Abstract
Purpose
The aim of this study was to evaluate the prognostic impact of inflammation-based markers, including the neutrophil-to-lymphocyte ratio (NLR) and prognostic nutritional index (PNI), in patients with recurrent gastric cancer (RGC).
Methods
This study reviewed 167 patients with RGC. A receiver operating characteristics (ROC) curve analysis was performed to determine the NLR and PNI cutoff values. The prognostic significance of the NLR and PNI was evaluated by a multivariate analysis.
Results
The optimal NLR and PNI cutoff values for predicting the 1-year survival after recurrence were 2.2 and 47, respectively. A univariate analysis revealed that the NLR (p < 0.001) and PNI (p < 0.001) were significantly associated with the survival time after recurrence, along with the histology, peritoneal recurrence, carbohydrate antigen 19-9, and chemotherapy for recurrence. In the multivariate analysis, a higher NLR (p < 0.001) and a lower PNI (p = 0.002) were independent predictors of a shorter survival time. Among the patients who underwent chemotherapy, the NLR and PNI were also independent prognostic factors.
Conclusions
Inflammation-based markers, including the NLR and PNI, are simple and useful clinical biomarkers that can be used to predict the survival time of patients with RGC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Even after curative gastrectomy, an appreciable proportion of patients with advanced gastric cancer develop recurrence. Palliative chemotherapy has been shown to prolong the survival time and improve the quality of life of patients with recurrent gastric cancer (RGC) [1, 2]. However, the prognosis of such patients remains extremely poor, and the median survival is only 1 year [3, 4]. In addition, chemotherapy may be associated with various adverse events. Thus, it is necessary to accurately predict the prognosis to determine the optimal treatment strategy and to provide important information to patients.
There is increasing evidence to support the notion that inflammation plays a critical role in the development and progression of many cancers [5, 6]. Recently, various inflammation-based markers, such as the neutrophil-to-lymphocyte ratio (NLR) and prognostic nutritional index (PNI), have been investigated and found to be associated with the clinical outcomes of various cancers [7,8,9,10]. In primary gastric cancer, these markers were identified as independent predictors of the postoperative prognosis [11, 12]. In addition, several studies have demonstrated that these markers were associated with the survival time in advanced gastric cancer patients who received palliative chemotherapy [13, 14]. However, few reports have investigated the role of inflammation-based markers in patients with RGC [15]. Thus, the present study evaluated the prognostic value of inflammation-based markers, including the NLR and PNI, in such patients.
Methods
A total of 1175 patients with gastric cancer underwent R0 or R1 gastrectomy between January 2001 and December 2015 in Nara Medical University Hospital. Among these patients, 201 (17.1%) were diagnosed with recurrent gastric cancer before September 2016. We excluded 25 patients lost to follow-up and 9 patients for whom the blood test data at recurrence or treatment for recurrence were unavailable. Thus, 167 patients were ultimately analyzed in the present study. This study was approved by the Local Ethics Committee on Clinical Investigation of Nara Medical University (no. 1425). Written informed consent was obtained from all of the patients.
The following clinicopathological characteristics were obtained retrospectively from the patients’ medical records: the age, sex, co-morbidities including cardiovascular disease, diabetes mellitus and chronic renal failure, histology, tumor depth, lymph node metastasis, distant metastasis, tumor stage, resectability of the tumor, preoperative and postoperative chemotherapy, recurrence pattern, treatment for recurrence and adverse events from chemotherapy. The stage of gastric cancer was classified according to the third edition of the Japanese Classification of Gastric Carcinoma [16]. Adverse events were evaluated according to the National Cancer Institute Common Toxicity Criteria version 4.0.
We also collected the results of blood tests performed at the time of recurrence, including the serum levels of albumin, carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9, and the neutrophil and lymphocyte counts in the peripheral blood. The NLR was calculated as the neutrophil count divided by the lymphocyte count [7]. The PNI was calculated using the following formula: 10 × serum albumin value (g/dl) + 0.005 × total lymphocyte count in the peripheral blood (per mm3) [17].
The recurrence of gastric cancer was diagnosed based on clinical imaging, gastroscopy with a biopsy, and/or a cytological examination of ascites, or intraoperative findings in patients who underwent reoperation. Recurrence was classified as peritoneal, hematogenous, lymphatic or local, according to the site of relapse.
The indications for the use of chemotherapy to treat recurrence were as follows: ≥20 years of age, Eastern Cooperative Oncology Group performance status 0–2, no uncontrolled infection or cardiopulmonary disease and adequate bone marrow as well as a renal and hepatic function. The chemotherapeutic regimen was selected by the patient’s surgeon based on the previous treatment, the patient’s general condition and request.
