Abstract
Tinnitus is an auditory phantom percept with a tone, hissing, or buzzing sound in the absence of any objective physical sound source. Two forms of low-intensity cranial electrical stimulation exist for clinical and research purposes: transcranial direct current stimulation (tDCS) and transcranial alternating current stimulation (tACS). In a recent study, it was demonstrated that a single session of tDCS over the dorsolateral prefrontal cortex (DLPFC) (anode over right DLPFC) yields a transient improvement in subjects with chronic tinnitus and that repeated sessions can possibly be used as a treatment. In the present study, the effect of a single-session individual alpha–modulated tACS and tDCS applied at the DLPFC bilaterally is compared with tinnitus loudness and tinnitus annoyance. A total of fifty tinnitus patients were selected and randomly assigned to the tACS or tDCS treatment. Our main result was that bifrontal tDCS modulates tinnitus annoyance and tinnitus loudness, whereas individual alpha-modulated tACS does not yield a similar result. This study provides additional insights into the role of DLPFC in tinnitus modulation as well as the intersection between tinnitus and affective/attentional processing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tinnitus is an auditory phantom percept with a tone, hissing, or buzzing sound in the absence of any objective physical sound source (Jastreboff 1990). The British Tinnitus Association estimates about 10 % of the UK adult population has chronic tinnitus. The constant awareness of this phantom sound often causes a considerable amount of distress. Between 6 and 25 % of the affected people report symptoms that are severely debilitating (Eggermont and Roberts 2004) and 2–4 % of the whole tinnitus population suffers in the worst degree, leading to a noticeable decrease in the quality of life (Axelsson and Ringdahl 1989). Psychological complications such as lifestyle detriment, emotional difficulties, sleep deprivation, work hindrance, interference with social interaction and decreased overall health have been attributed to tinnitus (Scott and Lindberg 2000).
Based on neurobiological research, it is generally accepted that most forms of tinnitus are attributable to maladaptive plasticity due to damage to the auditory system (Muhlnickel et al. 1998), as a mechanism to reduce uncertainty related to auditory deafferentation (De Ridder et al. 2012b). Changes in neural activity were also observed in non-auditory brain structures (Vanneste and De Ridder 2012). That is, tinnitus can be considered as an emergent network property of multiple, dynamically adaptive, partially overlapping, auditory and non-auditory brain networks, each representing a specific aspect of the tinnitus (De Ridder et al. 2011). These networks are made up of brain areas such as anterior cingulate cortex, posterior cingulate cortex, ventromedial prefrontal cortex, dorsal lateral prefrontal cortex, orbitofrontal cortex, and parahippocampus. In particular, the dorsolateral prefrontal cortex (DLPFC) seems to play a specific role in auditory processing. That is, the DLPFC has a bilateral facilitatory effect on auditory memory storage and contains auditory memory cells (Bodner et al. 1996). The DLPFC also exerts early inhibitory modulation of input to primary auditory cortex in humans (Knight et al. 1989) and has been found to be associated with auditory attention (Voisin et al. 2006) resulting in top-down modulation of auditory processing (Mitchell et al. 2005). This was further confirmed by electrophysiological data indicating that tinnitus might occur as the result of a dysfunction in the top-down inhibitory processes (Norena et al. 1999).
Two forms of low-intensity cranial electrical stimulation exist for clinical and research purposes: transcranial direct current stimulation (tDCS) (Nitsche et al. 2008) and transcranial alternating current stimulation (tACS) (Kanai et al. 2010). Both methods permit non-invasive brain stimulation and have been shown to be effective in modulating cortical excitability as well as guiding human perception and behavior. Many groups have studied and reviewed the neurophysiological and clinical effects of transcranial brain stimulation with direct current, and less effort in recent years has been dedicated to the study of stimulation with alternating current.
Depending on the polarity of the stimulation, tDCS can increase or decrease cortical excitability in the brain regions to which it is applied (Miranda et al. 2006). Currently, tDCS is usually applied through two surface electrodes, one serving as the anode and the other as the cathode, with the current flowing constantly from the anode to the cathode (George and Aston-Jones 2010). Some of the applied current is shunted through scalp tissue, and only a part of the applied current passes through the brain (Dymond et al. 1975). Anodal tDCS typically has an excitatory effect on the local cerebral cortex by depolarizing neurons, while under the cathode hyperpolarization is induced. The predominant effect on the oscillatory activity of the underlying brain area seems to be a decrease in gamma-band activity under the cathode and an increase in gamma-band activity under the anode (Vanneste et al. 2011b). This effect of tDCS typically outlasts the stimulation by an hour or longer after a single-treatment session of sufficiently long stimulation duration (Antal et al. 2004).
