Abstract
Background
Despite curative surgery for gastric cancer, many patients die of recurrent cancer. Few studies have investigated the time to recurrence after curative resection for gastric cancer.
Methods
Data were collected prospectively between December 1987 and December 2006. A total of 1,549 patients underwent curative resection of adenocarcinoma of the stomach at Taipei Veterans General Hospital. Among them, 419 patients had recurrence; they were divided into early recurrence (<2 years) and late recurrence (≥2 years). The clinicopathological characteristics, survival time after recurrence, and recurrence patterns were compared between the two groups.
Results
Multivariate analysis showed that stage III gastric cancer patients with early recurrence had larger tumors and more lymph node metastasis than patients with late recurrence, while no difference between early and late recurrence was observed in stage I and II patients. Early recurrence was associated with more distant metastasis than was late recurrence. Patients with advanced TNM stage tended to die within 2 years after recurrence.
Conclusions
Gastric cancer patients with larger tumors and more lymph node metastasis tended to have early recurrence, especially stage III patients. Advanced TNM stage was associated with early cancer death after recurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the second most common malignancy in the world, and surgery remains the main treatment with curative intent. Even after gastrectomy and lymphadenectomy, every year many patients die of recurrent gastric cancer [1]. To our knowledge, few studies have investigated early and late recurrence after curative surgery for gastric cancer [2–7]. In the current study, 419 patients with recurrent gastric cancer were analyzed after curative surgery. We hope to understand the clinicopathological characteristics, survival time after recurrence, and initial recurrence patterns of gastric cancer with early and late recurrence.
Materials and methods
A prospective gastric cancer database in the Department of Surgery of Taipei Veterans General Hospital recorded curative resection in 1,549 patients between December 1987 and December 2006. Among them, 419 (27%) had recurrence. The definitions of early and late recurrence vary between studies. Some studies [3] defined early recurrence as patients recurrence within 2 years after surgery, while other studies say 1 year [5, 6] or 3 years [2] after surgery. In our study, most recurrences (305/419, 72.8%) occurred within 2 years after curative surgery. For the purpose of achieving sufficient patient numbers for subsequent subgroup analysis of early and late recurrence in the same tumor stage, we defined early recurrence as recurrence within 2 years after surgery rather than 3 years. Late recurrence was defined as recurrence 2 years or more after surgery. Exclusion criteria included synchronous gastric double cancer, a previous history of surgery for gastric cancer, gastric stump cancer, or cancer anatomically classified as esophageal cancer according to the AJCC 7th ed. (Siewert type 1, type 2, and type 3 with Z-line invasion).
Before surgery, all patients had a chest radiograph, an abdominal sonogram, or a CT scan for tumor staging. Patients were evaluated on the basis of gender, age, tumor size, tumor location, operative method, combined organ resection, pathological tumor and lymph node stage, lymphovascular invasion, stromal reaction type, gross appearance, and recurrence pattern. For stromal reactions, cancer with a small number of stromal cells is classified as medullary type and cancer with a large number as scirrhous type. The intermediate type was histologically intermediate between the medullary and scirrhous types. The gross appearance of tumors was classified as well-defined (superficial type and Bormann types I and II) or ill-defined (Bormann types III and IV).
Follow-up
Overall survival was calculated from the time of surgery until death or the last follow-up contact. None of the patients received induction chemotherapy preoperatively. During the period of this study (1987–2006) adjuvant chemotherapy or radiotherapy after curative surgery was not performed routinely in our hospital, but only when tumor recurrence was diagnosed or highly suspected. Adjuvant therapy such as TS-1 was prescribed for stage II or stage III patients after curative surgery in our hospital since 2008 due to the proven survival benefit [7].
Follow-up assessments were performed every 3 months for the first 5 years after surgery, and every 6 months thereafter until the patient’s death. The follow-up procedures included a medical history, physical examination, routine blood tests, liver function tests, measurement of tumor marker levels (carcinoembryonic antigen and carbohydrate antigen 199), a chest radiograph, and other imaging studies. All routine procedures were performed by a surgeon, upper endoscopy was performed by a gastroenterologist, and upper gastrointestinal series, abdominal sonogram, and CT scans were performed by a radiologist.
