Abstract
Purpose
Nutrition and immunity significantly affect the progression of cancer in cancer patients. Therefore, the evaluation of the nutritional and immune status would be useful as a prognostic factor and to determine the optimal treatment strategy for patients with unresectable metastatic colorectal cancer who are receiving chemotherapy. The aim of this retrospective study was to evaluate the prognostic significance of the nutritional and immune status in patients with unresectable metastatic colorectal cancer treated with chemotherapy.
Methods
We retrospectively reviewed 80 patients with colorectal cancer. A total of 22 patients had metachronous unresectable cancer, and 58 patients had synchronous unresectable cancer. All patients underwent combination chemotherapy with oxaliplatin or irinotecan plus 5-fluorouracil/leucovorin as first-line chemotherapy. We then examined the correlations between the Onodera’s prognostic nutritional index (OPNI) and the patients’ clinicopathological features. The OPNI was calculated as follows: 10 × serum albumin (g/dl) + 0.005 × total lymphocyte count (per mm3). According to the receiver operating characteristic (ROC) curve analysis, the cutoff value for OPNI was 44.5.
Results
Patients with a pretreatment OPNI of ≧44.5 demonstrated a longer OS than those with a pretreatment OPNI of <44.5. Moreover, we categorized these patients into four groups according to the combination of the pre- and post-treatment OPNI. The patients in the group with both OPNIs ≥44.5 exhibited a better prognosis compared to the other group (p = 0.001).
Conclusion
The OPNI is considered to be a useful marker for predicting the long-term outcome in patients who receive chemotherapy for unresectable metastatic colorectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Japan, colorectal cancer is the one of the most of common cancers, accounting for the greatest number deaths from malignant neoplasms in females and the third greatest number of such deaths in males (Kayama et al. 2011). Although many patients with colorectal cancer undergo potentially curative resection, some of them relapse after surgery. Moreover, some patients have unresectable metastatic tumors at the time of diagnosis. Chemotherapy is necessary in patients with unresectable metastatic colorectal cancer to improve their prognosis. Furthermore, the number of patients with colorectal cancer is expected to increase, thus increasing the importance of chemotherapy as a therapeutic strategy.
Recently, the selection of chemotherapy regimens has widened, with different outcomes. Although the choice of chemotherapy regimen influences the prognosis, host factors, such as the nutritional and immune status, also play important roles in the effectiveness of chemotherapy (Ross et al. 2004). Therefore, nutrition and immunity are thought to affect the progression of disease in cancer patients. The evaluation of the nutritional and immune status is thus thought to be useful as a prognostic factor and to determine the optimal treatment strategies for patients with unresectable metastatic colorectal cancer who receive chemotherapy.
Onodera’s prognostic nutritional index (OPNI) is known to be an indicator that could be used to evaluate the nutritional and immune status. The OPNI can be determined from the serum albumin level and lymphocyte count. Therefore, the OPNI is easy to use in clinical practice. The OPNI was originally proposed as an indicator of postoperative complications, such as anastomotic leakage, pneumonia and wound infection, and a determinant of resection or anastomosis in patients undergoing gastrointestinal cancer (Onodera et al. 1984), although it has recently become widely used as an independent preoperative prognostic indicator in patients with various carcinomas (Kanda et al. 2011; Feng and Chen 2014; Nozoe et al. 2010, 2012; Maeda et al. 2014). The aim of this retrospective study was to evaluate the prognostic significance of the nutritional and immune status in patients with unresectable metastatic colorectal cancer treated with chemotherapy.
Methods
Patients
We reviewed all patients diagnosed with unresectable metastatic colorectal cancer between April 2003 and December 2012 at the Department of Surgical Oncology, Osaka City University Graduate School of Medicine, Japan. Of these patients, we identified 80 patients with unresectable metastatic colorectal cancer. The median age of the patients was 63 years, with a range of 36–80 years (Table 1). The patients included 44 males and 36 females. A total of 22 patients had metachronous unresectable cancer, and 58 patients had synchronous unresectable cancer. All patients underwent combination chemotherapy with oxaliplatin or irinotecan plus 5-fluorouracil/leucovorin as first-line chemotherapy. In particular, 42 patients received 5-FU + leucovorin + oxaliplatin (FOLFOX), 19 patients received capecitabine + oxaliplatin (CapeOX), eight patients received 5-FU + leucovorin + irinotecan (FOLFIRI) and 11 patients received other regimens.
