Abstract
Background
Major hepatectomy with extrahepatic bile duct resection for perihilar cholangiocarcinoma remains a highly morbid procedure. The association between preoperative sarcopenia and postoperative morbidity/mortality has been reported for various types of surgeries. The objective was to analyze the relationship between preoperative sarcopenia and postoperative morbidity/mortality in patients who underwent major hepatectomy with extrahepatic bile duct resection.
Methods
This study included 256 patients who underwent major hepatectomy with extrahepatic bile duct resection from 2008 to 2014. Preoperative sarcopenia was assessed by a measurement of the total psoas muscle area (TPA). The measured TPA was normalized by height. Preoperative sarcopenia was defined as the presence of a normalized TPA in the lowest sex-specific tertile.
Results
A total of 54 males and 31 females were determined to have preoperative sarcopenia. The length of the postoperative hospital stay for patients with sarcopenia was significantly longer than for those without sarcopenia (39 vs 30 days, p < 0.001). Patients with sarcopenia experienced a significantly higher rate of liver failure (ISGLS grade ≥ B) (33 vs 16 %), major complications with Clavien grade ≥ 3 (54 vs 37 %), and intra-abdominal abscess (29 vs 18 %) than those without sarcopenia (all p < 0.05). After a multivariate analysis, low normalized TPA (male <567 mm2/m2; female <395 mm2/m2) was identified as an independent risk factor for the development of liver failure (odds ratio 2.46).
Conclusions
This study demonstrated that preoperative sarcopenia increased the morbidity rate including the rate of liver failure, in patients who underwent major hepatectomy with extrahepatic bile duct resection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a result of advancements in the perioperative management of patients and in surgical techniques, liver surgery has become a relatively safe procedure. However, major hepatectomy with extrahepatic bile duct resection for perihilar cholangiocarcinoma remains a challenging procedure that is associated with a high rate of postoperative liver failure and infectious complications [1, 2].
In recent years, the association between preoperative sarcopenia and postoperative morbidity/mortality has been reported in different types of surgeries such as liver transplantation [3, 4], resection of pancreatic cancer [5], cystectomy [6], and hepatectomy [7, 8]. With respect to liver surgery, the impact of sarcopenia on the long-term outcome after hepatectomy for hepatocellular carcinoma [8] and colorectal liver metastasis [9] or after liver transplantation [4] has been reported. However, few reports have investigated the impact of sarcopenia on the short-term outcome after major hepatectomy [10]. Moreover, no published reports have investigated the effects of sarcopenia in patients who underwent a major hepatectomy with extrahepatic bile duct resection, which differs from a simple hepatectomy in terms of surgical insult.
In this study, after a measurement of the total psoas muscle area (TPA) on preoperative computed tomography scans, we retrospectively analyzed the relationship between preoperative sarcopenia and postoperative morbidity/mortality including liver failure, in patients who underwent major hepatectomy with extrahepatic bile duct resection at a single institution.
Patients and methods
Patients
This study included 256 consecutive patients who underwent major hepatectomy with extrahepatic bile duct resection from December 2008 to February 2014 in the Department of Surgery, Nagoya University Hospital in Nagoya, Japan. Patients who underwent hepato-pancreaticoduodenectomy were excluded. All patients underwent a preoperative abdominal/pelvic computed tomography scan within 30 days prior to surgery. When the patients had jaundice, an appropriate biliary drainage, either by endoscopic nasobiliary drainage (ENBD), endoscopic biliary stent (EBS), or percutaneous transhepatic biliary drainage (PTBD), was performed.
Recording of clinical data and postoperative complications
In this study, major hepatectomy included a resection of three or more Couinaud segments. In addition to the major hepatectomy, all patients underwent a caudate lobectomy en bloc. Preoperative and postoperative clinical data including information on demographics, type of treated diseases, type of biliary drainage, indocyanine green plasma disappearance rate (ICG-K) and retention value at 15 min (ICGR15), preoperative portal vein embolization (PVE), presence of preoperative cholangitis, extent of liver resection, type of surgical procedure, and data from laboratory tests were collected. The prognostic nutritional index (PNI), as an indicator of nutritional status, was assessed using the following equation as described previously [11]: PNI = 10 × serum albumin [mg/dl] + 0.005 × total lymphocyte count. Postoperative complications were scored using the Clavien–Dindo classification [12]. A major complication was defined as a complication with a Clavien grade ≥ 3. Liver failure and bile leakage were defined according to the definition of the International Study Group of Liver Surgery [13, 14]. Postoperative pancreatic fistula was defined according to the definition of the International Study Group of Pancreatic Fistula [15]. Postoperative infectious complications including pneumonia, surgical site infections (SSIs), and bacteremia as detected by the culture method, were also recorded. SSIs included superficial/deep incisional infections and organ/space infections.