Statistical analyses
Continuous variables with normal distribution were expressed as the mean and standard deviation (SD). The means were compared using the t test. Continuous variables without normal distribution were expressed as the median and interquartile range (IQR). Categorical variables were presented as numbers and percentages, and groups were compared using the Chi-squared test or Fisher’s exact test. To evaluate the sensitivity and specificity for predicting the 1-year survival after recurrence, receiver operating characteristic (ROC) curves were calculated, and the Youden index was estimated to determine the optimal NLR and PNI cut-off values [18].
The cut-off date was February 28, 2017. The overall survival (OS) was defined as the period from the recurrence of gastric cancer to death. The survival curves were estimated using the Kaplan–Meier method, and differences between the curves were analyzed using the Breslow’s generalized Wilcoxon test. Univariate and multivariate hazard ratios (HRs) were calculated using the Cox proportional hazards model. The following variables were included in the univariate analysis: age (<60 vs. ≥60 years), sex, histology, tumor depth, lymph node metastasis, distant metastasis, resectability of the tumor, preoperative chemotherapy, postoperative chemotherapy, duration from the operation to recurrence (<12 vs. ≥12 months), peritoneal recurrence, hematogenous recurrence, lymph node recurrence, multiple sites of recurrence, CEA and CA 19-9 levels, chemotherapy for recurrence, NLR and PNI. The variables with a p value of <0.1 in the univariate analysis were entered into the multivariate analysis. p values of <0.05 were considered to indicate statistical significance, and 95% confidence intervals (CI) were calculated. All of the statistical analyses were performed using the SPSS software program (version 22.0; SPSS, Chicago, IL, USA).
Results
Patient characteristics
There were 124 men and 43 women, with a mean age of 67.6 years (SD 11.3 years). The median duration from the operation to recurrence was 12.6 months (IQR 6.3–24.7 months). In 63 (43.2%) of the 146 patients who underwent R0 gastrectomy and 10 (47.6%) of the 21 patients with R1 gastrectomy, recurrence was detected within 12 months of the operation (p = 0.7). The sites of recurrence were as follows: peritoneum, n = 80 (47.9%); hematogenous, n = 51 (30.5%); lymph nodes, n = 48 (28.7%) and local, n = 6 (3.6%). Twenty (12.0%) patients had at least two concurrent sites of the recurrence. The median NLR was 2.2 (IQR 1.5–3.7). The mean PNI was 47.2 (SD 6.2). After the diagnosis of recurrence, a total of 132 (79.0%) patients underwent chemotherapy. The chemotherapy regimens included S-1, n = 38; paclitaxel, n = 18; UFT, n = 3; CPT-11, n = 2; 5-fluorouracil (5-FU), n = 1; a combination of S-1 and cisplatin, n = 21; a combination of CPT-11 and cisplatin, n = 21; a combination of S-1 and docetaxel, n = 11; a combination of paclitaxel and ramucirumab, n = 4; a combination of 5-FU and cisplatin, n = 3; a combination of S-1 and CPT-11, n = 3; a combination of S-1 and trastuzumab, n = 2; a combination of S-1 and oxaliplatin, n = 1; a combination of capecitabine and cisplatin, n = 1; a combination of capecitabine and oxaliplatin, n = 1; a combination of S-1, cisplatin and trastuzumab, n = 1 and a combination of docetaxel, cisplatin and S-1, n = 1. Eleven (6.6%) patients underwent surgical resection of metastatic lesions, and 7 (4.2%) underwent radiotherapy. The best supportive care was provided to 35 (21.0%) patients. Second-line chemotherapy was performed in 74 (56.1%) of the 132 patients who underwent chemotherapy for recurrence. The regimen included paclitaxel, n = 27; S-1, n = 6; docetaxel, n = 3; nab-paclitaxel, n = 3; CPT-11, n = 2; ramucirumab, n = 1; trastuzumab, n = 1; a combination of CPT-11 and cisplatin, n = 11; a combination of S-1 and docetaxel, n = 7; a combination of S-1 and CPT-11, n = 4; a combination of paclitaxel and ramucirumab, n = 4; a combination of S-1 and cisplatin, n = 3; a combination of paclitaxel and cisplatin, n = 1 and a combination of capecitabine and trastuzumab, n = 1.
ROC analyses
Using the 1-year survival as the endpoint, the area under the ROC curve for the NLR and PNI and was 0.728 and 0.665, respectively (Fig. 1). When the NLR was 2.2, the Youden index was maximum, with a sensitivity of 74.6% and specificity of 66.7%. When the PNI was 47, the Youden index was maximum, with a sensitivity of 69.8% and specificity of 55.6%. Thus, the NLR and PNI cut-off values were set at 2.2 and 47, respectively.