Biopsy sampling confirmed recurrent disease or distant metastases; biopsies were not obtained for new, multiple pulmonary lesions or for lesions characteristic of osseous metastases noted on CT or whole-body bone scans. Tumor recurrences in the hepatoduodenal ligament, celiac axis, and peripancreatic region were considered locoregional. We defined remote lymphatic metastasis (para-aortic, Virchow’s, and inguinal nodes) and pulmonary lymphangitic spread as distant lymphatic recurrence. By using metallic staples in both the proximal and the distal cut ends, we were able to identify the anastomotic sites readily and, therefore, could diagnose recurrence in the anastomotic sites or duodenal stumps. Recurrence of cancer was classified as locoregional, hematogenous, distant lymphatic, or peritoneal. Patients with tumor recurrence could receive 5-FU-based chemotherapy.
Statistical analysis
Statistical analysis was performed using SPSS v13.0 (SPSS Inc., Chicago, IL, USA). Data are presented as the mean ± standard deviation (SD). Categorical data were compared by a χ2 test with Yates correction or Fisher’s exact test, as indicated. Logistic regression multivariate analysis was conducted to determine the differences between the two groups. Analysis of survival was performed by the Kaplan–Meier method, and differences between the curves were tested using a two-tailed log-rank test. A P value <0.05 was considered to be statistically significant.
Results
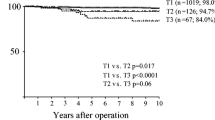
Most recurrences in our study group (304/419, 72.6%) occurred within 2 years after curative surgery. The majority of patients with recurrence of gastric cancer (361/419, 86.2%) died within 2 years after recurrence. We compared the clinicopathological differences between early recurrence (<2 years) and late recurrence (≥2 years). We also analyzed the differences between patients who died within 2 years and those who died ≥2 years after recurrence. Patients with early recurrence had a significantly worse 5-year overall survival than did those with late recurrence (5.4% vs. 40.1%, P < 0.001, Fig. 1).
Clinicopathological characteristics
Univariate analysis showed that early recurrence was associated with larger primary tumors, more ill-defined tumors, more lymphovascular invasion, more advanced T stage, more lymph node metastasis, and a more advanced TNM stage. Multivariate analysis showed that only a larger tumor and a more advanced TNM stage were prominent in early recurrence (Table 1).
We also compared the difference between the two groups in the same tumor stage. We found that there was no significant difference in clinicopathological characteristics between the two groups in stage I or stage II. Only for stage III gastric cancer were a larger tumor size and more lymph node metastasis more common in early recurrence than in late recurrence (Table 2).
Survival time after recurrence
The median survival time after recurrence was 4.8 months in early recurrence and 10.6 months in late recurrence (P = 0.022). Univariate analysis showed that patients with an ill-defined tumor, scirrhous stromal reaction, more advanced T stage, lymph node metastasis, and more advanced TNM stage tended to die within 2 years after recurrence (Table 3); however, multivariate analysis showed that only TNM stage was an independent risk factor for death within 2 years after recurrence.
With regard to locoregional recurrence, there was no significant difference in the median survival time after recurrence between early and late recurrence (5 vs. 11 months, P = 0.295); however, patients with late recurrence tended to survive longer after recurrence than did those with early recurrence. When there was distant metastasis, the median survival was 4 months after early recurrence versus 9.8 months after late recurrence (P = 0.010); for peritoneal dissemination, median survival was 2.8 months after early recurrence versus 10.5 months after later recurrence (P = 0.007); and for distant lymphatic recurrence, median survival was 2.9 months after early recurrence versus 9.5 months after late recurrence (P = 0.031) (Table 4).
Initial recurrence patterns
There is no significant difference between early and late recurrence with respect to locoregional recurrence. Distant metastasis is more common in early recurrence, including both peritoneal dissemination and hematogenous metastasis. Among patients with hematogenous metastasis, liver metastasis is more common in early recurrence (Table 5).