Image analysis and definition of sarcopenia
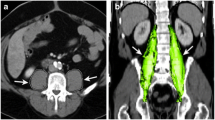
Preoperative sarcopenia was assessed by the measurement of the cross-sectional areas of the right and left psoas muscles (total psoas muscle area = TPA) using preoperative abdominal/pelvic computed tomography images. TPA was measured at the level of the third lumbar vertebra on the first image where both vertebral spines are visible. The border of the psoas muscle was manually outlined, and the TPA was automatically calculated. The measured TPA was normalized by height using the following equation as previously described; normalized TPA [mm2/m2] = measured TPA [mm2]/height [m]2 [16, 17]. Patients were stratified separately by tertiles according to the normalized TPA for males and females [3, 5, 18]. Preoperative sarcopenia was defined as the presence of a normalized TPA in the lowest sex-specific tertile.
Statistical analysis
The data were analyzed using Dr. SPSS II version 11.01 J (SPSS Inc., Chicago, IL, USA). Data were expressed as the mean ± standard deviation (SD) for continuous variables. Continuous data were compared between two groups using Student’s t test. When data were not normally distributed, a univariate analysis with a nonparametric test was used. Categorical data were compared using the χ 2 test. The impact of preoperative sarcopenia on postoperative morbidity and mortality was examined with univariate analyses. Preoperative and intraoperative risk factors for liver failure were explored with multivariate logistic regression analysis. The variables included age, gender, BMI, extent of liver resection, ICG-R15, preoperative cholangitis, normalized TPA, operation time, and blood loss. The optimal cut-off points of preoperative and intraoperative continuous data in the prediction of liver failure were determined using receiver operating characteristic (ROC) curve analysis, and the values were then used in the analysis of risk factors for liver failure. Additionally, preoperative and intraoperative risk factors for liver failure were also explored with multivariate logistic regression analysis. A p value <0.05 was considered statistically significant.
Results
Patient characteristics
The mean age of the study patients was 67 years (range 34–85 years), and 162 patients (63 %) were male. The mean body mass index (BMI) was 21.5 (range 15.1–30.8). The most frequently treated disease was bile duct cancers (n = 218, 85 %) including perihilar cholangiocarcinoma (n = 194, 76 %) and intrahepatic cholangiocarcinoma (n = 24, 9 %). Other diseases included gallbladder carcinoma (n = 17, 7 %), other malignant diseases (n = 6, 2 %), and benign diseases (n = 15, 6 %). Other malignant diseases included hepatocellular carcinoma (n = 3), colorectal liver metastasis (n = 2), and neuroendocrine tumor (n = 1). Benign diseases included intrahepatic stone (n = 6), IgG4-related sclerosing cholangitis (n = 3), and bile duct stenosis after cholecystectomy (n = 3). 231 (90 %) patients underwent preoperative biliary drainage including ENBD (n = 185, 72 %), EBS (n = 3, 1 %), and PTBD (n = 45, 18 %). The mean normalized TPA in male patients was 594 mm2/m2, whereas the mean normalized TPA in female patients was 422 mm2/m2 (p < 0.001). Male patients with a normalized TPA less than 536 mm2/m2 (n = 54) and female patients with a normalized TPA less than 378 mm2/m2 (n = 31) were included in the lowest tertile and were determined to have preoperative sarcopenia.
Sarcopenia and preoperative characteristics
The average normalized TPA of the patients without sarcopenia was 590 mm2/m2, whereas that of the patients with sarcopenia was 410 mm2/m2. Patients with sarcopenia had a significantly lower body weight, BMI, and PNI than those without sarcopenia. There were no differences between the two groups with respect to age, the type of treated diseases, preoperative liver function, the extent of liver resection, the proportion of patients who underwent preoperative PVE, or the presence of preoperative cholangitis (Table 1).
Sarcopenia and intraoperative characteristics
With regard to the intraoperative characteristics, the average operation time was significantly longer in patients with sarcopenia than in those without sarcopenia (Table 2). Other intraoperative variables including the surgical procedure, combined vascular resection, the total vascular occlusion time, and intraoperative blood loss were not different between the two groups.
Sarcopenia and postoperative outcomes
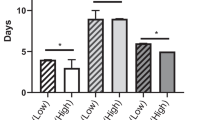
The postoperative hospital stay length among patients with sarcopenia was significantly longer than among patients without sarcopenia (39 vs 30 days, p < 0.001) (Table 3). Patients with sarcopenia revealed a significantly higher rate of liver failure (ISGLS grade ≥ B) (33 vs 16 %, p = 0.003), major complications with Clavien grade ≥ 3 (54 vs 37 %, p = 0.011), and intra-abdominal abscess (29 vs 18 %, p = 0.040) than those without sarcopenia.