The survival after recurrence and prognostic value of the inflammation-based markers
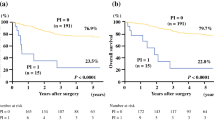
At the final follow-up, 146 (87.4%) patients had died, and 21 (12.6%) remained alive. Overall, the median survival time (MST) after recurrence was 8.1 months, and the 1- and 2-year OS rates were 39.6 and 14.1%, respectively. The associations between the clinicopathological variables and the survival time after recurrence are shown in Table 1. The patients with a higher NLR had a significantly shorter survival time than those with a lower NLR (p < 0.001; Fig. 2a). The survival time was significantly shorter in the patients with a lower PNI than in those with a higher PNI (p < 0.001; Fig. 2b). Other factors, including a younger age, undifferentiated type, tumor depth of T3–4, peritoneal recurrence, high CA 19-9 level and no chemotherapy for recurrence, were also associated with a shorter survival time after recurrence according to the univariate analysis. When adjusted for these factors, the NLR and PNI were independently associated with the survival time (Table 2).
Prognostic value of the inflammation-based markers among patients who underwent chemotherapy
We next evaluated the prognostic value of the inflammation-based prognostic markers among the 132 patients who received chemotherapy for recurrence. The NLR (p < 0.001) and PNI (p = 0.006) were significantly associated with the survival time (Fig. 3a, b). In the multivariate analysis, a higher NLR and a lower PNI were identified as independent predictors of a shorter survival time (Table 3).
Prognostic value of the inflammation-based markers among patients without treatment for recurrence
We further evaluated the prognostic value of the inflammation-based prognostic markers among the 35 patients who received best supportive care. The patients with a higher NLR had a significantly shorter survival time than those with a lower NLR (p = 0.009; Fig. 4a). There was no significant association between the survival time and PNI (p = 0.138; Fig. 4b).
Relationship between the inflammation-based markers and clinicopathological characteristics of the patient
We then evaluated the relationship between the inflammation-based markers and the clinicopathological characteristics of the patients (Table 4). The patients with a lower PNI were much older than the patients with a higher PNI. In addition, the prevalence of chronic renal failure was significantly higher among the patients with a lower PNI than among the patients with a higher PNI. The proportion of patients who underwent chemotherapy for the treatment of recurrence was significantly lower in patients with a higher NLR and lower PNI than in those without such factors. The rate of transition to second-line chemotherapy was significantly lower in the patients with a higher NLR than in the patients with a lower NLR. There was a significant association between the status of the NLR and PNI (p < 0.001).
Discussion
The present study evaluated the prognostic value of the inflammation-based markers, including the NLR and PNI, in patients with RGC and demonstrated that these inflammation-based markers are reliable predictors of the survival time. Previous studies have reported various prognostic factors in the patients with RGC, such as the performance status, time of recurrence, lymph node metastasis and the stage of the disease [15, 19, 20]. Recently, it has been increasingly recognized that the systemic inflammatory response influences the oncological outcomes in cancer patients. Some researchers have investigated the clinical significance of the inflammation-based markers and have found the NLR to be an independent prognostic factor in advanced gastric cancer patients receiving palliative chemotherapy [13, 14]. Although these studies included patients with RGC as well as those with unresectable gastric cancer, the prognostic value of these markers in RGC remains uncertain. One study reported the relationship between the systemic inflammation response and survival time in RGC. Kong et al. evaluated the prognostic significance of CRP in 72 patients with RGC and showed that an elevated CRP level was independently associated with a worse prognosis [15]. To our knowledge, the present study is the first to investigate the prognostic impact of the NLR and PNI in RGC. In our study, patients with a high NLR and a low PNI had a significantly shorter survival time after recurrence than those without such factors. Importantly, the multivariate analysis revealed that these markers were prognostic factors, independent of the age, histology, tumor depth, peritoneal recurrence, CA 19-9 and chemotherapy for recurrence.