Several tDCS studies focused on the DLPFC and have found successful results for treating major depression (Fregni et al. 2006b), mood changes in depression (Fregni et al. 2006a), as well as reducing impulsiveness (Beeli et al. 2008) and tinnitus (Fregni et al. 2006c). In a recent study, Vanneste and colleagues demonstrated that a single session of tDCS over the DLPFC (anode over right DLPFC) yields a transient improvement in subjects with chronic tinnitus (Vanneste et al. 2010b), and a pilot study of repeated sessions suggests that repeated sessions yield better results (Faber et al. 2011) and can possibly be used as a treatment (Frank et al. 2011).
A more recent application is tACS which also is potentially capable of interacting with rhythmic neuronal activity and has perceptual and behavioral consequences (Zaghi et al. 2010). This method relies on application of alternating currents through an electrode. Electrical currents are applied constantly at low intensities over a period of time, to achieve changes in cortical activity. The waveform of the stimulation is sinusoidal and different frequencies can be used during stimulation. As such, tACS is better suited to modulate functions that are closely related to brain oscillations at specific frequencies (Zaehle et al. 2010). Previous research already demonstrated that tACS strengthens the individual alpha frequency of the occipital areas (Zaehle et al. 2010).
Recent data indicate the involvement of the DLPFC in tinnitus (Faber et al. 2011), a region suggested to integrate cognitive and emotional processing (Gray et al. 2002). More precisely, it was shown that patients with tinnitus-related annoyance showed a decrease in the right DLPFC in the alpha frequency (Vanneste et al. 2010a). A recent TMS study demonstrated that low-frequency repetitive TMS (rTMS) of the auditory cortex in combination of high-frequency rTMS of the left DLPFC produces a better improvement of the tinnitus complaints in comparison with only low-frequency rTMS of the temporal cortex (Kleinjung et al. 2008). Furthermore, a study only stimulating the DLPFC by TMS (De Ridder et al. 2012a) and by implanting an electrode over the DLPFC (De Ridder et al. 2012c) exerts similar tinnitus-suppressing effects. As such, it might be that influencing this frontal alpha activity could modulate the tinnitus. As tACS elevates the endogenous alpha power, frontal tACS at the individual alpha frequency of the patient might suppress tinnitus as well.
In the present study, the effect of tACS and tDCS applied at the DLPFC bilaterally is investigated and compared with tinnitus loudness and tinnitus annoyance. We hypothesize that tACS might be better suited to suppress tinnitus than tDCS as tACS could theoretically normalize the alpha power which is known to be decreased in tinnitus (Lorenz et al. 2009; Weisz et al. 2011).
Methods and materials
Participants
A total of fifty tinnitus patients (N = 50; 26 females and 24 males) with a mean age of 51.37 (SD = 12.99 years) were selected from the multidisciplinary Tinnitus Research Initiative (TRI) Clinic of the University Hospital of Antwerp, Belgium. Patients had a mean tinnitus duration of 2.85 years (SD = 2.10 years). Individuals with pulsatile tinnitus, Ménière disease, otosclerosis, chronic headache, neurological disorders such as brain tumors, and individuals being treated for mental disorders (i.e., neuropsychiatric diseases) were not included in the study in order to obtain a homogeneous sample. All patients had tinnitus for more than 1 year and have a tinnitus that is constantly present. No psychoactive neuropharmaca were added or removed during the trial period in both tACS and tDCS groups. Four patients took medication at the time of the experiment (2 patients were assigned to the sham tDCS, one person in the real and one sham tACS).
All patients were investigated for the extent of hearing loss using audiograms. Tinnitus matching was performed looking for tinnitus pitch (frequency) and tinnitus loudness. Participants were requested to refrain from alcohol consumption 24 h prior to recording and from caffeinated beverages on the day of recording.
