre versus post. No results were also found for patients receiving tDCS with the anode overlying the left and cathode overlying the right DLPFC for, respectively, VAS intensity and VAS distress pre versus post. Yet, patients receiving tDCS, anode right and cathode left, a paired two-sample t test of participants revealed a significant decrease for VAS intensity when comparing VAS intensity pre (M = 6.54; SD = 2.15) and post (M = 6.02; SD = 2.90) (t = 7.11, P < 0.001), revealing a reduction of 7.95%. A similar analysis yielded also a significant decrease for VAS distress when comparing pre (M = 6.03; SD = 2.42) versus post (M = 5.55; SD= 3.02) (t = 6.41, P < 0.001), demonstrating a suppression effect of 7.95% (see Fig. 1 for overview).
Of these patients receiving tDCS, anode right and cathode left, 134 (29.9%) responded to the tDCS treatment and 314 (70.1%) did not. This latter group had a suppression of 27.56 and 31.26% for, respectively, intensity and distress. A regression analysis (responders and non-responders included) revealed that the amount of suppression was independent of gender, age, tinnitus type, tinnitus laterality and tinnitus duration for, respectively, tinnitus perception and tinnitus-related distress (see Table 2). Logistic analysis verifying whether the independent variables gender, age, tinnitus type, tinnitus laterality and tinnitus duration could discriminate between responders and non-responders yielded no significant effect for respectively tinnitus perception and tinnitus-related distress (see Table 2).
For the responders, a univariate ANOVA on the amount of reduction between pre and post VAS intensity (=VAS intensity pre–post) with type (pure tone vs. narrow band noise) and laterality (unilateral vs. bilateral) as fixed factors and tinnitus durations as a covariate revealed no significant effect. However, when conducting a similar analysis on the amount of difference between pre and post VAS distress (=VAS distress pre–post), the interaction effect type X laterality almost yielded significance (F = 3.59, P < 0.06; see Fig. 2). A simple contrast revealed a significant effect for pure tone (F = 5.02, P < 0.05), indicating less reduction for unilateral tinnitus in comparison with bilateral tinnitus. No significant effect was obtained for narrow band noise between unilateral and bilateral tinnitus.
To better understand unilateral tinnitus has less reduction than bilateral tinnitus for pure tone, we verified whether there was a difference between left- and right-side tinnitus. An independent sample t test with as dependent variable amount of reduction between pre and post VAS distress and independent variable tinnitus unilaterality (left vs. right) demonstrated a significant effect for unilaterality (t = 2.22, P < 0.05). This analysis showed that there was more suppression on left-sided tinnitus (M = 1.38, SD = 1.27) than on right-sided (M = 0.17, SD = 0.41) tinnitus.
Discussion
Our results show that bilateral tDCS of the DLPFC (anodal F4, cathodal F3) can decrease tinnitus in one-third of the patients with chronic tinnitus, while bilateral tDCS of DLPFC (anodal F3, cathodal F4) has no influence on tinnitus. For bilateral tDCS of the DLPFC (anodal F4, cathodal F3), gender, age, tinnitus type, tinnitus laterality and tinnitus duration on had no effect the amount of tinnitus perception and tinnitus-related distress as well as could not predict responders versus non-responders. For the responders only to bilateral tDCS of the DLPFC (anodal F4, cathodal F3) a reduction was found for both tinnitus-related distress and tinnitus intensity. In addition, it is revealed that the amount of suppression for tinnitus-related distress but not for tinnitus intensity is moderated by an interaction between tinnitus type and tinnitus laterality. For the latter effect it is shown that unilateral pure tone tinnitus is less suppressed than bilateral pure tone as well as unilateral and bilateral narrow band noise. A closer look at the data indicates that the reduced suppression effect for unilateral pure tone tinnitus can mainly be explained by a very limited suppressive effect on right-sided tinnitus.