Long-term survival (>5 years) after recurrence
A total of 16 patients survived more than 5 years after recurrence: ten with early recurrence and six with late recurrence. Among the six patients with late recurrence, the recurrence patterns included one Virchow’s lymph node metastasis, two liver metastases, one para-aortic lymph node metastasis, one hepatoduodenal ligament recurrence, and one anastomosis recurrence with liver invasion. Among the ten patients with early recurrence, the recurrence pattern included hepatoduodenal ligament recurrence, lung metastasis, anastomosis recurrence, liver metastasis, abdominal wall recurrence, and peritoneal seeding. Among these patients, only one had peritoneal seeding with abdominal wall recurrence and received chemotherapy after recurrence. The patient died 5 years after recurrence. We also precisely analyzed the different recurrence patterns and management as follows:
Anastomosis recurrence
All three anastomosis recurrences occurred at the gastrojejunostomy site. Each of these patients underwent subsequent radical total gastrectomy. One of these patients also had lateral segmentectomy of the liver and subtotal pancreatectomy because of tumor invasion. Two of the three patients died of pneumonia more than 10 years after the second operation. The third patient remains alive at this time, more than 5 years after the second operation.
Liver metastasis
Of the two patients with liver metastasis, one patient received four rounds of percutaneous ethanol injection therapy, and the other patient developed multiple liver metastases after surgery and received three courses of chemotherapy and then shifted to oral UFUR after a partial response. The follow-up CT scan showed complete response. Both patients are alive at the present time.
Hepatoduodenal ligament recurrence
Among the six patients with hepatoduodenal ligament recurrence, four patients received chemotherapy and radiotherapy; one patient had concurrent lung metastasis and received chemotherapy and radiotherapy; and one patient had a second operation for removal of the single metastatic lymph node on the common hepatic artery and received chemotherapy after surgery. Among the six patients, four patients were alive at the last follow-up.
Discussion
In the current study, stage III patients with a larger tumor and more lymph node metastasis tended to have early cancer recurrence, but this was not observed in stage I and stage II gastric cancer patients. Patients with a more advanced TNM stage were more likely to die within 2 years after recurrence.
In our study, early recurrence is associated with larger tumor size and more advanced TNM stage than was late recurrence. As seen in Table 1, there is a big difference in cancer stage between the early and late recurrence groups. It is a matter of course that the patients with much more advanced stage cancer manifested early recurrence. As a result, we further investigated the difference between early and late recurrence for patients at the same cancer stage, a unique angle and never been mentioned in other series. It is interesting that only for stage III gastric cancer patients was early recurrence associated with larger tumor size and more advanced N stage than late recurrence (Table 2). There was no difference in clinicopathological characteristics between early and late recurrence in stage I and II patients. This might be because the difference in tumor size and pathological N stage is not significant between stage I and II. Otsuji et al. [4] demonstrated that tumor depth was one of the most important predictors of early recurrence; however, some investigators have reported tumor depth not to be an indicator of the timing of recurrence after gastrectomy [2, 3, 6]. The importance of tumor depth as an independent risk factor for early recurrence remains controversial; however, there is agreement in these studies that more advanced tumors tend to have early tumor recurrence. Larger tumor size is associated with deeper cancer invasion and a more advanced tumor stage. Our results showed that both tumor size and TNM stage are more important than tumor depth with regard to early cancer recurrence.
The current study has demonstrated that in patients with cancer recurrence, TNM stage was the most important prognostic factor affecting survival, similar to the findings of the study by Shiraishi et al. [3]. It is understandable that the tumor is more aggressive in advanced gastric cancer, leading to early cancer death after recurrence. Sakar et al. [8] reported that lymph node metastasis is the most important risk factor of death within 2 years after recurrence. Although our results also show that lymph node metastasis is one of the risk factors of early cancer death after recurrence by univariate analysis, multivariate analysis demonstrated that TNM stage more than lymph node metastasis plays the most important role in early cancer death after recurrence.
Eom et al. [6] reported that hematogenous metastasis was more common in early recurrence, which was similar to our observations. Our data showed that patients with early recurrence had more distant metastasis than those with late recurrence, including peritoneal dissemination and hematogenous metastasis. Our results further point out that patients should be carefully monitored for distant metastasis, especially in the first 2 years after surgery. Because gastric cancer has various patterns of recurrence, close follow-up is important for early detection of recurrence and subsequent tailored treatment should be provided as soon as possible.