Evaluation
The clinicopathological classification was determined according to the TNM classification of malignant tumors, as described by the International Union Against Cancer (UICC) (Sobin et al. 2009). We collected data from blood tests conducted within 1 week before start of the first-line chemotherapy and at 8 weeks after the first day of chemotherapy, including the serum albumin level, total lymphocyte count in the peripheral blood and carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9 levels. Computed tomography was carried out for response evaluation after four cycles of chemotherapy and/or at the time of clinical deterioration.
The clinicopathological factors included in the univariate analyses were as follows: age, gender, primary lesion, location of the primary tumor, timing of detection of unresectable, histology, number of metastatic sites, CEA level (cutoff level was 5.0 ng/ml), CA 19-9 level(cutoff level was 37 U/ml) and the OPNI (high/low).
The OPNI was calculated as follows: 10 × serum albumin (g/dl) + 0.005 × total lymphocyte count (per mm3) (Onodera et al. 1984). The differential white blood cell (WBC) count was analyzed using an XE-5000 hematology analyzer (Sysmex, Kobe, Japan).
The pretreatment OPNI was defined as the value of the OPNI calculated within 1 week before the start of the first-line chemotherapy, and the post-treatment OPNI was defined as the value of the OPNI calculated 8 weeks after the first day of chemotherapy.
Overall survival (OS) was defined as the time from the date of initiation of first-line chemotherapy to death from any cause. Progression-free survival (PFS) was defined as the time from the date of initiation of first-line chemotherapy to disease progression.
We defined the total treatment period (TTP) as the interval from the start of chemotherapy to the provision of best supportive care.
The response to treatment was evaluated using the response evaluation criteria in solid tumors (RESIST) criteria (Eisenhauer et al. 2009).
Statistical analysis
The associations between the OPNI and the clinicopathologic parameters were assessed using the χ 2 test or Fisher’s exact test. A univariate survival analysis was performed using the Kaplan–Meier method, and differences were evaluated according to the log-rank test. A multivariate survival analysis was performed using Cox’s proportional-hazard model. The hazard ratio (HR), along with the 95 % confidence interval (95 % CI), was used as a measurement of the association in this study. The data were analyzed statistically using the JMP® 10 software program (SAS Institute Inc., Cary, NC, USA) A p value of <0.05 was considered to be statistically significant.
Results
Among all patients, the median OS was 30.5 months and the median PFS was 8.7 months. The overall response rate was 38.7 %, and there was no significant difference between the pretreatment OPNI and response rate (Table 2). In this study, stratification of the OPNI was informed by the best predictive values for overall survival calculated using ROC curves analyses, which found the most sensitive and specific values based on the peak and cutoff points. Based on this analysis, the cutoff value for OPNI was 44.5 (AUC = 0.563, sensitivity: 73.3 %, specificity: 42 %).
There were 42 patients with an OPNI of 44.5 or higher (52.5 %) and 38 patients with an OPNI of <44.5 (47.5 %). We analyzed the correlations between the OPNI and the characteristics of the patients. No significant differences were observed except for the gender (Table 3).
We then examined the correlations between the clinicopathological factors and the prognosis. In the univariate analysis, age and OPNI were found to be significantly associated with the OS (Table 4). A multivariate analysis demonstrated that an age of more than 70 years (HR 2.52, CI 1.36–4.50; p = 0.004), and OPNI (HR 2.37, CI 1.35–4.14; p = 0.002) was independent risk factors for a worse survival.