Suppression of tinnitus by tDCS seems to be related to enhancing excitability of right prefrontal cortex and reducing excitability of the left prefrontal cortex as we only found a tinnitus-suppressing effect with the anode overlying the right and the cathode overlying the left DLPFC. However, it is possible that tDCS stimulation of bilateral DLPFC with anode left and cathode right might produce only clinically beneficial effects after repetitive tDCS (rtDCS), similar to what is noted in tDCS for depression. A recent study demonstrated that anodal tDCS of the left DLPFC has an effect on depressive symptoms after five sessions (Fregni et al. 2006). As tinnitus can be accompanied with depression, it might be interesting to perform a repetitive tDCS study in the future with anode overlying the left DLPFC and cathode overlying the right DLPFC. As such verifying whether this would modulate tinnitus-related distress directly and subsequently tinnitus perception.
It is known that the DLPFC plays an important role in anxiety, depression (Fregni et al. 2006; Avery et al. 2007), and the unpleasantness related to pain (Freund et al. 2007). Furthermore, DLPFC activity correlates with the emotional perception of pain (Lorenz et al. 2003) and DLPFC is involved in aversive auditory stimuli, and hypothetically in tinnitus (Mirz et al. 2000). As such it is possible that bilateral tDCS of DLPFC might interfere with tinnitus. Our results confirm indeed a reduction not only for tinnitus intensity, but also for tinnitus-related distress after bilateral tDCS of the DLPFC. The effect on tinnitus intensity might be via the DLPFC’s inhibitory modulation of the auditory cortex (Knight et al. 1989), which is involved in tinnitus intensity coding (van der Loo et al. 2009), while tinnitus-related distress might be more directly suppressed similar to depression (Fregni et al. 2006; Avery et al. 2007).
If tDCS and TMS share common working mechanism, an alternative hypothetical explanation for the effect observed might be provided by the fact that tDCS could influence the neural activity in the anterior cingulate. A study already confirms that high-frequency prefrontal TMS influences the anterior cingulate (Paus et al. 2001). The anterior cingulate is critically involved in the emotional control of sensory processing. As top-down inhibitory mechanism that originates from the prefrontal lobe, the anterior cingulate has been shown to play an important role in auditory processing (Grunwald et al. 2003) and in the integration of tinnitus (Norena et al. 1999). Furthermore, the emotional responses are regulated by limbic system of the brain, such as the anterior cingulate, amygdala and hypothalamus (Liberzon et al. 2000). The DLPFC might regulate structures involved in the emotional perception of tinnitus, including the anterior cingulated cortex, insula and amygdala (Lorenz et al. 2003) (Vanneste et al. submitted). Thus, bilateral tDCS of DLPFC might strengthen on one hand deficient inhibitory top-down mechanisms in tinnitus, making it possible to induce auditory sensory gating in the anterior cingulate cortex (Tanaka et al. 2008). On the other hand, tDCS might also interfere with the emotional processing of tinnitus (i.e. tinnitus-related distress) by modulating the corticosubcortical and corticocortical pathways, as DLPFC may have a dampening effect on the activity of the midbrain-(dorso)medial thalamic pathway, as has been shown for the somatosensory system (Lorenz et al. 2003; Casey et al. 2003). The dorsomedial nucleus of the thalamus also contains specific auditory processing cells, and it is conceivable that a similar pathway exists for auditory input. As such, bilateral tDCS of DLPFC might suppress both tinnitus-related distress and tinnitus intensity.
It has been shown that frontal lobotomies, which undercut the connections to the prefrontal cortex, do not seem to change the tinnitus intensity but rather the emotional (distress) component of the tinnitus (Beard 1965; Elithorn 1953). Therefore, the emotional component could be generated in network involving a prefrontal hub.
Compared with narrow band noise modulating pure tone tinnitus by tDCS seems complicated as the amount of distress suppression depends on the tinnitus laterality: bilateral tDCS of DLPFC can suppress right-sided tinnitus less than left-sided or bilateral pure tone tinnitus patients. As to why this is, no clear answers can be provided yet since not enough is known on the differences in the pathophysiology of unilateral versus bilateral tinnitus and pure tone versus narrow band noise tinnitus. However a hypothesis can be proposed. Previous research already revealed that the DLPFC is connected with the ipsilateral auditory cortex via the parahippocampus (Grunwald et al. 2003; Boutros et al. 2005, 2008; Korzyukov et al. 2007). Our results reveal that tDCS works only with the anode activating the right and cathode inactivating the left DLPFC. It is possible that by stimulating the right DLPFC the right auditory cortex is modulated. Furthermore, a recent study revealed that tinnitus intensity is related to gamma band activity in the contralateral auditory cortex (van der Loo et al. 2009). As left-sided tinnitus is better suppressed than right-sided tinnitus in this study and tinnitus intensity is correlated to the contralateral auditory cortex, i.e. the right auditory cortex, this would suggest a connection between the right DLPFC and the right auditory cortex 1.