When accompanied by distant metastasis, late recurrence was associated with longer survival after recurrence than was early recurrence in our study, especially for those with peritoneal dissemination or distant lymphatic recurrence, but not hematogenous metastasis. We should explain this result, because patients with distant metastasis usually had poor survival. In our study, most patients (70/75, 93.3%) with distant lymphatic recurrence within 2 years after surgery had recurrence at more than one site, significantly more than those (7/20, 35%) with distant lymphatic recurrence more than 2 years after surgery (P < 0.001). This might be the reason why patients with late distant lymphatic recurrence had significantly longer survival than did those with early distant lymphatic recurrence. Moreover, for patients with peritoneal recurrence, the prognosis is usually rather negative. Our results showed that patients with early peritoneal recurrence (<2 years) had a larger tumor, more lymphovascular invasion, and more advanced TNM stage than those with late peritoneal recurrence (≥2 years). As a result, patients with early peritoneal recurrence are more likely to have a more aggressive tumor than those with late peritoneal recurrence, thus leading to worse survival.
In the current study, 16 patients had long-term survival of >5 years after recurrence. Among them, all three patients with recurrence at the gastric stump were diagnosed and underwent repeat surgery within 3 years after the first surgery. We suggest that close follow-up with panendoscopy is important, especially in the first 3 years. Early detection of gastric stump cancer could increase the possibility of curative total gastrectomy and, thus, prolong survival. In the current study, despite poor survival after recurrence of cancer and unfavorable response to the chemotherapy in some patients, others did have partial or even complete response after chemotherapy and had long-term survival.
There is still room for improvement in chemotherapy for gastric cancer. S-1 therapy has been included as adjuvant therapy of gastric cancer in the 2010 NCCN guidelines on the basis of its survival benefit and decreased incidence of peritoneal dissemination and distant lymphatic metastasis [7]. Peritoneal recurrence is known to be associated with poor survival. In our study group, only one patient survived 5 years after peritoneal seeding. We hope that in the future, adjuvant chemotherapy, such as S-1, will benefit more patients, not only by increasing survival but also by helping to maintain an acceptable quality of life.
In conclusion, close postoperative systemic follow-up is important for patients with gastric cancer, especially in the first 3 years, and for those with deep cancer invasion and lymph node metastasis.
References
Nakamura K, Ueyama T, Yao T et al (1992) Pathology and prognosis of gastric carcinoma. Findings in 10,000 patients who underwent primary gastrectomy. Cancer 70:1030–1037
Adachi Y, Oshiro T, Mori M et al (1996) Prediction of early and late recurrence after curative resection for gastric carcinoma. Cancer 77:2445–2448
Shiraishi N, Inomata M, Osawa N et al (2000) Early and late recurrence after gastrectomy for gastric carcinoma. Univariate and multivariate analyses. Cancer 89:255–261
Otsuji E, Kuriu Y, Ichikawa D et al (2004) Time to death and pattern of death in recurrence following curative resection of gastric carcinoma: analysis based on depth of invasion. World J Surg 28:866–869. doi:10.1007/s00268-004-7359-7
Yokota T, Saito T, Teshima S et al (2002) Early and late recurrences after gastrectomy for gastric cancer: a multiple logistic regression analysis. Upsala J Med Sci 107:17–22
Eom BW, Yoon H, Ryu KW et al (2010) Predictors of timing and patterns of recurrence after curative resection for gastric cancer. Dig Surg 27:481–486
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Sakar B, Karagol H, Gumus M et al (2004) Timing of death from tumor recurrence after curative gastrectomy for gastric cancer. Am J Clin Oncol 27:205–209
Acknowledgments
This research was supported by the Center of Excellence for Cancer Research at Taipei Veterans General Hospital (DOH100-TD-C-111-007) and the grants from Taipei Veterans General Hospital (99DHA0100632).
Disclosure
The authors have no financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiang, CY., Huang, KH., Fang, WL. et al. Factors Associated with Recurrence Within 2 Years After Curative Surgery for Gastric Adenocarcinoma. World J Surg 35, 2472–2478 (2011). https://doi.org/10.1007/s00268-011-1247-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-011-1247-8