This study was approved by the local ethical committee (Antwerp University Hospital) and was in accordance with the declaration of Helsinki. Patients gave oral informed consent before the procedure.
tACS
EEGs (Mitsar, Nova Tech EEG, Inc, Mesa) were obtained 1 week before the tACS stimulation in a fully lighted room with each participant sitting upright in a comfortable chair. The EEG was sampled with 19 electrodes (Fp1, Fp2, F7, F3, Fz, F4, F8, T7, C3, Cz, C4, T8, P7, P3, Pz, P4, P8, O1, O2) in the standard 10–20 international placements referenced to linked lobes, and impedances were checked to remain below 5 kΩ. Data were collected for 100 2-s epochs eyes closed, sampling rate = 1,024 Hz, and band passed 0.15–200 Hz. Data were resampled to 128 Hz, band-pass filtered (fast Fourier transform filter) to 2–44 Hz. These data were transposed into Eureka Software (Congedo 2002), plotted and carefully inspected manually for artifact. All episodic artifacts including eye blinks, eye movements, teeth clenching, body movement, or ECG artifacts were removed from the stream of the EEG.
To determine the frequency of stimulation, the individual alpha frequency (IAF) peak was identified according to literature guidelines (Klimesch et al. 1999). This individual alpha frequency peak was defined as the frequency within the range of 6–13 Hz range of the EEG spectrum showing maximum power for the priori chosen electrodes F3 and F4.
Alternating current was transmitted by a saline-soaked pair of surface sponge (35 cm2) and delivered by specially developed, battery-driven, constant current stimulator with a maximum output of 10 mA (NeuroConn; http://www.neuroconn.de/). For each patient receiving tACS, one electrode was placed over the left DLPFC and one was placed on the right DLPFC as determined by the International 10/20 Electroencephalogram System corresponding to F3 and F4, respectively. The frequency of the tACS was set to the IAF. In both real tACS and sham, the AC current was initially increased in a ramp-like fashion over several seconds (10 s) until reaching 2 mA. In tACS, stimulation was maintained for a total of 20 min; in sham, it was turned off after 30 s. These parameters for sham stimulation were chosen based on previous reports that the perceived sensations on the skin, such as tingling, fade usually out in the first 30 s of tACS (Nitsche et al. 2003). Additionally, we collected the EEG for those patients received real tACS immediately after the treatment.
tDCS
Patients who received tDCS underwent an EEG measurement similar to the tACS group. Direct current was transmitted by a saline-soaked pair of surface sponge (35 cm²) and delivered by specially developed, battery-driven, constant current stimulator with a maximum output of 10 mA (NeuroConn; http://www.neuroconn.de/). For each patient receiving tDCS, the cathode was placed over the left DLPFC and the anode was placed on the right DLPFC as determined by the International 10/20 Electroencephalogram System corresponding to F3 and F4, respectively. In both real tDCS and sham, the DC current was initially increased in a ramp-like fashion over several seconds (10 s) until reaching 2 mA. In tDCS, stimulation was maintained for a total of 20 min; in sham, it was turned off after 30 s. These parameters for sham stimulation were chosen based on previous reports that the perceived sensations on the skin, such as tingling, fade usually out in the first 30 s of tDCS (Nitsche et al. 2003).
Evaluation
Patients were randomly assigned to the tACS or tDCS treatment. Thirteen patients underwent real tACS, 13 patients received sham tACS, 12 patients received real tDCS, and 12 patients received sham tDCS. A numeric rating scale (NRS) for tinnitus intensity (‘How loud is your tinnitus? 0 = no tinnitus and 10 = as loud as imaginable’) and tinnitus annoyance (‘How annoying is your tinnitus? 0 = not annoying 10 = suicidal annoying’) was asked before (pre) and directly after (post) tDCS stimulation.
Statistical analysis
Calculations were performed using SPSS software package. A repeated measure ANOVA was conducted with stimulation (pre vs. post) as the within-subjects variable and condition (real vs. sham) and device (tACS vs. tDCS) as the between-subjects variables for both annoyance and loudness in one model. We used simple contrast analyses as this method allows us to test the statistical significance of predicted specific differences in particular parts of our complex design.
Results
The mean tinnitus loudness on a NRS scale was 7.30 (SD = 1.44) (‘How loud is your tinnitus?’) and the tinnitus annoyance on a NRS scale was 7.02 (SD = 1.32) (‘How annoying is your tinnitus?’).