Risk factors for liver failure
Post-hepatectomy liver failure is a major complication after major hepatectomy with extrahepatic bile duct resection. Therefore, in the next step, preoperative and intraoperative risk factors that may be associated with the development of liver failure were evaluated (Table 4). The optimal cut-off points of continuous data in the prediction of liver failure were determined using ROC curve analysis. Univariate analysis indicated that the extent of liver resection (>50 %), ICG-R15 (>9 %), preoperative PVE, preoperative cholangitis, normalized TPA (male < 567 mm2/m2; female < 395 mm2/m2), operation time (>600 min), and intraoperative blood loss (>1,200 ml) were associated with the development of liver failure. After a multivariate analysis, with logistic regression analysis, the extent of liver resection (p = 0.003), normalized TPA (p = 0.012), operation time (p = 0.005), and intraoperative blood loss (p < 0.001) were identified as independent risk factors of liver failure. The odds ratio of low normalized TPA (male < 567 mm2/m2; female < 395 mm2/m2) was 2.44 with 95 % confidence intervals (1.20–4.99).
Discussion
The different types of hepatectomies include various extents of invasion that range from a minimum hepatic parenchymal resection to a systematic major hepatectomy. In the treatment for most of perihilar biliary malignancies, major hepatectomies with extrahepatic bile duct resection are essential procedures. In addition, either a combined portal vein resection or a hepatic artery resection is required in highly advanced cases. Therefore, a major hepatectomy with extrahepatic bile duct resection for biliary malignancies is obviously a more invasive procedure than a simple hepatectomy, which requires only a parenchymal dissection. In spite of advancements in the perioperative management of patients and in surgical techniques, the morbidity and mortality rates after major hepatectomies with extrahepatic bile duct resection remain high (morbidity rate, 44 %; mortality rate, 3 %) (1). The rate of postoperative liver failure (grade ≥ B) has also been reported to be high (38 %) [1]. In this procedure, preoperative patient management may be improved to minimize the postoperative complications. Therefore, in this study, we focused on the preoperative sarcopenia that may potentially be improved by a collaboration of patients and medical staff such as nutritionists and physical therapists, among others. According to the findings of this study, normalization of the sarcopenia status is recommended to minimize postoperative complications, including liver failure, following major hepatectomies with extrahepatic bile duct resection.
In the present study, preoperative sarcopenia was defined as the presence of normalized TPA in the lowest sex-specific tertile. The definition of preoperative sarcopenia differs depending on study [3, 5, 6, 8–10, 19, 20]. According to The European Working Group on Sarcopenia in Older People (EWGSOP), the evaluation of the presence of both low muscle mass and muscle function is recommended for a diagnosis of sarcopenia [21]. The definition of sarcopenia used in this study also included the conceptual staging of pre-sarcopenia, as suggested by EWGSOP. Although EWGSOP also suggests the use of handgrip strength to evaluate muscle strength, most of the present study was investigated retrospectively; therefore, the evaluation of muscle function was difficult.
In the present study, patients with sarcopenia had a lower PNI than those without sarcopenia. This result may reflect the malnutrition status in patients with preoperative sarcopenia, which may have negative impact on postoperative recovery including tissue repair [22] and immunological responses [23]. This hypothesis may partly explain the mechanisms of poor postoperative outcome including the incidence rate of liver failure and intra-abdominal abscess in patients with sarcopenia. EWGSOP and several studies have proposed that supplementation with amino acids, vitamin D, testosterone, and growth hormone has the potential to improve muscle mass and function. Among the amino acids, branched chain amino acids (BCAA) have been reported to be associated with muscle mass [24]. The plasma BCAA to tyrosine ratio has been reported to be significantly correlated with preoperative skeletal muscle mass [4]. Kaido et al. reported that perioperative nutritional therapy with BCAA and synbiotics significantly improved overall survival after liver transplantation in patients with sarcopenia [4]. In this regard, the perioperative administration of BCAA may also improve the short-term outcome after highly invasive surgery such as hepatectomy with extrahepatic bile duct resection.
Despite similar surgical invasiveness between the two groups, e.g., combined vascular resection and total vascular occlusion time, the operation time was likely longer in patients with sarcopenia. Moreover, the average intraoperative blood loss tended to be higher in patients with sarcopenia than in patients without sarcopenia, although this difference was not significant. It was hypothesized that patients with sarcopenia may have an impaired coagulability that may be due to a deteriorated ability to synthesize coagulation factors. To confirm this hypothesis, further prospective data collection with an evaluation of preoperative coagulability tests is necessary.