One possible explanation for why the inflammation-based markers were associated with the prognosis in the patients with RGC is that these markers were associated with the frequency of chemotherapy. In the present study, the patients with a high NLR and a low PNI received chemotherapy less frequently than those without such factors. It has been established that palliative chemotherapy can prolong the survival time of patients with RGC [2]. In addition, a previous study reported that the presence of chemotherapy was independently associated with the survival time after the recurrence of gastric cancer [21]. Similarly, our study showed that patients who received chemotherapy had a significantly longer survival time than those who did not receive chemotherapy and that the administration of chemotherapy was an independent prognostic factor. In our institute, the administration of chemotherapy was decided based on each patient’s general condition and request. The PNI is considered to be an indicator not only for systemic inflammation, but also the patient’s nutritional status. One study showed that patients with sarcopenia had a significantly lower PNI than those without sarcopenia among patients who underwent major hepatectomy [22]. In the present study, we observed that the PNI was significantly associated with patient age and the prevalence of chronic renal failure, while there were no associations between the NLR and any patient factors. However, several studies have evaluated the relationship between the inflammation-based markers and the performance status. Jeong et al. reported that patients with a higher NLR were more likely to have a PS of ≥2 than those with a lower NLR [13]. These findings suggest that the inflammation-based markers may reflect a patient’s general condition; however, we were unable to evaluate their association with the performance status in the present study.
Another explanation is that the inflammation-based markers are associated with the transition to the second-line chemotherapy. In the present study, a subgroup analysis revealed that the NLR and PNI were independently associated with the survival time among patients who received chemotherapy. In patients treated with palliative chemotherapy, second-line or subsequent chemotherapy had a greater effect on the survival time. In fact, we observed that the patients who received second-line chemotherapy had a significantly longer survival time than those who did not among patients who received any chemotherapy (MST 14.1 vs. 7.4 months, p = 0.002). One study reported that the rate of transition to second-line chemotherapy in the high-PNI group was significantly higher than in the low-PNI group among patients with unresectable colorectal cancer, suggesting that patients with a high PNI were able to continue to a second- or third-line treatment because of an adequate physical reserve [23]. In the present study, we found a significant correlation between the NLR and the rate of the transition to second-line chemotherapy, while no association was observed between the PNI and the transition to second-line chemotherapy. These data also suggest an association between the inflammation-based markers and the patient’s general condition.
In addition, the presence of the systemic inflammation has been reported to be associated with the tolerability and response to anti-cancer treatment in various cancers [9, 10]. It has recently been shown that the systemic inflammation may impair the activity of cytochrome p450 3A4 (CYP3A4), and its reduced activity was found to be associated with an impeded drug response or increased toxicity [24]. However, in the present study, we observed no association between the inflammation-based markers and the incidence of grade ≥3 adverse events from chemotherapy. In contrast, Cho et al. demonstrated that the low-NLR group had a significantly higher disease control rate than the high-NLR group among metastatic advanced gastric cancer who underwent palliative chemotherapy [14]. These data suggest that inflammation-based markers may be useful for identifying patients who are likely to benefit from palliative chemotherapy. Thus, inflammation-based markers can help decision-making in relation to treatment for RGC. For example, an aggressive chemotherapeutic regimen can be administered to patients with a low NLR and a high PNI. However, the present study was unable to evaluate the response to chemotherapy. In addition, the chemotherapeutic regimens used in the present study were heterogeneous, and the efficacy of each regimen might, therefore, not be uniform. Further studies are, therefore, required.
Furthermore, it has been suggested that the inflammation-based markers may reflect the tumor burden and aggressive behavior. Inflammation promotes cancer cell proliferation, tumor angiogenesis and metastasis [5, 6]. Systemic inflammation can increase the number of neutrophils and decrease the number of lymphocytes. Lymphocytes play a central role in antitumor immunity, and lymphocytopenia reflects the impairment of cellular adaptive immunity against cancer cells [5, 6]. Neutrophilia may inhibit the immune system by suppressing the cytolytic activity of immune cells and promote cancer cell growth and metastasis [25]. Furthermore, it has been shown that proinflammatory cytokines, such as interleukin-6 and tumor necrosis factor-α, can decrease the serum albumin level by reducing the production of serum albumin by the hepatic cells [26]. Thus, a high NLR and a low PNI may indicate greater malignant potential of a tumor. Some studies have shown that the NLR and PNI were associated with the tumor depth, lymph node metastasis, distant metastasis and elevated levels of tumor markers in the clinical setting [11, 12]. In the present study, however, these markers were not associated with any tumor-related factors. The NLR and PNI may be associated with the extent of tumor spread at the time of recurrence, such as the number and size of liver and lymph node metastases and the amount of peritoneal fluid, although we were unable to evaluate these associations. Further investigations are, therefore, required to clarify the association between the inflammation-based markers and tumor aggressiveness.
The present study also showed that the NLR was significantly associated with the survival time in patients who received the best supportive care. Among the patients who did not receive treatment for recurrence, the survival time after recurrence was significantly shorter in the patients with a higher NLR than in those with a lower NLR. These findings also suggest that the NLR may reflect the tumor burden and aggressive behavior. In contrast, no significant association was observed between the PNI and the survival time. This may be due to the small number of patients that were not treated for recurrence. In addition, the overall number of patients included in the present study was also relatively small. Therefore, we were unable to draw any definite conclusions based on these findings.