The overall survival of the patients with a pretreatment OPNI ≥44.5 was significantly (p = 0.005) better than that of the patients with a pretreatment OPNI of <44.5 (Fig. 1a). The median survival time (MST) was 37.0 and 22.8 months, respectively. There were no significant associations between the OPNI and the PFS (Fig. 1b). The rate of transition to secondary treatment was 96.0 and 76.6 %, respectively, in the high and low OPNI groups (p = 0.012).
The high pretreatment OPNI group had a significantly longer TTP than the low pretreatment OPNI group. The median TTP from the initiation of chemotherapy was 22.2 and 16.8 months, respectively, in the high and low OPNI groups (p = 0.043, Fig. 2).
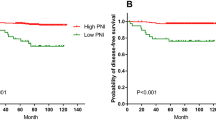
We categorized the patients into four groups according to the combination of their pre- and post-treatment OPNI: group (1) both OPNIs ≥ 44.5; group (2) pretreatment OPNI ≥44.5 and post-treatment OPNI <44.5; group (3) pretreatment OPNI <44.5 and post-treatment OPNI ≥44.5; group (4) both OPNIs <44.5.
The patients in group 1 exhibited a better prognosis compared to the other groups (p = 0.001, Fig. 3). The MST was 18.5, 24.2 and 21.3 months in groups 2, 3 and 4, respectively, compared with 51.3 months in group 1 (Fig. 3).
Discussion
There have been many reports on the prognostic factors for colorectal cancer. Some of them reported a correlation between the prognosis and tumor-related factors such as the depth of tumor invasion, lymph node metastasis, the extent of distant metastasis, lymphovascular invasion, the histological type, microsatellite instability (MSI), KRAS mutation and BRAF mutation (Zhang et al. 2010; Mehrkhani et al. 2009; Zlobec et al. 2010). Moreover, some authors described the correlations between the prognosis and treatments such as surgery and chemotherapy (Kuhry et al. 2008; Meyerhardt and Mayer 2005).
Recently, the immune and nutritional status have been recognized as host-related factors that affect the prognosis. In general, the lymphocyte count reflects the immune status, while the albumin level reflects the nutritional status. Therefore, it is considered that the OPNI is a good index which reflects both the immune and nutritional status of the host. The OPNI has been reported to be a useful preoperative prognostic marker for patients with various cancers (Kanda et al. 2011; Feng and Chen 2014; Nozoe et al. 2010, 2012; Maeda et al. 2014). In this study, we demonstrated that the OPNI is a useful prognostic marker in patients with unresectable metastatic colorectal cancer treated with chemotherapy.
We revealed that the patients with a pretreatment OPNI <44.5 had a poor prognosis. Since the OPNI is composed of both the lymphocyte count and serum albumin level, lymphocytopenia and hypoalbuminemia cause a reduction of the value of the OPNI. In addition, it has been reported that lymphocytopenia and hypoalbuminemia are independent prognostic factors (Oñate-Ocaña et al. 2007; Gupta and Lis 2010; Ceze et al. 2011).
There are several possible causes of hypoalbuminemia. In cancer patients, a low serum albumin concentration may be mainly due to the production of proinflammatory cytokines, such as interleukin-1 (IL-1), interleukin-6 (IL-6) and tumor necrosis factor (TNF-α), which modulate the production of albumin by hepatocytes (Oñate-Ocaña et al. 2007; Rothschild et al. 1988; Chojkier 2005; Fearon et al. 1998; Forones et al. 2001; Bauer and Herrmann 1991). Moreover, chronic inflammatory diseases, including cancer, result in reductions in the serum albumin level (Chojkier 2005). Regarding the serum albumin level, some authors have indicated that a low serum albumin level is associated with a poor outcome in cancer patients (Oñate-Ocaña et al. 2007; Gupta and Lis 2010).