After a multivariate analysis, the extent of liver resection, low normalized TPA, long operation time, and excessive blood loss were identified as independent risk factors for the development of liver failure. The extent of the liver resection, the operation time, and intraoperative blood loss are variables that are dependent on surgical invasiveness; in other words, they are determined by tumor aggressiveness. Therefore, among the four independent risk factors of liver failure which are identified in this study, only preoperative sarcopenia can be improved by a comprehensive preoperative patient management. Preoperative nutrition therapy [4] as well as preoperative rehabilitation (i.e., prehabilitation) [25, 26] should be incorporated into future preoperative patient management plans. These results indicated that the low normalized TPA, likewise the extent of hepatectomy, is an important risk factor in predicting postoperative liver failure. In addition to the incidence of liver failure, low normalized TPA (cut-off values determined by ROC curve analysis; male < 580 mm2/m2, female < 396 mm2/m2) was also identified as an independent risk factor for postoperative morbidity with Clavien grade ≥ 3 by multivariate analysis (the odds ratio 1.78; 95 % confidence intervals 1.07–2.98; p = 0.028) (table not shown).
In conclusion, this study demonstrated that preoperative sarcopenia increased morbidity rate, including that of liver failure, after major hepatectomy with extrahepatic bile duct resection. Among the risk factors of liver failure that were identified in this study, only sarcopenia can be improved by preoperative patient management. It seems that preoperative nutritional therapy and rehabilitation are essential to improve the short-term outcome following highly invasive surgery such as major hepatectomy with extrahepatic bile duct resection.
References
Yokoyama Y, Ebata T, Igami T et al (2014) Predictive power of prothrombin time and serum total bilirubin for postoperative mortality after major hepatectomy with extrahepatic bile duct resection. Surgery 155:504–511
Nagino M, Ebata T, Yokoyama Y et al (2013) Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg 258:129–140
Krell RW, Kaul DR, Martin AR et al (2013) Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl 19:1396–1402
Kaido T, Ogawa K, Fujimoto Y et al (2013) Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transpl 13:1549–1556
Peng P, Hyder O, Firoozmand A et al (2012) Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg 16:1478–1486
Smith AB, Deal AM, Yu H et al (2014) Sarcopenia as a predictor of complications and survival following radical cystectomy. J Urol 191(6):1714–1720
Voron T, Tselikas L, Pietrasz D et al (2014) Sarcopenia Impacts on Short- and Long-term Results of Hepatectomy for Hepatocellular Carcinoma. Ann Surg. doi:10.1097/SLA.0000000000000743
Harimoto N, Shirabe K, Yamashita YI et al (2013) Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg 100:1523–1530
van Vledder MG, Levolger S, Ayez N et al (2012) Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg 99:550–557
Peng PD, van Vledder MG, Tsai S et al (2011) Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford) 13:439–446
Roy LB, Edwards PA, Barr LH (1985) The value of nutritional assessment in the surgical patient. JPEN J Parenter Enter Nutr 9:170–172
Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196
Rahbari NN, Garden OJ, Padbury R et al (2011) Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 149:713–724
Koch M, Garden OJ, Padbury R et al (2011) Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 149:680–688
Bassi C, Dervenis C, Butturini G et al (2005) Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138:8–13
Baumgartner RN, Koehler KM, Gallagher D et al (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755–763
Mourtzakis M, Prado CM, Lieffers JR et al (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33:997–1006
Dodson RM, Firoozmand A, Hyder O et al (2013) Impact of sarcopenia on outcomes following intra-arterial therapy of hepatic malignancies. J Gastrointest Surg 17:2123–2132
Dello SA, Lodewick TM, van Dam RM et al (2013) Sarcopenia negatively affects preoperative total functional liver volume in patients undergoing liver resection. HPB (Oxford) 15:165–169
Lieffers JR, Bathe OF, Fassbender K et al (2012) Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 107:931–936
Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 39:412–423
Yoshida R, Yagi T, Sadamori H et al (2012) Branched-chain amino acid-enriched nutrients improve nutritional and metabolic abnormalities in the early post-transplant period after living donor liver transplantation. J Hepatobiliary Pancreat Sci 19:438–448
Reynolds JV, O’Farrelly C, Feighery C et al (1996) Impaired gut barrier function in malnourished patients. Br J Surg 83:1288–1291
Lustgarten MS, Price LL, Chale A et al (2014) Branched chain amino acids are associated with muscle mass in functionally limited older adults. J Gerontol A 69:717–724
Mayo NE, Feldman L, Scott S et al (2011) Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery 150:505–514
Carli F, Brown R, Kennepohl S (2012) Prehabilitation to enhance postoperative recovery for an octogenarian following robotic-assisted hysterectomy with endometrial cancer. Can J Anaesth 59:779–784
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Otsuji, H., Yokoyama, Y., Ebata, T. et al. Preoperative Sarcopenia Negatively Impacts Postoperative Outcomes Following Major Hepatectomy with Extrahepatic Bile Duct Resection. World J Surg 39, 1494–1500 (2015). https://doi.org/10.1007/s00268-015-2988-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-015-2988-6