In previous studies, neither the preoperative NLR nor PNI was found to be an independent prognostic factor in patients with stage IV gastric cancer who underwent gastrectomy [12, 27]. These results conflict with our present findings. This discrepancy may be due to the patient selection, as all of the patients included in those studies underwent gastrectomy, while only 6.6% of the patients in the present study underwent surgical resection. However, the precise reason for this discrepancy remains uncertain.
In conclusion, the present study demonstrated that the inflammation-based markers, including the NLR and PNI, are simple and useful clinical biomarkers that can predict the survival time of patients with RGC. Inflammation-based markers may be useful for decision-making in relation to the treatment for RGC. Further large-scale studies are necessary to validate our results.
References
Wohrer SS, Raderer M, Hejna M. Palliative chemotherapy for advanced gastric cancer. Ann Oncol. 2004;15:1585–95.
Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–9.
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44.
Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil–lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–4.
Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O’Reilly DS, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow inflammation outcome study. Eur J Cancer. 2011;47:2633–41.
Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011;104:1288–95.
Shimizu K, Okita R, Saisho S, Yukawa T, Maeda A, Nojima Y, et al. Prognostic nutritional index before adjuvant chemotherapy predicts chemotherapy compliance and survival among patients with non-small-cell lung cancer. Ther Clin Risk Manag. 2015;11:1555–61.
Shimada H, Takiguchi N, Kainuma O, Soda H, Ikeda A, Cho A, et al. High preoperative neutrophil–lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13:170–6.
Migita K, Takayama T, Saeki K, Matsumoto S, Wakatsuki K, Enomoto K, et al. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. 2013;20:2647–54.
Jeong JH, Lim SM, Yun JY, Rhee GW, Lim JY, Cho JY, et al. Comparison of two inflammation-based prognostic scores in patients with unresectable advanced gastric cancer. Oncology. 2012;83:292–9.
Cho IR, Park JC, Park CH, Jo JH, Lee HJ, Kim S, et al. Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer. 2010;17:703–10.
Kong F, Gao F, Chen J, Zheng R, Liu H, Li X, et al. Elevated serum C-reactive protein level predicts a poor prognosis for recurrent gastric cancer. Oncotarget. 2016;7:55765–70.
Association Japanese Gastric Cancer. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12.
Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85:1001–5.
Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163:670–5.
Sakar B, Karagol H, Gumus M, Basaran M, Kaytan E, Argon A, et al. Timing of death from tumor recurrence after curative gastrectomy for gastric cancer. Am J Clin Oncol. 2004;27:205–9.
Chiang CY, Huang KH, Fang WL, Wu CW, Chen JH, Lo SS, et al. Factors associated with recurrence within 2 years after curative surgery for gastric adenocarcinoma. World J Surg. 2011;35:2472–8.
Bilici A, Salman T, Oven Ustaalioglu BB, Unek T, Seker M, Aliustaoglu M, et al. The prognostic value of detecting symptomatic or asymptomatic recurrence in patients with gastric cancer after a curative gastrectomy. J Surg Res. 2013;180:e1–9.
Otsuji H, Yokoyama Y, Ebata T, Igami T, Sugawara G, Mizuno T, et al. Preoperative sarcopenia negatively impacts postoperative outcomes following major hepatectomy with extrahepatic bile duct resection. World J Surg. 2015;39:1494–500.
Ikeya T, Shibutani M, Maeda K, Sugano K, Nagahara H, Ohtani H, et al. Maintenance of the nutritional prognostic index predicts survival in patients with unresectable metastatic colorectal cancer. J Cancer Res Clin Oncol. 2015;141:307–13.
Rivory LP, Slaviero KA, Clarke SJ. Hepatic cytochrome P450 3A drug metabolism is reduced in cancer patients who have an acute-phase response. Br J Cancer. 2002;87:277–80.
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99.
McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223–6.
Tanaka H, Muguruma K, Toyokawa T, Kubo N, Ohira M, Hirakawa K. Differential impact of the neutrophil–lymphocyte ratio on the survival of patients with stage IV gastric cancer. Dig Surg. 2014;31:327–33.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Migita, K., Matsumoto, S., Wakatsuki, K. et al. The prognostic significance of inflammation-based markers in patients with recurrent gastric cancer. Surg Today 48, 282–291 (2018). https://doi.org/10.1007/s00595-017-1582-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-017-1582-y