Lymphocytes are crucial components of the host’s cellular adaptive immunity against cancer cells and can both attack malignant cells and eliminate nascent tumor cells (Smyth et al. 2006; Rosenberg 2001). Therefore, a low lymphocyte count may be responsible for an insufficient immunologic reaction to tumors (Hoffmann et al. 2002). Ceze showed that pretreatment lymphocytopenia is an independent risk factor for a shorter survival following palliative chemotherapy in patients with colorectal cancer (Ceze et al. 2011). In this way, the immune function and nutritional status are associated with the prognosis and progression of cancer.
In this study, there were significant differences in the OS with respect to the pretreatment OPNI; however, no differences were observed in the PFS. This observation may be due to the fact that second-line or subsequent therapy has greater effects on the OS. The rate of transition to secondary treatment was significantly different between the high and low OPNI groups. In addition, a tendency toward a longer TTP was noted in the high OPNI group compared with that observed in the low OPNI group. Even when the primary treatment was discontinued due to side effects and/or disease progression, the high OPNI group was able to continue to a second and third line of treatment because they had an adequate physical reserve. As a result, high OPNI contributed to improving the OS.
We subsequently evaluated the internal changes between the pre- and post-treatment OPNI. As a result, the group that maintained a high OPNI had a better prognosis than the other groups. Even in the patients with a high pretreatment OPNI, a decrease in the OPNI after chemotherapy was associated with a worse OS. Disease progression due to a compromised immune function and difficulty in continuing treatment as a result of a decreased physical condition are thought to account for the decrease in OS. Therefore, in patients who receive chemotherapy, it is important to maintain a good nutritional and immune status before and during treatment.
Cachexia reduces the quality of life in cancer patients and may result in the discontinuation of treatment. It has been suggested that cachexia is the direct cause of death in 20 % of cancer patients (Haggar and Boushey 2009). Cancer anorexia-cachexia syndrome impedes the response to chemotherapy due to frequent toxicities and severe complications, which results in a shortened survival time (Ciccolallo et al. 2005; Haggar and Boushey 2009). Hence, with respect to the management of chemotherapy patients, worsening of the nutritional status should be avoided to the extent possible. Dintinjana reported that nutritional counseling, supplemental feeding and pharmacological support can be used to temporarily stop weight loss and improve appetite in patients with colorectal cancer (Dintinjana et al. 2008). Therefore, providing nutritional support may be effective in improving the nutritional status, thereby reducing chemotherapy-induced morbidities (Dintinjana et al. 2013).
In this study, having a good pretreatment nutritional and immunological status and maintaining them during treatment allowed for the use of continuous treatment and improved the OS. However, we were unable to conclude whether nutritional intervention is effective in patients who receive chemotherapy, and no large studies have prospectively investigated of the influence of nutritional intervention. It is difficult to directly improve immunocompetence; however, it has been previously reported that improvements in the nutritional status lead to improvements in immunity (Klek et al. 2014). Therefore, we believe that it is necessary to evaluate whether nutritional intervention contributes to improving the OPNI.
In conclusion, the OPNI is a useful marker for predicting the long-term outcome in patients who receive chemotherapy for unresectable metastatic colorectal cancer. Maintenance of the nutritional and immunological status may be important in such patients.