In summary, this study encourages further exploration of tDCS for the treatment of tinnitus. In addition, this study supports the involvement of the prefrontal cortex in the pathophysiology of tinnitus. We are aware of the fact that the conclusions drawn from this study are preliminary, as results were not placebo-controlled. Compared with TMS on the temporal cortex for tinnitus 17% has a placebo effect (Vanneste et al. submitted), while for sham tDCS for tinnitus with the electrode placed on the left temporal lobe no patients responded to the sham tDCS (Fregni et al. 2006). Nevertheless, further research is needed which includes a sham tDCS.
References
Alain C, Woods DL, Knight RT (1998) A distributed cortical network for auditory sensory memory in humans. Brain Res 812(1–2):23–37
Antal A et al (2004) Excitability changes induced in the human primary visual cortex by transcranial direct current stimulation: direct electrophysiological evidence. Invest Ophthalmol Vis Sci 45(2):702–707
Avery DH et al (2007) Transcranial magnetic stimulation reduces pain in patients with major depression: a sham-controlled study. J Nerv Ment Dis 195(5):378–381
Axelsson A, Ringdahl A (1989) Tinnitus—a study of its prevalence and characteristics. Br J Audiol 23(1):53–62
Beard AW (1965) Results of leucotomy operations for tinnitus. J Psychosom Res 9(1):29–32
Beeli G et al (2008) Modulating presence and impulsiveness by external stimulation of the brain. Behav Brain Funct 4:33
Bodner M, Kroger J, Fuster JM (1996) Auditory memory cells in dorsolateral prefrontal cortex. Neuroreport 7(12):1905–1908
Boggio PS et al (2008) Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. Eur J Neurol 15(10):1124–1130
Boggio PS, Zaghi S, Fregni F (2009) Modulation of emotions associated with images of human pain using anodal transcranial direct current stimulation (tDCS). Neuropsychologia 47(1):212–217
Boutros NN et al (2005) Sensory gating in the human hippocampal and rhinal regions. Clin Neurophysiol 116(8):1967–1974
Boutros NN et al (2008) Sensory gating in the human hippocampal and rhinal regions: regional differences. Hippocampus 18(3):310–316
Casey KL, Lorenz J, Minoshima S (2003) Insights into the pathophysiology of neuropathic pain through functional brain imaging. Exp Neurol 184(suppl 1):S80–S88
De Ridder D et al (2004) Magnetic and electrical stimulation of the auditory cortex for intractable tinnitus. Case report. J Neurosurg 100(3):560–564
De Ridder D et al (2007a) Theta, alpha and beta burst transcranial magnetic stimulation: brain modulation in tinnitus. Int J Med Sci 4(5):237–241
De Ridder D et al (2007b) Do tonic and burst TMS modulate the lemniscal and extralemniscal system differentially? Int J Med Sci 4(5):242–246
Elithorn A (1953) Prefrontal leucotomy in the treatment of tinnitus. Proc R Soc Med 46(10):832–833
Fregni F et al (2006a) Transient tinnitus suppression induced by repetitive transcranial magnetic stimulation and transcranial direct current stimulation. Eur J Neurol 13(9):996–1001
Fregni F et al (2006b) Cognitive effects of repeated sessions of transcranial direct current stimulation in patients with depression. Depress Anxiety 23(8):482–484
Fregni F et al (2006c) Treatment of major depression with transcranial direct current stimulation. Bipolar Disord 8(2):203–204
Freund W et al (2007) Cortical correlates of perception and suppression of electrically induced pain. Somatosens Mot Res 24(4):203–212
Friedland DR et al (2007) Feasibility of auditory cortical stimulation for the treatment of tinnitus. Otol Neurotol 28(8):1005–1012
Grunwald T et al (2003) Neuronal substrates of sensory gating within the human brain. Biol Psychiatry 53(6):511–519
Kleinjung T et al (2008) Transcranial magnetic stimulation: a new diagnostic and therapeutic tool for tinnitus patients. Int Tinnitus J 14(2):112–118