Pre- versus post-stimulation
A significant within effect (pre vs. post; F(2, 45) = 11.06, p < .001) was obtained on the tinnitus NRS scales. A univariate test of within-subjects revealed that both annoyance [F(1, 46) = 21.50, p < .001] as well as loudness [F(1, 46) = 14.94, p < .001] changed significantly. It was shown that post-stimulation (M = 6.41 for annoyance, M = 6.29 for loudness) patients had lower scores on the NRS than pre-stimulation (M = 7.30 for annoyance, M = 7.02 for loudness). No significant main effect was obtained for condition (real vs. sham) and device (tACS vs. tDCS) on both tinnitus loudness and tinnitus annoyance. No significant interaction effect was found between condition × device on both tinnitus loudness and tinnitus annoyance.
Pre- versus post-stimulation dependent on the condition (real or sham)
Further analysis indicated that this effect was mediated significantly by condition. We found a two-way interaction between stimulation (pre vs. post) × condition (real vs. sham) [F(2, 45) = 6.00, p < .01]. A univariate test demonstrated that this was so for both annoyance [F(1, 46) = 4.38, p < .05] as well as for loudness [F(1, 46) = 12.19, p < .01]. Simple contrasts indicated both annoyance [F(1, 46) = 21.17, p < .001] and loudness [F(1, 46) = 25.93, p < .001] significantly decreased after real stimulation in comparison with pre-stimulation. However, no significant effects were obtained when comparing pre- and post-stimulation in the sham condition for both annoyance and loudness. Also no significant effect was obtained when comparing real versus sham condition before stimulation. However, a significant effect was obtained when comparing the real versus the sham condition post-stimulation for both annoyance [F(1, 46) = 4.38, p < .05] as well as loudness [F(1, 46) = 12.19, p < .01], indicating that that real stimulation yielded more decrease than sham stimulation. No significant effect was demonstrated for stimulation (pre vs. post) × device.
Pre- versus post-stimulation dependent on the condition (real or sham) as well as on the device (tACS or tDCS)
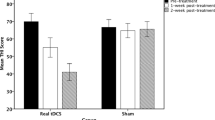
Most importantly, our analysis yielded a significant three-way interaction [F(2, 45) = 4.66, p < .05] between stimulation (pre vs. post) × device (tACS vs. tDCS) × condition (real vs. sham) for both annoyance [F(1, 46) = 6.10, p < .05] and loudness [F(1, 46) = 9.13, p < .01] (see Fig. 1). Simple contrast for tDCS revealed that both annoyance [F(1, 46) = 29.65, p < .001] and loudness [F(1, 46) = 29.37, p < .001] were significantly suppressed during real stimulation but not during sham stimulation. For tACS, simple contrasts revealed no significant effects, indicating that there were no differences on both annoyance as well as loudness during real or sham stimulation. To further explore these effects, simple contrasts were calculated for both annoyance and loudness post-stimulation between tDCS and tACS. The analyses revealed that for both annoyance [F(1, 46) = 8.10, p < .01] as well loudness [F(1, 46) = 6.12, p < .05], there was a significant suppression after tDCS in comparison with tACS post-stimulation.
EEG results pre- versus post-tACS
Although tACS does not yield a significant clinical effect, it is possible that it induces neurophysiological changes. Hence, we look at the power changes at the F3 and F4 electrodes determined by the International 10/20 Electroencephalogram System that overlay the DLPFC. A comparison of the power between pre- and post-tACS for, respectively, F3 and F4 revealed no significant effect for each frequency separately (see Fig. 2). It is, however, possible that due to volume conduction, posterior or motor regions might interfere with the findings on F3 and F4. Therefore, also a current source density was calculated specifically for left and right DLPFC before and after tACS stimulation. However, a comparison between, respectively, the left and right DLPFC before and after stimulation demonstrated no significant effect for each frequency separately (see Fig. 2).
Discussion
We have reported the first side-by-side comparison of tACS with tDCS and its effect on the suppression of annoyance and loudness in a sham-controlled fashion. Results indicate that a single session of bifrontal tDCS can induced suppression of both annoyance and loudness, while tACS had no effect on both measurements (Table 1).