References
Bauer J, Herrmann F (1991) Interleukin-6 in clinical medicine. Ann Hematol 62:203–210
Ceze N, Thibault G, Goujon G, Viguier J, Watier H, Dorval E, Lecomte T (2011) Pre-treatment lymphopenia as a prognostic biomarker in colorectal cancer patients receiving chemotherapy. Cancer Chemother Pharmacol 13:1305–1313
Chojkier M (2005) Inhibition of albumin synthesis in chronic diseases. J Clin Gastroenterol 39:S143–S146
Ciccolallo L, Capocaccia R, Coleman MP et al (2005) Survival differences between European and US patients with colorectal cancer: role of stage at diagnosis and surgery. Gut 54:268–273
Dintinjana RD, Guina T, Krznarić Z, Radić M, Dintinjana M (2008) Effects of nutritional support in patients with colorectal cancer during chemotherapy. Coll Antropol 32:737–740
Dintinjana RD, Trivanovic D, Zelić M et al (2013) Nutritional support in patients with colorectal cancer during chemotherapy: does it work? Hepatogastroenterology 60:475–480
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours, revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Fearon KCH, Falconer JS, Slater C (1998) Albumin synthesis rates are not decreased in hypoalbuminemic cachectic cancer patients with an ongoing acute-phase protein synthesis. Ann Surg 227:249–254
Feng JF, Chen QX (2014) Significance of the prognostic nutritional index in patients with esophageal squamous cell carcinoma. Ther Clin Risk Manag 10:1–7
Forones NM, Mandowsky SV, Lourenco LG (2001) Serum levels of interleukin-2 and tumor necrosis factor-alpha correlate to tumor progression in gastric cancer. Hepatogastroenterology 48:1199–1201
Gupta D, Lis CG (2010) Pretreatment serum albumin as a predictor of cancer survival : a systemic review of the epidemiological literature. Nutr J 9:69
Haggar FA, Boushey RP (2009) Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg 22:191–197
Hoffmann TK, Dworacki G, Tsukihiro T, Meidenbauer N, Gooding W, Johnson JT, Whiteside TL (2002) Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res 8:2553–2562
Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A (2011) Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg 98:268–274
Kayama T, Wakao F, Sobue T, Katanoda K, Tsukuma H, Mikami H, Kitai A (2011) Cancer statistics in Japan, 2011. Foundation for Promotion of Cancer Research, Tokyo
Klek S, Szybinski P, Szczepanek K (2014) Perioperative immunonutrition in surgical cancer patients: a summary of a decade of research. World J Surg 38:803–812
Kuhry E, Schwenk W, Gaupset R, Romild U, Bonjer J (2008) Long-term outcome of laparoscopic surgery for colorectal cancer: a Cochrane systematic review of randomised controlled trials. Cancer Treat Rev 34:498–504
Maeda K, Shibutani M, Otani H et al (2014) Low Nutritional Prognostic Index correlates with poor survival in patients with stage IV colorectal cancer following palliative resection of the primary tumor. World J Surg 38:1217–1222
Mehrkhani F, Nasiri S, Donboli K, Meysamie A, Hedayat A (2009) Prognostic factors in survival of colorectal cancer patients after surgery. Color Dis 11:157–161
Meyerhardt JA, Mayer RJ (2005) Systemic therapy for colorectal cancer. N Engl J Med 3:476–487
Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T (2010) Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today 40:440–443
Nozoe T, Kohno M, Iguchi T, Mori E, Maeda T, Matsukuma A, Ezaki T (2012) The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today 42:532–535
Oñate-Ocaña LF, Aiello-Crocifoglio V, Gallardo-Rincón D et al (2007) Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol 14:381–389
Onodera T, Goseki N, Kosaki G (1984) Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 85:1001–1005
Rosenberg SA (2001) Progress in human tumour immunology and immunotherapy. Nature 411:380–384
Ross PJ, Ashley S, Norton A et al (2004) Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer 90:1905–1911
Rothschild MA, Oratz M, Schreiber SS (1988) Serum albumin. Hepatology 8:385–401
Smyth MJ, Dunn GP, Schreiber RD (2006) Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol 90:1–50
Sobin L, Gospodarowicz M, Wittekind C (2009) TNM classification of malignant tumors, 7th edn. Wiley, International Union Against Cancer, New York, pp 73–77
Zhang S, Gao F, Luo J, Yang J (2010) Prognostic factors in survival of colorectal cancer patients with synchronous liver metastasis. Color Dis 12:754–761
Zlobec I, Bihl MP, Schwarb H, Terracciano L, Lugli A (2010) Clinicopathological and protein characterization of BRAF- and K-RAS-mutated colorectal cancer and implications for prognosis. Int J Cancer 15:367–380
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ikeya, T., Shibutani, M., Maeda, K. et al. Maintenance of the nutritional prognostic index predicts survival in patients with unresectable metastatic colorectal cancer. J Cancer Res Clin Oncol 141, 307–313 (2015). https://doi.org/10.1007/s00432-014-1799-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1799-8