Knight RT, Scabini D, Woods DL (1989) Prefrontal cortex gating of auditory transmission in humans. Brain Res 504(2):338–342
Korzyukov O et al (2007) Generators of the intracranial P50 response in auditory sensory gating. Neuroimage 35(2):814–826
Lewis JW, Beauchamp MS, DeYoe EA (2000) A comparison of visual and auditory motion processing in human cerebral cortex. Cereb Cortex 10(9):873–888
Liberzon I et al (2000) Limbic activation and psychophysiologic responses to aversive visual stimuli. Interaction with cognitive task. Neuropsychopharmacology 23(5):508–516
Lockwood AH et al (1998) The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology 50(1):114–120
Lorenz J, Minoshima S, Casey KL (2003) Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 126(Pt 5):1079–1091
Miranda PC, Lomarev M, Hallett M (2006) Modeling the current distribution during transcranial direct current stimulation. Clin Neurophysiol 117(7):1623–1629
Mirz F et al (2000) Functional brain imaging of tinnitus-like perception induced by aversive auditory stimuli. Neuroreport 11(3):633–637
Mitchell TV et al (2005) Functional magnetic resonance imaging measure of automatic and controlled auditory processing. Neuroreport 16(5):457–461
Muhlnickel W et al (1998) Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci USA 95(17):10340–10343
Nitsche MA, Paulus W (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527:633–639
Nitsche MA, Paulus W (2001) Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57(10):1899–1901
Nitsche MA et al (2003) Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol 114(4):600–604
Nondahl DM et al (2002) Prevalence and 5-year incidence of tinnitus among older adults: the epidemiology of hearing loss study. J Am Acad Audiol 13(6):323–331
Norena A, Cransac H, Chery-Croze S (1999) Towards an objectification by classification of tinnitus. Clin Neurophysiol 110(4):666–675
Paus T, Castro-Alamancos MA, Petrides M (2001) Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. Eur J Neurosci 14(8):1405–1411
Salvi RJ, Wang J, Ding D (2000) Auditory plasticity and hyperactivity following cochlear damage. Hear Res 147(1–2):261–274
Schlee W et al (2009) Abnormal resting-state cortical coupling in chronic tinnitus. BMC Neurosci 10:11
Scott B, Lindberg P (2000) Psychological profile and somatic complaints between help-seeking and non-help-seeking tinnitus subjects. Psychosomatics 41(4):347–352
Sindhusake D et al (2004) Factors predicting severity of tinnitus: a population-based assessment. J Am Acad Audiol 15(4):269–280
Smits M et al (2007) Lateralization of functional magnetic resonance imaging (fMRI) activation in the auditory pathway of patients with lateralized tinnitus. Neuroradiology 49(8):669–679
Tanaka E et al (2008) A transition from unimodal to multimodal activations in four sensory modalities in humans: an electrophysiological study. BMC Neurosci 9:116
van der Loo E et al (2009) Tinnitus intensity dependent gamma oscillations of the contralateral auditory cortex. PLoS ONE 4(10):e7396
Vanneste S, Plazier M, Ost J, van der Loo E, Van de Heyning P, Congedo M, De Ridder D (submitted) The neural correlates of tinnitus-related distress
Voisin J et al (2006) Listening in silence activates auditory areas: a functional magnetic resonance imaging study. J Neurosci 26(1):273–278
Weisz N et al (2007) The neural code of auditory phantom perception. J Neurosci 27(6):1479–1484
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vanneste, S., Plazier, M., Ost, J. et al. Bilateral dorsolateral prefrontal cortex modulation for tinnitus by transcranial direct current stimulation: a preliminary clinical study. Exp Brain Res 202, 779–785 (2010). https://doi.org/10.1007/s00221-010-2183-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-010-2183-9