In regard to tDCS, our results obtained confirm previous findings that bifrontal tDCS with the anodal electrode placed over the right DLPFC and the cathodal electrode placed over the left DLPFC can modulate tinnitus annoyance and tinnitus loudness (Vanneste et al. 2010b, 2011a; Faber et al. 2011; Plazier et al. 2011). It has been stated that the prefrontal cortex is important for the integration of sensory and emotional aspects of tinnitus (Jastreboff 1990; Vanneste et al. 2010a). The DLPFC might regulate structures involved in the emotional perception of tinnitus, including the anterior cingulate cortex, amygdala and insula (Lorenz et al. 2003). TDCS of the DLPFC can reduce tinnitus annoyance, interfering with the emotional processing of tinnitus (i.e., tinnitus-related distress), analogous to tDCS for depression (Fregni et al. 2006a). This can also be supported to some extent by frontal lobotomy studies in which it has been shown that by cutting the connections to the prefrontal cortex, the tinnitus loudness does not change but rather the emotional ‘distress’ component of tinnitus (Beard 1965).
However, our results indicate that tACS does not generate the same results as tDCS both clinically and neurophysiologically. Previous research already demonstrated that tDCS affects neural tissue via a sustained modulation of the membrane voltage of neurons while tACS most probably yields its effect via an up- and down-regulation of certain synapses (Zaehle et al. 2010) similar to TMS (Thut and Pascual-Leone 2010). It has been shown that a single session of bifrontal tDCS exerts a clear neurophysiological effect in tinnitus patients immediately under the electrodes as well as at distal areas (Vanneste and De Ridder 2011). Our results support the idea that tDCS and tACS might have a different working mechanism with a different impact on the stimulated neural tissue.
It has been suggested that tACS is better suited to modulate functions that are closely related to brain oscillations at specific frequencies (Basar et al. 2001) and is potentially capable of interacting with rhythmic neuronal activity and has perceptual and behavioral consequences (Zaghi et al. 2010). Previous research in tinnitus patients indicates that the alpha frequency in frontal brain area is an important aspect of tinnitus-related distress (Vanneste et al. 2010a). Our findings, however, revealed that frontal tACS modulating the alpha frequency is not a good option to modulate tinnitus annoyance clinically. It is possible that a different frequency setting might obtain better results, although there is a good rationale to use the alpha frequency setting for frontal neuromodulation. Alternatively, it could also be that the current for tACS needs to be higher in comparison with tDCS to resort the same results. Further research needs to be conducted to test these hypotheses.
In summary, this study explored the effect of tDCS and tACS for the treatment of tinnitus. This study provides additional insights into the role of DLPFC in tinnitus modulation as well as the intersection between tinnitus and affective/attentional processing. Our main result was that bifrontal tDCS modulates tinnitus annoyance and tinnitus loudness, while individual alpha-modulated tACS does not yield a similar result.
References
Antal A, Kincses TZ, Nitsche MA, Bartfai O, Paulus W (2004) Excitability changes induced in the human primary visual cortex by transcranial direct current stimulation: direct electrophysiological evidence. Invest Ophthalmol Vis Sci 45:702–707
Axelsson A, Ringdahl A (1989) Tinnitus—a study of its prevalence and characteristics. Br J Audiol 23:53–62. doi:10.3109/03005368909077819
Basar E, Basar-Eroglu C, Karakas S, Schurmann M (2001) Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol 39:241–248
Beard AW (1965) Results of leucotomy operations for tinnitus. J Psychosom Res 9:29–32
Beeli G, Casutt G, Baumgartner T, Jancke L (2008) Modulating presence and impulsiveness by external stimulation of the brain. Behav Brain Funct 4:33. doi:10.1186/1744-9081-4-33
Bodner M, Kroger J, Fuster JM (1996) Auditory memory cells in dorsolateral prefrontal cortex. NeuroReport 7:1905–1908
Congedo M (2002) EureKa! (Version 3.0) [Computer Software]. NovaTech EEG Inc, Knoxville, TN. http://www.NovaTechEEG
De Ridder D, Elgoyhen AB, Romo R, Langguth B (2011) Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci USA 108:8075–8080. doi:10.1073/pnas.1018466108
De Ridder D, Song JJ, Vanneste S (2012a) Frontal cortex TMS for tinnitus. Brain Stimul. doi:10.1016/j.brs.2012.07.002
De Ridder D, Vanneste S, Freeman W (2012b) The Bayesian brain: phantom percepts resolve sensory uncertainty. Neurosci Biobehav Rev. doi:10.1016/j.neubiorev.2012.04.001
De Ridder D, Vanneste S, Plazier M, Menovsky T, van de Heyning P, Kovacs S, Sunaert S (2012c) Dorsolateral prefrontal cortex transcranial magnetic stimulation and electrode implant for intractable tinnitus. World Neurosurg 77:778–784. doi:10.1016/j.wneu.2011.09.009
Dymond AM, Coger RW, Serafetinides EA (1975) Intracerebral current levels in man during electrosleep therapy. Biol Psychiatry 10:101–104
Eggermont JJ, Roberts LE (2004) The neuroscience of tinnitus. Trends Neurosci 27:676–682. doi:10.1016/j.tins.2004.08.010
Faber M, Vanneste S, Fregni F, De Ridder D (2011) Top down prefrontal affective modulation of tinnitus with multiple sessions of tDCS of dorsolateral prefrontal cortex. Brain Stimul. doi:10.1016/j.brs.2011.09.003
Frank E, Schecklmann M, Landgrebe M et al (2011) Treatment of chronic tinnitus with repeated sessions of prefrontal transcranial direct current stimulation: outcomes from an open-label pilot study. J Neurol. doi:10.1007/s00415-011-6189-4
Fregni F, Boggio PS, Nitsche MA, Marcolin MA, Rigonatti SP, Pascual-Leone A (2006a) Treatment of major depression with transcranial direct current stimulation. Bipolar Disord 8:203–204. doi:10.1111/j.1399-5618.2006.00291.x
Fregni F, Boggio PS, Nitsche MA, Rigonatti SP, Pascual-Leone A (2006b) Cognitive effects of repeated sessions of transcranial direct current stimulation in patients with depression. Depress Anxiety 23:482–484. doi:10.1002/da.20201
Fregni F, Marcondes R, Boggio PS et al (2006c) Transient tinnitus suppression induced by repetitive transcranial magnetic stimulation and transcranial direct current stimulation. Eur J Neurol 13:996–1001. doi:10.1111/j.1468-1331.2006.01414.x
George MS, Aston-Jones G (2010) Noninvasive techniques for probing neurocircuitry and treating illness: vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). Neuropsychopharmacology 35:301–316. doi:10.1038/npp.2009.87
Gray JR, Braver TS, Raichle ME (2002) Integration of emotion and cognition in the lateral prefrontal cortex. Proc Natl Acad Sci USA 99:4115–4120
Jastreboff PJ (1990) Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res 8:221–254
Kanai R, Paulus W, Walsh V (2010) Transcranial alternating current stimulation (tACS) modulates cortical excitability as assessed by TMS-induced phosphene thresholds. Clin Neurophysiol 121:1551–1554. doi:10.1016/j.clinph.2010.03.022
Kleinjung T, Eichhammer P, Landgrebe M et al (2008) Combined temporal and prefrontal transcranial magnetic stimulation for tinnitus treatment: a pilot study. Otolaryngol Head Neck Surg 138:497–501. doi:10.1016/j.otohns.2007.12.022
Klimesch W, Doppelmayr M, Schwaiger J, Auinger P, Winkler T (1999) ‘Paradoxical’ alpha synchronization in a memory task. Brain Res Cogn Brain Res 7:493–501
Knight RT, Scabini D, Woods DL (1989) Prefrontal cortex gating of auditory transmission in humans. Brain Res 504:338–342
Lorenz J, Minoshima S, Casey KL (2003) Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 126:1079–1091
Lorenz I, Muller N, Schlee W, Hartmann T, Weisz N (2009) Loss of alpha power is related to increased gamma synchronization-A marker of reduced inhibition in tinnitus? Neurosci Lett 453:225–228. doi:10.1016/j.neulet.2009.02.028
Miranda PC, Lomarev M, Hallett M (2006) Modeling the current distribution during transcranial direct current stimulation. Clin Neurophysiol 117:1623–1629. doi:10.1016/j.clinph.2006.04.009
Mitchell TV, Morey RA, Inan S, Belger A (2005) Functional magnetic resonance imaging measure of automatic and controlled auditory processing. NeuroReport 16:457–461
Muhlnickel W, Elbert T, Taub E, Flor H (1998) Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci USA 95:10340–10343
Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W (2003) Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol 114:2220–2222 (author reply 2222–2223)
Nitsche MA, Cohen LG, Wassermann EM et al (2008) Transcranial direct current stimulation: state of the art 2008. Brain Stimul 1:206–223. doi:10.1016/j.brs.2008.06.004
Norena A, Cransac H, Chery-Croze S (1999) Towards an objectification by classification of tinnitus. Clin Neurophysiol 110:666–675
Plazier M, Joos K, Vanneste S, Ost J, De Ridder D (2011) Bifrontal and bioccipital transcranial direct current stimulation (tDCS) does not induce mood changes in healthy volunteers: a placebo controlled study. Brain Stimul. doi:10.1016/j.brs.2011.07.005
Scott B, Lindberg P (2000) Psychological profile and somatic complaints between help-seeking and non-help-seeking tinnitus subjects. Psychosomatics 41:347–352
Thut G, Pascual-Leone A (2010) A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr 22:219–232. doi:10.1007/s10548-009-0115-4
Vanneste S, De Ridder D (2011) Bifrontal transcranial direct current stimulation modulates tinnitus intensity and tinnitus-distress-related brain activity. Eur J Neurosci 34:605–614. doi:10.1111/j.1460-9568.2011.07778.x
Vanneste S, De Ridder D (2012) The auditory and non-auditory brain areas involved in tinnitus. An emergent property of multiple parallel overlapping subnetworks. Front Syst Neurosci 6:31. doi:10.3389/fnsys.2012.00031
Vanneste S, Plazier M, der Loo E, de Heyning PV, Congedo M, De Ridder D (2010a) The neural correlates of tinnitus-related distress. Neuroimage 52:470–480. doi:10.1016/j.neuroimage.2010.04.029
Vanneste S, Plazier M, Ost J, van der Loo E, Van de Heyning P, De Ridder D (2010b) Bilateral dorsolateral prefrontal cortex modulation for tinnitus by transcranial direct current stimulation: a preliminary clinical study. Exp Brain Res 202:779–785. doi:10.1007/s00221-010-2183-9
Vanneste S, Focquaert F, Van de Heyning P, De Ridder D (2011a) Different resting state brain activity and functional connectivity in patients who respond and not respond to bifrontal tDCS for tinnitus suppression. Exp Brain Res 210:217–227. doi:10.1007/s00221-011-2617-z
Vanneste S, Langguth B, De Ridder D (2011b) Do tDCS and TMS influence tinnitus transiently via a direct cortical and indirect somatosensory modulating effect? A combined TMS-tDCS and TENS study. Brain Stimul 4:242–252. doi:10.1016/j.brs.2010.12.001
Voisin J, Bidet-Caulet A, Bertrand O, Fonlupt P (2006) Listening in silence activates auditory areas: a functional magnetic resonance imaging study. J Neurosci 26:273–278. doi:10.1523/JNEUROSCI.2967-05.2006
Weisz N, Hartmann T, Muller N, Lorenz I, Obleser J (2011) Alpha rhythms in audition: cognitive and clinical perspectives. Front Psychol 2:73. doi:10.3389/fpsyg.2011.00073
Zaehle T, Rach S, Herrmann CS (2010) Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLoS ONE 5:e13766. doi:10.1371/journal.pone.0013766
Zaghi S, de Freitas Rezende L, de Oliveira LM, El-Nazer R, Menning S, Tadini L, Fregni F (2010) Inhibition of motor cortex excitability with 15 Hz transcranial alternating current stimulation (tACS). Neurosci Lett 479:211–214. doi:10.1016/j.neulet.2010.05.060
Acknowledgments
The authors received funds from Research Foundation Flanders (FWO), the Stavros Niarchos Foundation (SNF), Tinnitus Research Initiative (TRI), and Royal Society Industry Fellowship (VW).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vanneste, S., Walsh, V., Van De Heyning, P. et al. Comparing immediate transient tinnitus suppression using tACS and tDCS: a placebo-controlled study. Exp Brain Res 226, 25–31 (2013). https://doi.org/10.1007/s00221-013-3406-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-013-3406-7